Abstract
Fossils of pan-carettochelyid turtles are known from most continents of the world, except Antarctica. The fossils that have been described indicate a group of estuarine turtles that have little modified their body form since the Cretaceous. The only species for which ecological data exist is the extant Carettochelys insculpta, found in estuarine or fresh waters in Australia and New Guinea. Here we report the discovery of an incomplete skull of a previously unknown carettochelyid Carettochelys niahensis sp. nov. from an undated fossil deposit within or beneath a Miocene marine limestone formation in Niah Great Cave, Sarawak, Malaysia. The skull exhibits many anatomical features characteristic of this turtle group but differs from previously known taxa in that it has a broad, nasal orifice that is proportionately wider than in other carettochelyids and relatively shallow but wider temporal arches. Some aspects of the palaeoecology of this turtle are inferred.
Arthur White [[email protected]] Earth and Sustainability Science Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney 2052, Australia;
Michael Archer [[email protected]] Earth and Sustainability Science Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney 2052, Australia;
Suzanne J. Hand [[email protected]] Earth and Sustainability Science Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney 2052, Australia;
Henk Godthelp [[email protected]];
Anna K. Gillespie [[email protected]] Earth and Sustainability Science Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney 2052, Australia.
CARETTOCHELYID turtles (sensu Joyce et al. Citation2021) have a long fossil history, yet, for most species little is known about their palaeoecology. There is only one living species, Carettochelys insculpta Ramsay, 1886 from northern Australia and southern New Guinea. It is primarily a riparian/estuarine species that lives in rivers but sometimes forages out into marine environments (Georges & Kennett Citation1989, Eisemberg Citation2010). Fossil carettochelyids are known from North America, Europe, Asia, Africa and Australasia (Joyce Citation2014, Rule et al. Citation2022, Joseph-Ouni et al. Citation2023). Many of the fossil localities imply still, freshwater paleoenvironments, in contrast to the habitat of C. insculpta, which will inhabit estuarine and saline environments.
Few of the extinct carettochelyids are complete, and many have not been fully described or figured (Joyce Citation2014). Those that have been described, and in particular the Caenozoic taxa, demonstrate an overall conservation of body form. For example, Joyce et al. (Citation2018) compared the skull of the Middle Eocene Anosteira pulchra (Clark, Citation1932) from fluviatile sediments (Roehler Citation1992) in the Washakie Formation of North America to that of C. insculpta. Despite being separated by more than 18 000 km and 42 million years, the major difference noted was the relative placement of the posterior internal carotid foramen. The authors did, however, also note that the skull was narrower, and the nares and orbits differed slightly in orientation. Danilov et al. (Citation2017) described the cranial anatomy of Anosterira maomingensis Chow & Liu, Citation1955 and re-examined the cranial features of pan-Carettochelyid turtles but was restricted in the number of cranial characters that could be used in developing a phylogeny for this group.
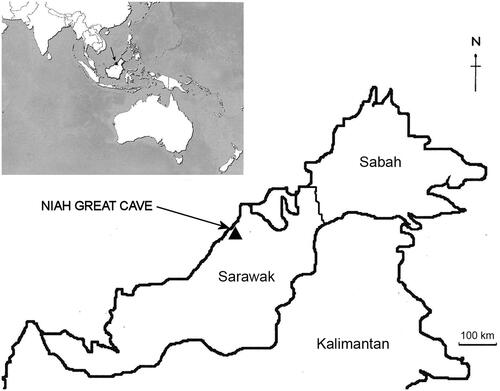
In 1989, we discovered a partial carettochelyid skull in a fossil deposit in Niah Great Cave, Sarawak. The geology of the Niah Cave system has been described in general (Wilford Citation1964), but the deposit that contained the turtle fossils described herein has not previously been known. Although the surrounding sediments are Miocene marine limestones, the deposit containing these turtle remains may be unrelated to or even underlie these Miocene limestones. Hence, the age and even the petrogenesis of this deposit are at present unknown.
In this paper, we describe the partial skull from Niah Great Cave and examine anatomical features that may provide insights into the palaeoecology of this animal.
Institutional abbreviations
UNSW, University of New South Wales, Sydney, Australia; SM, Sarawak Museum, Kuching, Sarawak, Malaysia.
Material and methods
The previously unknown fossiliferous deposit was discovered as a partially exposed outcrop at floor level beneath an overhanging massif within the interior of Niah Great Cave. Because of its recessed position, we were only able to retrieve a few pieces of matrix for examination. When it was clear that these contained fossil bones, with permission from the State Secretary of the Chief Minister’s Office, and the Directors of SM, Forestry Department, and National Parks, these were transported to UNSW for preparation and study. The carbonate matrix was subjected to dissolution using dilute (∼5%) acetic acid. The fossil bones were found to be intact and permineralized, requiring no additional preparation or reconstruction.
Comparative skull material of Carettochelys insculpta was obtained from preserved specimens that had been part of a previous research programme (Heaphy Citation1990) at the University of New South Wales, Sydney. Two skulls prepared from this material, one from an adult male and one from an adult female, are held in the zoology collections at UNSW. Nomenclature for cranial anatomy follows that used by Gaffney (Citation1979).
Anatomical abbreviations
bs, basisphenoid; bo, basioccipital; fr, frontal; j, jugal; max, maxilla; pa, parietal; pal, palatine; pf, pre-frontal; pm, premaxilla; p-o, post-orbital; q-j, quadrato-jugal; qu, quadrate; s-o, supraoccipital; sq, squamosal; v, vomer.
Systematic palaeontology
Order TESTUDINES Linnaeus, Citation1758
Infraorder CRYPTODIRA (Cope, Citation1868)
Superfamily TRIONYCHIA Fitzinger, Citation1826
Family CARETTOCHELYIDAE Boulenger, Citation1887
Carettochelys niahensis sp. nov.
(, )
Diagnosis
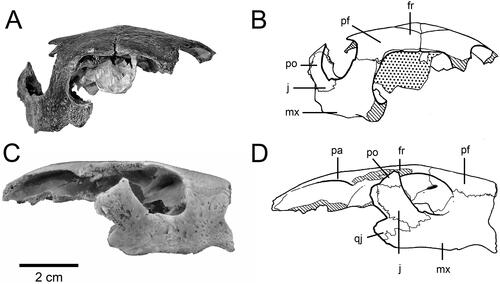
Carettochelys niahensis is distinguished from Carettochelys insculpta (), the only other species in the genus, as follows. Carettochelys niahensis has a relatively flat skull ( and ) in contrast to the skull of C. insculpta (), which is steeply angled from the orbit back. In C. niahensis, the frontal bones are wider than the prefrontals along the mid-line, and the post-orbitals lie mainly in the dorsal plane, whereas in C. insculpta, they lie mainly in the lateral plane. The frontals also make a broader contribution to the orbit. The ventral ridge of the frontals (crista cranii) forms a broad contact with the pre-frontal, such that the contact area forms a vertical sheet of bone that almost completely ossifies the medial wall of the orbit, this feature being much more pronounced than in C. inscultpa. There is also a foramen present between the posteroventral process of the pre-frontal and the crista cranii. Unlike in C. insculpta, this foramen appears to provide a direct connection between the orbit and the nasal cavity. The parietals are not raised and are scarcely higher than the upper cranium (a narrow canal still exists, however, for the passage of the main external muscle for the lower jaw). The jugal is relatively large and square in contrast to the condition in C. insculpta where the jugal is smaller and rectangular in shape (see ). The jugal makes up most of the posterior wall of the orbit. The orbit is deeply recessed and there is a distinct maxillary ridge on the lower rim of the orbit. The ventral line of the maxilla is relatively straight, although there is a distinct notch immediately posterior to the premaxillary suture. In C. insculpta this suture is also present but there is an additional downwards curve on the maxilla below the orbit giving the maxilla a sinusoidal profile. The pre-frontal struts contacting the vomer are curved rather than almost straight as they are in C. insculpta (). The top of the inner orbit is flat, unlike the condition in C. insculpta where the orbit is evenly rounded.
Etymology
The species name refers to the type locality, the Niah Great Cave in Sarawak.
Holotype
SM cb 2.9.a, upper third of an intact, adult skull, collected on 11 December 1989 by a team from UNSW.
Type locality, unit and age
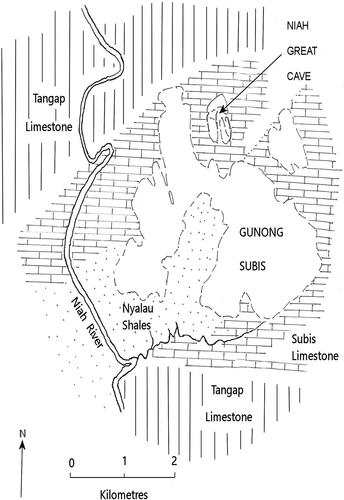
Batu Mangantung Dalaman, Niah Great Cave, Sarawak, Malaysia (). Niah Great Cave () is a massive karst system that includes several long passageways, some up to 3.5 km long that link to the main chamber. This cave occurs within the Goonong Subis topographic unit, which is regarded to be Eocene to Miocene in age (Wilford Citation1964; ). The latter consists of two limestone units: an underlying marine limestone that is part of the Nyalau Formation; and an overlying marine limestone, the Subis Limestone, that is part of the Tangap Formation. Niah Great Cave is contained within the Subis Limestone. Relatively recent stratigraphic analysis indicates that the Subis Limestone is early Miocene (Aquitanian), while the more basal Nyalau Formation is late Oligocene (Chattian) (Mihaljevic et al. Citation2014). The Subis Limestone member is rich with foraminiferan remains and shows weak evidence of marine erosion post deposition. Solution grooves and planes are evident and consistent with decreasing water levels associated with regional uplifting of the limestone beds in the middle to late Pleistocene (Wilford Citation1964). If this interpretation is correct, the Goonong Subis area must have been an isolated island surrounded by shallow, marine bays until the Pleistocene. The Niah Great Cave itself may not have formed until sometime during the Pleistocene (Mihaljevic et al. Citation2014).
Many of the lower (and presumably more recent) solution passages of the Niah Great Cave are filled with alluvial fine-grained sediments containing fragments of chert. In some areas, this has become consolidated into cave breccia.
At present, we do not know which of the known geological units noted above, if any, was the primary source for this carettochelyid material. It is even possible, given its very basal position in the cave system itself, that the deposit that contained these bones pre-dates all of the Goonong Subis units exposed in Niah Great Cave. Before the age of this deposit can be more precisely determined, further investigation of the type locality within the cave will be required. Until that time, we have tentatively concluded that the deposit is late Oligocene or younger in age.
Description
The holotype comprises the upper third of an adult turtle skull (). The skull measured 77 mm from the anterior edge of the right pre-frontal to the broken posterior margin of the right parietal (). The right maxilla, frontal and jugal are complete and intact. The left pre-frontal and frontal are also intact. The left maxilla and jugal are missing, but the left post-orbital is present. The skull shows clean breaks along the right palato-maxillary suture, the right jugal-post-orbital suture, the right vomerine-maxillary suture, the left jugal-post-orbital suture and the left pre-frontal-maxillary suture. The lack of weathering or abrasion of the skull indicates that the missing skull pieces are probably still in the limestone matrix at the type locality. The holotype was collected by the four junior authors on this paper, and it is planned to revisit the site to locate additional fossil material.
Figure 2. Skull of Carettochelys niahensis, sp. nov., SM cb2.9.a, Niah Great Cave, Sarawak, Malaysia. A–B, Dorsal view. C–D, Ventral view.
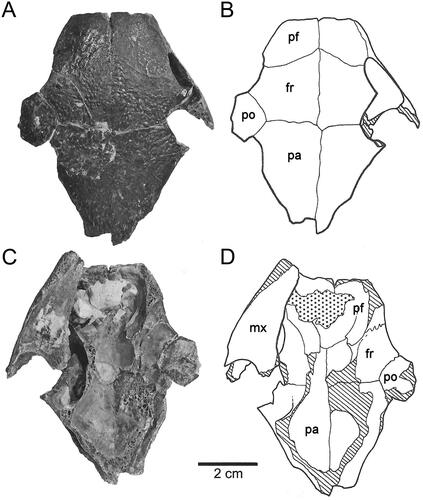
Figure 4. Planar view of the Niah National Park with major stratigraphic units marked (after Wilford, Citation1964).
The dorsal surface of the skull is fairly flat. The prefrontals are quite large, measuring 14 mm along the mid-line and being 18 mm wide at the frontal suture. The prefrontals contact the frontals, premaxilla and maxilla. The frontals are also large and flat, being 23 mm along the mid-line. Frontals contact the prefrontals anteriorly, the post-orbitals and parietals posteriorly. The incomplete parietals are even larger, taking up 40 mm of the mid-line (). The parietals are triangular in dorsal aspect but have a shallow ventrolateral recess along their entire length. Anteriorly, the parietals contact the frontals and post-orbitals, while the dorsal extension of the parietals meets with supracoccipital in the dorsal plane. This recess expands quickly to form the upper braincase. The medial border of the posterior skull emargination formed by the parietal is evident.
The nasal opening is very large, being 30 mm in width (). The nasal bones (and lacrimal bones) are absent. The edges of the nasal opening are level with the inner margin of the orbit. The descending process of the prefrontals barely occludes this wide nasal chamber. The sulcus olefactorius is very wide, being almost as wide as the posterior portion of the cavum cranii.
Figure 5. Skull features of Carettochelys spp., in lateral view. A, Carettochelys insculpta. B, C. niahensis.
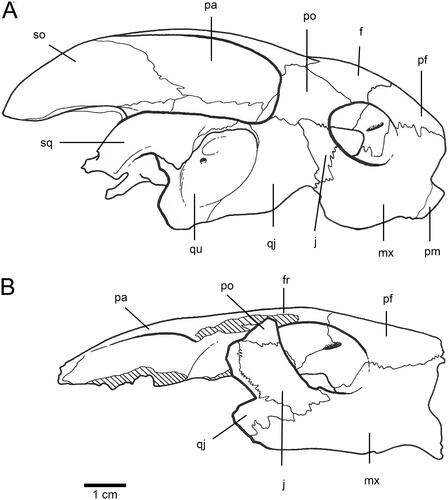
The orbit is relatively large, being 20 mm in diameter. It is deeply recessed behind a lip of the maxilla. The maxilla forms almost the entire floor of the orbit. The squat jugal makes up most of the posterior wall of the orbit, while the post-orbital lies in a more dorsal plane forming the upper posterior margin of the orbit. There is a large foramen high on the inner recess of the pre-frontal. This foramen lies between the posteroventral process of the pre-frontal and crista cranii, and appears as an oblique opening connecting the orbit to the nasal cavity (). The general orientation of the orbit is dorso-lateral.
The ventral line of the maxilla is not straight. It is indented anteriorly, anterior to the suture with the premaxilla (). The maxillae are relatively wide (15 mm) anterior to the orbit. The palatine process of the maxilla is relatively narrow. Suture contacts between the vomer and prefrontals are present. The contact between the vomer and premaxillae does not appear possible.
Figure 6. Skull features of Carettochelys spp., in dorsal (A–B) and ventral (C–D) views. A, C, Carettochelys insculpta. B, D, Carettochelys niahensis.

The temporal arch is relatively shallower (in contrast to the condition in C. insculpta). This arch is the conduit for the two main jaw closing muscles; the m. adductor mandibulae Pars profundus and the m. adductor mandibulae externus Pars superficialis. Compression of this arch restricts the muscle supply to the mandible and hence limits the biting power of the jaws. The narrowing of the temporal arch also restricts transverse movement of the mandible.
Unfortunately, the pterygoids are not present; hence no assessment of the location or orientation of the internal carotid arteries or facial nerves is possible (see Joyce et al. Citation2018).
Referral to Carettochelys
Assignment of this skull to Testudines has been made on the basis of the extent of skull roofing (Joyce Citation2017). The extensive posterior emargination of the skull and the presence of a pre-frontal strut making contact with the vomer indicate placement in the Cryptodira (Gaffney Citation1975, Joyce Citation2007).
Referral to Trionychia can be made by a process of elimination. Among cryptodiranturtles, only the families Carettochelyidae, Chelydridae, Dermatemydidae, Emydidae, Kinosternidae and Trionychidae have extensive posterior emargination of the skull where the emargination is anterior to the quadrate (Gaffney Citation1975). Of these families, only the Carettochelyidae and the Kinosternidae have contact between the quadratojugal and the maxilla.
Joyce (Citation2014) recognized additional shared-derived characters in the skull that he regarded to be trionychian features, including the lack of cheek emargination and the extensive participation of the palatines in making up the braincase. The new species described here qualifies on all these points to be regarded as a carettochelyid.
Confident placement of the new taxon in Carettochelyidae can be made on the basis of several distinctive features: there are deep, temporal emarginations in the skull but relatively weak cheek emarginations (Walther Citation1922, Havlik et al. Citation2014), and there are incomplete sutures between the jugal and quadratojugal and between the maxilla and the jugal. This indicates that there is a strong contact between the quadratojugal and the maxilla below the jugal (Gaffney Citation1975). In addition, the dorsal surface of the parietal, frontal and pre-frontal has shallow crenulations, similar to those present in Allaeochelys Noulet, Citation1867 (Havlik et al. Citation2014) and Carettochelys insculpta (Joyce Citation2014),
The skull of Carettochelys niahensis has contact between the maxilla and quadratojugal (). This contact is also present in Allaeochelys species (Harrassowitz Citation1922) and C. insculpta (Walther Citation1922), but is not present in Anosteira Leidy, Citation1871 species (Joyce et al. Citation2018).
The classical determination (sensu Williams Citation1950) of subfamilial affiliation within the Carettochelyidae is based on shell and vertebral structures. These elements are not available for C. niahensis. The inclusion of C. niahensis within Carettochelyinae is confirmed by the contact between the quadratojugal and maxilla (Danilov et al. Citation2017).
Placement within the genus Carettochelys is more problematic. Inclusion in Carettochelys is indicated by the extent of the quadratojugal-maxilla contact (Gaffney Citation1975). However, it appears that this feature is also present in Allaeochelys species (Harrassowitz Citation1922; unpublished observations; W. Joyce, pers. comm.). However, the extent of contact between these bones may be informative; in both C. insculpta and C. niahensis, there is broad contact between the quadratojugal and the maxilla, whereas in Allaeochelys, the contact is quite narrow. The sulcus olfactorius as formed by the crista cranii is relatively wide in C. niahensis, similar to C. insculpta, whereas in Allaeochelys, the sulcus is much narrower (Havlik et al. Citation2014).
Six apomorphies were used to diagnose Allaeochelys libyca Havlik, Joyce & Bohme, Citation2014 (Havlik et al. Citation2014), but owing to the incompleteness of the skull of C. niahensis, none of these features are available for comparison. The described skull of A. libyca lacks the post-orbital region; hence comparisons with the enlarged jugal and quadratojugal placement present in C. niahensis are not possible either. Theoretically Carettochelys (Boulenger Citation1887) could even prove to be a junior synonym of Allaeochelys (Noulet Citation1867). Given these uncertainties, we nevertheless decided to at least tentatively allocate the Niah species to Carettochelys rather than Allaeochelys based solely on the extrinsic factor of the far greater geographic proximity of Sarawak to Sahul than to Africa.
Discussion
Using skull size as a guide, Carettochelys niahensis was a moderately large turtle. A modern pig-nose turtle (Carettochelys insculpta) with the same size skull (i.e., about 10 cm long and 6 cm wide) would have a straight shell length of about 50 cm. However, there are a number of distinctive differences between the skulls of C. niahensis and C. insculpta that are suggestive of ecological and functional differences between the two species. The Sarawak turtle had a broader head than an equivalent sized pig-nose turtle (). Orientation of the orbits indicates that C. niahensis had relatively more extensive vision above its head and correspondingly less lateral vision. This arrangement of the orbits is most commonly found in bottom-dwelling turtles, rather than free-swimming species (Pritchard Citation1979). The orientation of the orbits is only moderately upwards directed, compared with species that are totally bottom-dwellers, and so it is inferred that C. niahensis spent most of its time in deeper water and rarely occupied shallow or surface waters.
The relatively flat and broad maxillae () suggest that C. niahensis did not chew its food. Instead, its jaws appear to have been better suited for crushing soft food or swallowing food whole. The greater nasal aperture and nasal cavities suggest that the snout was movable and probably used for both olfaction and touch.
Perhaps the most interesting aspect of the skull of C. niahensis is the relatively small space available for the major adductor muscles for the lower jaw (). In the modern pig-nose turtle, the main external adductor muscles pass anteriorly from their attachment points on the supraoccipital and parietals to the occiput, over the trochlear apparatus to attach to the coronoid process on the lower jaw via an adductor tendon. These muscles have a large attachment area at the back of the skull, extending from the sagittal crest (comprising both supraoccipital and parietal crests) around and over the posterior blade of the supraoccipital. A large trough exists between the cranium and the skull roof to accommodate these muscles. This trough is created by the extension of the dorsal processes of the parietal and supraoccipital. The trochlear is posterior to the orbit leaving a large space for the converging muscle fibres leading to the lower jaw.
The dorsal process of the parietal is more ventral, and the trochlear process is much closer to the orbit and below the line of skull emargination. Assuming that all of the adductor muscles are equally restricted by this arrangement, the biting strength of the Sarawak turtle must have been much less than that of the living species. This suggests that softer (or smaller) food items made up the bulk of its diet. In the wild, C. insculpta feeds primarily on river weed but will also opportunistically scavenge flesh (Georges & Wombey Citation1993). Given the catholic diet of C. insculpta, it is likely that the Niah Carettochelys also fed upon a range of soft foods such as aquatic invertebrates, small molluscs and soft (non-fibrous) plant matter. It probably did not scavenge harder foods as much as its modern cousin.
Fossil history and zoogeography of carettochelyid turtles
Carettochelyid turtles have an extensive fossil history commencing in the Mesozoic. The oldest known specimen is an unknown species of Kizylkumemys Nesov, Citation1977 from Early Cretaceous sediments in northeastern Thailand (Tong et al. Citation2009). Other Mesozoic specimens include Kizylkumemys schultzi Nesov, Citation1977, a Cretaceous turtle from Uzbekistan (Nesov Citation1977) and Kizylkumemys khoratensis Tong, Sutheethorn, Claude, Buffetaut & Jintasakul, Citation2005 also from northeastern Thailand.
Carettochelyids have traditionally been considered close allies of the soft-shelled turtles (Trionychidae) (Ramsey Citation1886, Meylan Citation1987), and although it has been challenged by some authors (e.g., Nesov Citation1977), molecular data (e.g., Shaffer et al. Citation1997, Pereira Citation2017, Thomson et al. Citation2021) strongly support the sister relationship between the trionychids and carettochelids. Both families flourished in Eurasia during the late Mesozoic and Palaeogene (Nesov Citation1977).
By Eocene times, carettochelyids had radiated extensively in the Northern Hemisphere and had entered North America. Eocene fossils are known from France (de Broin Citation1977), Germany (Zangler Citation1959), Spain (Jiminez-Fuentes Citation1971), India (Lydekker Citation1887), Pakistan (de Broin Citation1987), Mongolia (Zangler Citation1947, Shuvalov & Chkhikvadze Citation1979), China (Chow Citation1956) and North America (Clark Citation1932). Eighteen extinct genera have been described (Meylan Citation1988).
Previous studies have proposed that carettochelyids had already differentiated into two recognizable groups by the beginning of the Palaeogene (Joyce Citation2014). These groups, Anosteira species and the subfamily Carettochelyinae, differ in size, scale patterns, carapacial spines (Meylan Citation1988) and cervical vertebrae (Williams Citation1950). Both groups are present in fossil sites throughout Eurasia, but only the Anosteira turtles reached North America (Meylan Citation1988). Carettochelys is a carettochelyine genus but has some features that are unusual. For example, adult carettochelyine turtles lack scutes and have rounded shells, whereas in Carettochelys insculpta, some scutes may be retained, and the carapace retains a smooth, dorsal keel (Meylan Citation1988). A more recent study (Danilov et al. Citation2017) has also made the case that the Anosteira species group is paraphyletic.
Carettochelyids were still widespread in the Miocene. Fossil material is known from Europe, Africa, the Middle East and Australia, but none is known from Asia. Carettochelyid fossils known from south of Wallace’s Line are rare and comprise shell pieces of a carettochelyine turtle from Papua New Guinea (Glaessner Citation1942), and recent discoveries of carettochelyid remains from Miocene Victoria (Rule et al. Citation2022) and Pliocene Queensland (Joseph-Ouni et al. Citation2023). Gaffney (Citation1981) refutes claims by Gorter & Nicol (Citation1978) that fossils found in Windjana Gorge in Western Australia are carettochelyid, and we agree.
Presuming the Niah fossil deposit is Neogene in age, the Niah fossil is the only Neogene carettochelyine turtle skull known from Southeast Asia. Its presence suggests that carettochelyine turtles dispersed south into Africa and the Middle East, and from China into Southeast Asia and the Indonesian Archipelago by Neogene time where Carettochelys evolved.
Godinot & de Lapparent de Broin (Citation2003) argued that carettochelyids originated in Asia and spread westwards and southwards during the Tertiary. Their movements appear to coincide with the development of the new tropics in Southeast Asia, and it may be that they were able to exploit the new freshwater habitats created there. Current interpretation (Audley-Charles Citation1988, Metcalfe Citation1994, Hall Citation2017) suggests that parts of the island of Borneo existed as small land fragments that were isolated by large marine barriers between the late Jurassic until the late Cretaceous. However, the only contact with other land masses occurred in the late Eocene when the small land unit that is now north Borneo abutted Southeast Asia. Contact was of short duration owing to sea floor spreading associated with the formation of the South China Sea. This appears to be the only opportunity for ancestral carettochelyids to enter Borneo directly. From the Eocene onwards, access into Borneo would have had to have been by sea. By the late Oligocene, the Malay Archipelago had broken up into an extensive island chain separated by wide, shallow seas. Carettochelyids would have been required to make long sea journeys between islands, and freshwater river retreats may have been uncommon.
The living C. insculpta is primarily a freshwater species in Australia (Georges & Kennett Citation1989), although it is known to be salt-tolerant and swims in coastal estuaries in New Guinea (Eisemberg Citation2010), and it may nest on coastal beaches (Georges Citation1987). If ancestral carettochelyids were similar in lifestyle, they may not have been able to complete large ocean crossings but would have been able to island-hop between neighbouring islands and the continent of Australia, providing that freshwater rivers were present at land fall.
Recent discoveries of carettochelyid remains in Miocene (Rule et al. Citation2022) and Pliocene (Joseph-Ouni et al. Citation2023) deposits in Australia indicate an entry into this land mass by the Miocene. The Miocene fragments from Beaumaris in Victoria could not be assigned to a genus but are the southernmost record of carettochelyids anywhere in the world (Rule et al. Citation2022). The Pliocene material was ascribed to Carettochelys and is notable in that it represents the most inland location in Australia for this family, being 250 km from the nearest coastal area.
The spread of carettochelyids southwards after the Eocene is paralleled by a similar but later radiation of trionychid turtles. Trionychids, the sister-group to carettochelyids, are known from Eocene deposits in Australia (White Citation2002) but died out there by the Pleistocene, perhaps in response to the reduction in rainfall over much of the Australian continent (Georges & Wombey Citation1993). The entry of trionychids into Southeast Asia appears to have occurred in the Miocene, after the arrival of carettochelyids. Southward dispersal appears to be a repeated event. For example, one living trionychid, Pelochelys bibroni (Owen, 1853), has relatively recently dispersed throughout Indonesia and New Guinea (Moll & Vijaya Citation1986) but has not yet reached Australia.
Acknowledgements
The Australian Research Council provided funding to SJH and MA for palaeontological and zoological research in Sarawak. We thank Sarawak’s State Secretary of the Chief Minister’s Office, Director of SM, Director of Forestry, and Director of National Parks for their support and help in obtaining permits and organizing contacts during our research. The rangers and staff in Niah National Park were also very helpful during site inspection and fossil collecting. We thank Wong Siew Fui (SM) and G. M. Drawhorn (California State University Sacramento), for assisting with specimen registration and advice. Constructive criticism by Serjoscha Evers (University of Freiburg) and an anonymous reviewer greatly improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Audley-Charles, M.G., 1988. Evolution of the southern margin of the Tethys (North Australian Region) from the Early Permian to Late Cretaceous. In Gondwana and Tethys. Audley-Charles, M. G. and Hallam, A. eds, Geological Society of London Special Publication No. 37, Oxford, UK, 79–100.
- Boulenger, G., 1887. On a new family of pleurodiran turtles. Annals and Magazine of Natural History 19, 170–172.
- Chow, M.C., 1956. Supplementary notes on Anosteira maomingensis. Acta Palaeontologica Sinensis 4, 233–238.
- CHOW, M.-C. & LIU, C.-L., 1955. A new anosteirine turtle from Maoming, Kwangtung. Acta Palaeontologica Sinica 3, 275–282.
- Clark, J., 1932. A new anosteirid from the Uinta Eocene. Annals of the Carnegie Museum 21, 161–170.
- Cope, E.D., 1868. Sixth contribution to knowledge of the marine Miocene fauna of North America. Proceedings of the American Philosophical Society 35, 139–146.
- Danilov, I.G., Obraztsova, E.M., Chen, W. & Jianhua, J., 2017. The cranial morphology of Anosteira maomingensis (testudines, Pan-Carettochelys) and the evolution of Pan-Caretochelyid turtles. Journal of Vertebrate Paleontology 37, e1335735.
- De Broin, F., 1977. Contribution a l’etude palaeontologique des chelonians, cheloniens continenteaux du cretace et du tertiare de France. Memoirs de la Musee de naturelle Histoire (Naturalist Series C) 38, 1–366.
- De Broin, F., 1987. Lower vertebrates from the Early-Middle Eocene Kuldana Formation of Kohat (Pakistan): Chelonia. University of Michigan Contributions to Vertebrate Paleontology 27, 169–185.
- Eisemberg, C.C., 2010. Nesting Ecology, Harvest and Conservation of the Pig-Nosed Turtles (Carettocehlys insculpta) in the Kikori region, Papua New Guinea. Unpublished PhD thesis, University of Canberra, Canberra.
- Fitzinger, L.J., 1826. Newe classification der reptilien. G. H. Heuber Verlag, Wein.
- Gaffney, E.S., 1975. A phylogeny and classification of the higher categories of turtles. Bulletin of the American Museum of Natural History 155, 391–436.
- Gaffney, E.S., 1979. Comparative cranial morphology of recent and fossil turtles. Bulletin of the American Museum of Natural History 164, 65– 376.
- Gaffney, E.S., 1981. A review of the fossil turtles of Australia. American Museum Novitates 2720, 1–39.
- Georges, A. & Kennett, R., 1989. Dry-season distribution and ecology of Carettochelys insculpta (Chelonia: Carettochelydidae) in Kakadu National Park, Northern Australia. Wildlife Research 16, 323–325.
- Georges, A. & Wombey, J., 1993. Family Carettochelyidae. In Fauna of Australia, Vol. 2A eds, Glasby, C. J., Ross, G. J. B. & Beesley, P. L., Australian Government Publishing Service, Canberra, 2–11.
- Georges, A., 1987. The pig-nosed turtle Warradjan. Australian Natural History 22, 230–234.
- Glaessner, M.F., 1942. The occurrence of the New Guinea turtle (Carettochelys) in the Miocene of New Guinea. Records of the Australian Museum 21, 106–109.
- Godinot, M., De Lapperent De Broin, F., 2003. Arguments for a mammalian and reptilian dispersal from Asia to Europe during the Palaeocene–Eocene boundary interval. Deinsea 10, 255–275.
- Gorter, J.D. & Nicol, R.S., 1978. Reptilian fossils from Windjana Gorge, Western Australia. Journal of the Royal Society of Western Australia 60, 97–104.
- Hall, R., 2017. Southeast Asia: new views of the geology of the Malay Archipelago. Annual Review of Earth and Planetary Sciences 45, 331–358.
- Harrassowitz, H., 1922. Die Schildkrotengattung Anosteira von Messel bei Darmstadt und ihre stammesgeschtliche Bedeutung. Abhandlungen der Hessischen Geologischen Landesanstalt zu Darmstadt 6, 132–239.
- Havlik, P.E., Joyce, W.G. & Bohme, M., 2014. Allaeochelys libyca, a new carettochelyine turtle from the Middle Miocene (Langhian) of Libya. Bulletin of the Peabody Museum of Natural History 55, 201–214.
- Heaphy, L., 1990. The Ecology of the Pig Nose Turtle, Carettocehlys insculpta, in Northern Australia. PhD thesis, University of New South Wales, Sydney. (unpublished)
- Jiminez-Fuentes, E., 1971. Primer pseudotrionyx espagnol Allaeochelys casasecai, nov. sp. de Luteciense de Corrales (Zamora). Estudies Geologique Instituta “Lucas Mallada” 27, 153–166.
- Joseph-Ouni, M., White, A., Mccord, W.P. & Spring, K., 2023. Meadow fairy: a new fossil species of Carettochelys (Testudinata: Carettochelyidae) from Queensland, Australia. Part E: Return to the Darling Downs XIX – New Fossil Taxa Special Supplement 3. The Australian Fossil Turtles Record 3. The Batagur Monographs 9, 168–183.
- Joyce, W.G., 2007. Phylogenetic relationships of Mesozoic turtles. B. Peabody Museum of Natural History 48, 3–102.2.0.CO;2]
- Joyce, W.G., 2014. A review of the fossil record of turtles of the clade Pan-Carettochelys. Bulletin of the Peabody Museum of Natural History 55, 3–33.
- Joyce, W.G., 2017. A review of the fossil record of basal Mesozoic turtles. Bulletin of the Peabody Museum of Natural History 58, 65–113.
- Joyce, W.G., Anquetin, J., Cadena, E.-A., Claude, J., Danilov, I.G., Evers, S.W., Ferreira, G.S., Gentry, A.D., Georgalis, G.L., Lyson, T.R., Pérez-García, A., Rabi, M., Sterli, J., Vitek, N.S. & Parham, J.F., 2021. A nomenclature for fossil and living turtles using phylogenetically defined clade names. Swiss Journal of Palaeontology 140, 5.
- Joyce, W.G., Volpato, V.S. & Rollot, Y., 2018. The skull of the carettochelyid turtle Anosteira pulchra from the Eocene (Uintan) of Wyoming and carotid canal system of carettochelyid turtles. Fossil Record 21, 301–310.
- LEIDY, J., 1871. Remarks on extinct turtles from Wyoming Tertiary, Anosteira ornata and Hybemys arenarius. Proceedings of the Academy of Natural Sciences of Philadelphia 1871, 102–103.
- Linnaeus, C., 1758. Systema naturae. 10th ed. Vol. 1. Laurentius Salvius, Stockholm.
- Lydekker, R., 1887. Eocene Chelonia from the Salt Range. Memoirs of the Geological Survey of India (Ser. 10) 4, 58–65.
- Metcalfe, I., 1994. Gondwanaland origins, dispersion and accretion of East and Southeast Asia continental terranes. Journal of South American Earth Sciences 7, 333–347.
- Meylan, P., 1987. Phylogenetic relationships of soft-shelled turtles. Bulletin of the American Museum of Natural History 186, 1–101.
- Meylan, P., 1988. Peltochelys Dollo and the relationships among the genera of the Carettochelidae (Testudines: Reptilia). Herpetologica 44, 440–450.
- Mihaljevic, M., Renema, W., Welsh, K. & Pandolfi, J.M., 2014. Eocene–Miocene shallow-water carbonate platforms and increased habitat diversity of Sarawak, Malaysia. Palaios 29, 378–391.
- Moll, E.E. & Vijaya, J., 1986. Distributional records of some Indian turtles. Journal of the Bombay Natural History Society 8, 538–552.
- Nesov, L.A., 1977. A new genus of pitted-shelled turtle from the Upper Cretaceous of Karakalpakia. Paleontological Journal 1, 96–107.
- NOULET, J.B., 1867. Nouveau genre de tortues fossiles sous le nom d' Allaeochelys. Mémoires de l'Académie des Sciences, 6e Série 5, 172–177.
- Pereira, A.G., Sterli, J., Moreira, F.R. & Schrago, C.G., 2017. Multilocus phylogeny and statistical biogeography clarify the evolutionary history of major lineages of turtles. Molecular Phylogenetics and Evolution 113, 59–66.
- Pritchard, P.C.H., 1979. Encyclopedia of Turtles. T. F. H. Publications, Neptune.
- Ramsey, E.P., 1886. On a new genus and species of freshwater tortoise from the Fly River, New Guinea. Proceedings of the Linnean Society of New South Wales (Series 2) 1, 158–162.
- Roehler, H.W., 1992. Description and Correlation of Eocene Rocks in Stratigraphic Reference Sections for the Green River and Washakie Basins, Southwest Wyoming. U. S. Geological Survey Professional Paper 1506-D, Washington, DC, 83 p.
- Rule, J., Kool, L., Parker, W.M.G. & Fitzgerald, E.M.G., 2022. Turtles all the way down: Neogene pig-nosed turtle fossil from southern Australia reveals cryptic freshwater turtle invasions and extinctions. Papers in Paleontology e1414, 7 pp.
- Shaffer, H.B., Meylan, P. & Mcknight, M.L., 1997. Tests of turtle phylogeny: molecular, morphological, and paleontological approaches. Systematic Biology 46, 235–268.
- Shuvalov, V.T. & Chkhikvadze, V.M., 1979. On the stratigraphical and systematical position of some freshwater turtles from new Cretaceous localities in Mongolia. Trudy Sovmestnoy Sovetsko-Mongol’skoy Paleontologicheskov Ekspeditsiis 8, 58–76. [in Russian).
- Thomson, R.C., Spinks, P.O. & Shaffer, H.B., 2021. A global phylogeny of turtles reveals a burst of climate-associated diversification on continental margins. Proceedings of the National Academy of Science 118, e2012215118.
- Tong, H.J., Claude, J., Buffetaut, E., Suteethorn, V., Naksri, W. & Chitsing, S., 2005. Fossil turtles of Thailand: an updated review. In Papers from the 2005 Heyuan International Dinosaur Symposium. Lu, J. C., Kobayashi, Y., Hunag, D. & Lee, Y. N., eds, Geological Publishing House, Beijing, 183–194.
- Tong, H.J., Claude, J., Suteethorn, V., Naksri, W. & Buffetaut, E., 2009. Turtle assemblages of the Khorat Group (Late Jurassic–Early Cretaceous) of north-eastern Thailand and their palaeobiogeographical significance. Geological Society, London, Special Publications 315, 141–152.
- Walther, W.G., 1922. Die Neu-Guinea-Schildkrote Carettochelys insculpta Ramsay. Novae-Guinea 13, 607–704.
- White, A.W., 2002. A new Eocene soft-shelled turtle (Trionychidae) from Murgon, south-eastern Queensland. Memoirs of the Association of Australasian Palaeontologists 25, 37–43.
- Wilford, G.E., 1964. The Geology of Sarawak and Sabah Caves. Geological Survey, Borneo Region, Malaysia. Bulletin No. 6, 1–181.
- Williams, E., 1950. Variation and selection on the cervical central articulations of living turtles. Bulletin of the American Museum of Natural History 94, 505–562.
- Zangler, R., 1947. A new anosteirine turtle from Manchuria. Field Geology 10, 13–21.
- Zangler, R., 1959. Rudimentäre carapaxschuppung bei jungen exemplaren von Carettochelys und inhre morphogenetische bedeutung. Naturforschen Gest Zurich 104, 138–147.