Abstract
Six species of monotremes, three newly described here, occur in the Cenomanian fossil fauna from Lightning Ridge in northeastern New South Wales, Australia, making it the most diverse monotreme assemblage on record. Four species are known from a single specimen, suggesting that diversity remains underrepresented. No other mammal lineages are known from the deposit, although the absence of smaller taxa is likely due to sampling biases introduced by the opal mining process. Early-Late Cretaceous monotremes thus clearly diversified in Australia during the absence of other large-bodied mammalian competitors; and subsequently occupied a wider range of ecological niches than at any other time in their evolutionary history. One new taxon described herein represents a previously unknown monotreme family that combines marked elongation and torsion of the dentary with teinolophid character states, including the retention of five molars. Another shares dental features with ornithorhynchids, while the third is a possible diminutive steropodontid and simultaneously represents the smallest-bodied post-Barremian monotreme. Additional material of Steropodon galmani is also documented, confirming that a Meckelian groove is rudimentary or absent in this taxon, thus adding to the morphological understanding of this unusual monotreme. Lastly, we posit that the loss of teeth in ornithorhynchids may have occurred during the Pleistocene as a result of competition with aquatic hydromyin rodents dispersing to Australia from New Guinea.
Timothy F. Flannery [[email protected]]
Kristofer M. Helgen [[email protected]], and
Matthew McCurry [[email protected]] Australian Museum, 1 William Street Sydney, New South Wales 2000, Australia; Earth and Sustainability Research Centre, School of Biological Earth and Environmental Science, The University of New South Wales, Sydney New South Wales 2052, Australia
Thomas H. Rich [[email protected]], Museums Victoria, PO Box 666, Melbourne, Victoria 3001, Australia;
Patricia Vickers-Rich [[email protected]], School of Earth, Atmosphere and Environment, Monash University, Clayton, Victoria 3800, Australia; Museums Victoria, PO Box 666, Melbourne, Victoria 3001, Australia; Curtin University, Bentley Street, Perth, Western Australia 6102, Australia
Elizabeth T Smith [[email protected]; [email protected]] Australian Opal Centre, PO Box 229, Lightning Ridge, New South Wales 2834, Australia.
UNTIL the discovery of Steropodon galmani Archer, Flannery, Ritchie & Molnar, Citation1985, the evolutionary history of pre-Oligocene monotremes remained entirely unknown. Over the last few decades a modest diversity of 11 extinct genera (Patagorhynchus Chimento, Agnolín, Manabe, Tsuihiji, Rich, Vickers-Rich & Novas, Citation2023 together with all previously named genera listed by Flannery et al. Citation2022a) ranging in age from the Barremian (Early Cretaceous) to Pleistocene and distributed across Australia, New Guinea and South America, have been documented. Long before even the bare outlines of the pre-Pleistocene monotreme fossil record was known, Darlington (Citation1957, p. 339) speculated about the group’s past diversity, noting: ‘It may be guessed that, wherever they originated, monotremes radiated in Australia before marsupials did, and that the old Australian monotreme fauna may have been at least as diverse as the later marsupial fauna…’ Darlington’s ruminations were based in large part on the contrasting morphologies of the echidnas and platypus. These taxa, however, are now known to be terminal twigs on a deep evolutionary tree that molecular studies suggest diverged between 73–13 million years ago (Ma) (Phillips et al. Citation2009), or around 55 Ma (Zhou et al. Citation2021) depending upon preferred constraints. A much greater morphological diversity of monotremes has now been shown to lie outside of this clade (Flannery et al. Citation2022a). Yet, until now the evidence for Darlington’s (Citation1957) vision has been limited. New findings documented here thus provide the first substantial support for Darlington’s (Citation1957) hypothesis.
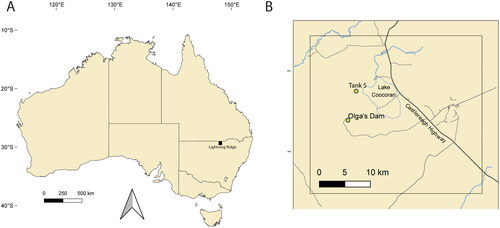
The Finch Clay facies of the Wallangulla Sandstone Member in the Griman Creek Formation at Lightning Ridge in northeastern New South Wales () has produced a unique fossil assemblage that inhabited a high palaeolatitude setting around 60°S during the Cenomanian (Kitchener et al. Citation2019). The identified vertebrates included teleosts, chondrichthyans, dipnoans (Kemp & Berrell Citation2020), turtles (Smith Citation2010; Smith & Kear Citation2013), leptocleidian and elasmosaurid plesiosaurs (Kear Citation2003, Citation2006, Citation2016, Kear & Hamilton-Bruce Citation2011), pterosaurs (Brougham, Smith and Bell Citation2017), sauropods (Frauenfelder et al. Citation2021), theropods (Brougham, Smith & Bell Citation2019), ankylosaurs (Bell et al. Citation2018), ornithopods (Bell et al. Citation2018, Bell et al. Citation2019a), crocodylomorphs (Hart Citation2020), enantiornithine birds and monotremes, amongst other taxa (Bell et al. Citation2019b, Poropat et al. Citation2023). Three monotreme species have been described from the Finch Clay facies: Steropodon galmani (Steropodontidae); Kollikodon ritchiei Flannery, Archer, Rich & Jones, Citation1995 (Kollikodontidae); and Stirtodon elizabethae Rich, Flannery & Vickers-Rich, Citation2020a (?Teinolophidae: Flannery et al. Citation2022a).
Fig. 1. Map of Australia showing the location of Lightning Ridge and nearby localities. A, Lightning Ridge. B, Tank 5 and Olga’s Dam sites. Adapted from Hart et al. Citation2021.
The Finch Clay facies fossils are preserved in SiO2•nH2O (hydrated silica dioxide), either as ‘potch’ or common opal, or as precious opal displaying plays of colour. These formed as pseudomorphs (amorphous potch replacements), or as opal replacements in which silica mineralization was coupled to decomposition. If the opal is translucent, fine internal anatomical details are sometimes visible. Opalized fossils are also usually three-dimensional and undistorted by compaction or diagenesis, but may show a variable degree of pre-burial weathering. The majority of recovered specimens have passed through mechanical agitators—large rotating barrels that wash out and remove softer sediments to leave tailings of harder claystone and opal, including fossils. This process breaks delicate fossils, rounds edges and often removes the crowns of teeth and other projections. All of the fossil monotreme jaws described herein exhibit fresh breaks due to mechanical excavation and processing. However, alveoli containing remnant claystone indicate that the teeth were lost prior to burial. Notably, the opal-mining process also biases against the recovery of smaller vertebrates, with the smallest mammal remains recovered to date (see below) having posterior molars estimated at almost twice as long as the largest molars of Australian Cretaceous tribosphenidans (Flannery et al. Citation2022b). The Finch Clay facies also produces perinatal (possibly embryonic) ornithopod dinosaurs with an estimated body mass of 113 g (Kitchener et al. Citation2019), and tiny elements from fish, turtles, crocodylomorphs and birds (Bell et al. Citation2019b). We suspect that tribosphenidans were present in the Lightning Ridge faunal assemblage, and that careful excavation may yet yield evidence of them.
We assign all of our named Lightning Ridge mammal taxa to Monotremata based on their very large dental canals and other diagnostic features of the clade. However, the often fragmentary condition of the fossils makes identification of tooth positions difficult. Consequently, we interpret dental homologies based on comparisons with Teinolophos trusleri Rich, Vickers-Rich, Constantine, Flannery, Kool & van Klaveren, Citation1999, which is known from multiple dentaries (Flannery et al. Citation2022a), the tooth-bearing holotype dentary (AM F66763) of S. galmani, and the juvenile dentition of Ornithorhynchus anatinus Shaw, Citation1799.
Finally, we have not undertaken a phylogenetic analysis of the Lightning Ridge monotreme fossils because our interests principally lie in taxonomy and zoogeography. The outgroup to Monotremata is disputed, with two very different mammalian groups having been put forward—Southern Hemisphere tribosphenids (Luo et al. Citation2001), and dryolestids (Archer et al. Citation1993). This makes determinations of character state polarity (e.g., molar number) uncertain. Moreover, the very small number of identified taxa and their fragmentary preservation produces excessive missing data that compromises tree resolution (Kearney & Clark Citation2003). Consequently, we adopt the approach of Flannery et al. (Citation2022b) in focusing on only the few unambiguous functional/adaptive character state complexes to diagnose taxa.
Institutional abbreviation
AM, Australian Museum ('F’ prefix denotes the Palaeontology Collection), Sydney, Australia.
Systematic palaeontology
MAMMALIA Linnaeus, Citation1758
MONOTREMATA Bonaparte, Citation1837 or 1838
ORNITHORHYNCHOIDEA superfam. nov.
Diagnosis
Distinguished amongst other Monotremata by torsion of the dentary, whereby the lingual surface of the distal portions of the dentaries faces dorsally and are dorsoventrally flattened (except Tachyglossidae); and the distal portion of the masseteric canal being divided into three wide moieties (except Tachyglossidae).
Remarks
Ornithorhynchoidea includes Opalionidae and fam. nov. crown group monotremes: (1) Tachyglossidae, including Tachyglossus Illiger, Citation1811, Zaglossus Gill, Citation1877, Megalibgwilia Griffiths, Wells & Barrie, 1991, and Murrayglossus Flannery, Rich, Vickers-Rich, Ziegler, Veatch & Helgen, Citation2022a, all of which have highly reduced dentaries, but likely split from Ornithorhynchidae during the Cenozoic (Phillips et al. Citation2009); (2) Ornithorhynchidae, which includes Ornithorhynchus Blumenbach, Citation1800 and Obdurodon Woodburne & Tedford, 1975, plus the stem ornithorhynchids (sensu Flannery et al. Citation2022a) Monotrematum Pascual, Archer, Jaureguizar, Prado, Godthelp & Hand, Citation1992, Patagorhynchus, and Dharragarra gen. nov.—these taxa share key synapomorphies with crown-group ornithorynchids. Given that divergences within Ornithorhynchoidea probably occurred during the Cretaceous, multiple clades might be recognized within the systematic scope of Monotremata.
OPALIONIDAE fam. nov.
Opalios splendens gen. et sp. nov.
()
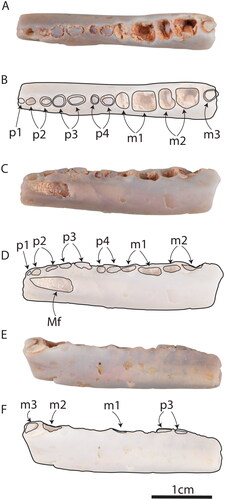
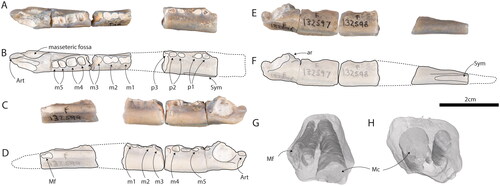
Fig. 2. AM F132596-9, dentary of Opalios splendens gen. et sp. nov. in A, B, occlusal, C, D, buccal, and E, F, lingual views. The internal morphology of the mandibular canal is shown in both G, distal oblique and H, mesial oblique views. G and H are not to scale. Art = articular facet of dentary. Mc = mandibular canal. Mf = mental foramen. Sym = symphysis.
Diagnosis
Differs from all monotremes except Teinolophos trusleri in possessing five molars rather than four or fewer (). Differs from T. trusleri in being much larger and possessing: a lower ascending ramus; an articular facet below the level of the toothrow (); a hemispherical depression for insertion of the masseteric musculature on the buccal side of the dentary (); the anterior portion of the dentary twisted (torsion) such that the lingual surface faces dorsally and the anterior dentary is dorsoventrally flattened (); a much reduced or absent Meckelian groove (); an extremely dorsoventrally shallow mandibular symphysis (). Differs from all other monotremes except stem and crown ornithorhynchids by exhibiting torsion of the horizontal ramus of the dentary. We consider Opalios splendens to be the basal-most diverging taxon within Ornithorhynchoidea.
Etymology
The genus name refers to ‘opal’ in Greek. The species name refers to the large size and spectacular translucency of the holotype (AM F132596–AM F132599), which provides views of its internal structure.
Holotype
AM F132596–AM F132599, a left dentary broken into four pieces. AM F132596 comprises the posterior-most dentary including the rear margin of the m5 alveolus. AM F132597 includes the m5, m4 and posterior wall of the m3 alveoli. AM F132598 preserves the anterior wall of the m3, as well as m2, m1 and posterior wall of the p4 alveolus. AM F132599 bears the p1–3 alveoli. The anterior-most part of the dentary is missing.
Type locality, unit and age
AM F132596–AM F132599 were found in 2001 during sorting of a tailings heap at the Tank Five processing dam (29.406629°S, 147.779965°E) on the Coocoran Opal Fields west of Lightning Ridge township. The tailings had been deposited by the opal miner Dusan Malinovik, and mined from his claim at the nearby Smith’s Opal Field (29.437973°S, 147.771212°E). AM F132596–AM F132599 were recovered over the course of a few days in sequence of AMF132596, AMF132598 (discovered by Clytie Smith of Lightning Ridge), AMF132597 and AMF132599 (discovered by ETS). Although the entire 10 m2 area was searched over subsequent weeks, no further dentary sections were located. Finch Clay facies, Wallangulla Sandstone Member, Griman Creek Formation; Cenomanian (Upper Cretaceous), 100.2–96.6 Ma (Bell et al. Citation2019b).
Description
The ventral margin of the dentary is gently arcuate, flattening anteriorly. The alveolar margins of the molars also form an arc with high points at the anterior and posterior ends of the molar row, as in Kollikodon ritchiei (Flannery et al. Citation1995). A shallow groove at the level of the termination of the tooth roots is present on the labial surface (). The total length of the dentary as reconstructed is around 76 mm. The length of three missing sections below m3, below p4, most of p3, and the anterior-most part of the dentary adds some uncertainty to this estimate. The least problematic of these gaps is that below m3 because both the posterior surface of the posterior alveolus, and the anterior surface of the anterior alveolus is preserved, and the segments of the lingual surface of the dentary almost occlude, constraining the length of the missing portion. The length of the missing section which includes the posterior premolars is more difficult to estimate. Our estimate is based on the contour of the adjacent preserved dentary fragments, and assumes a smooth transition from the circumference of one section to the next. The length of the missing distal section has been estimated by comparing the specimen with the dentaries of both Ornithorhynchus anatinus and Teinolophos trusleri (Rich et al. Citation2016), in the latter of which approximately 15% of the dentary lies anterior to p1. We thus estimate that the dentary of Opalios splendens would have extended approximately a centimetre beyond its anterior-most break.
Judging from their alveoli, the molars all had two roots. The alveolar lengths of the five molars are: m1 4.3 mm, m2 5.1 mm, m3 5.4 mm (estimate), m4 6.4 mm, m5 5.2 mm. Judging from the preserved alveoli, there was a step change in size between the molars and the premolars (as is seen in all monotremes where the dentary is known). Because the alveoli and mandibular canal are filled with white claystone and the specimen is glassy and translucent, features are clearly visible inside the bone, especially in the midsection of the dentary. The tooth roots were apparently short and thick, extending slightly lingual (medial) to the mandibular canal (). Broken cross-sections indicate that the roots actually contact and press into the top section of the mandibular canal. Tooth roots of p1 and p2 are extremely short and butt against the mandibular canal.
AM F132596 was damaged in the agitator in such a way that the angle of the ascending ramus cannot be accurately estimated (). Judging from what remains, the ascending ramus would have extended from just posterior to m5 to just anterior to the articular facet, and was modest in height (). A slight ridge is present on the ventrolingual margin of the dentary (). It is unlikely that this extended into an angle. There is no internal coronoid process. The mandibular condyle is extremely low-set, lying about midway between the dorsal and ventral edges of the horizontal ramus (). The articular surface is situated near the buccal margin of the bone and is elongated anteroposteriorly (length 3.5 mm, width 2.6 mm) with a flat articular surface whose buccal margin is lower than the lingual one. A deep, hemispherical mandibular sulcus () indicates the insertion point of the masseters. A roughened tubercle is present on the buccal side of the dentary at the rear, onto which a portion of the masseteric musculature presumably attached. There is a well-developed lingual shelf present, dorsal to a shallow groove, which may be a remnant Meckelian groove, or a myohyloid groove. This groove shallows both anteriorly and posteriorly: it does not reach the margin of the mandibular foramen, and attenuates before terminating below m5 (). The mandibular foramen is large and bounded buccally by a ridge, buccal to which is a fossa, presumably for insertion of the mandibular musculature. This fossa extends from a point buccal to the articular facet, anteriorly to the point at which the ascending ramus is visible when viewed lingually ().
AM F132597 fits precisely onto AM F132596. The part of the dentary housing the dental canal bulges in its posterior section. The alveoli indicate that the anterior and posterior alveoli of m5 are subequal in size, while the posterior alveolus of m4 is larger than the anterior one. There are pronounced crenulations or imbrications (irregular vertical striae) on the anterior and lingual walls of the alveoli for m4.
AM F132598 is separated from AM F132597 by a gap where the m3 would have been situated. Both the posterior wall of the posterior alveolus, and the anterior wall of the anterior alveolus of m3, survive. The alveoli of m2 indicate that this tooth was smaller than m4, and that its anterior moiety was narrower than its posterior one. The alveoli for the roots of m1 indicate that it was narrower than the m2, with its anterior moiety being narrower than its posterior one (). The anterior root of the m1 is preserved in its alveolus ().
Table 1. Measurements for monotreme specimens mentioned in the text.
A gap of uncertain length exists between AM F132598 and AM F132599. Teinolophos trusleri has four premolars (Rich et al. Citation2016). We interpret O. splendens to have also had four premolars. In T. trusleri, the p4 is closely adpressed against m1 (Rich et al. Citation2016). No evidence of p4 survives in O. splendens, the section of the dentary that presumably supported it being missing. AM F132599 (the anterior-most preserved fragment of the dentary) has alveoli for three premolars, interpreted here p1–3. The posterior-most root of p2 and the anterior-most root of p1 are preserved. The posterior margin of AM F132599 preserves the anterior alveolus of p3, which is not separated from p2 by a diastema. A diastema of about half a premolar length separates p2 from p1, and a diastema of uncertain length is present anterior to p1. A large dental foramen is present adjacent to the anterior root of p1, and continues anteriorly to where the dentary fragment terminates. The dental foramen is more dorsally placed than in O. anatinus. A narrow symphysial region, 9.2 mm long, is preserved on the ventrolingual margin of the anterior end of AM F132599. There is no indication that the distal portions of the dentaries diverged to support the broad bill, as occurs in O. anatinus, and it seems likely that the mandibular symphysis in O. splendens extended to the mandibular tip, as it does in T. trusleri (Rich et al. Citation2016).
When the fragments of the dentary are assembled, it is evident that the horizontal ramus displays torsion (its long axis is vertically oriented under the molars, but twisted anticlockwise in the holotype left dentary to an angle of around 90° below p1). The symphysial regions of the dentaries would have aligned closely, although they are not sutured. The lingual side of this anterior-most preserved section of the dentary has a peculiar rough, dimpled surface texture, extending posteriorly to m1 on AMF192598. Because of torsion, this dimpled section would have formed part of the floor of the oral cavity. Strong texturing of the surface of the dentary at this location is unusual, and its functional significance remains unknown.
The mandibular canal can be seen in cross section in several fragments. A CT scan reveals that it trifurcates anteriorly, a branch exiting at the dental foramen, while a smaller dorsal branch and a much larger ventral branch do not exit at a foramen but can be seen in cross section at the anterior-most break of the dentary ().
Remarks
Because the four fragments that comprise the dentary were recovered separately, a rationale for their association is required. Opalized vertebrate bones are generally uncommon in the Finch Clay facies of the Griman Creek Formation, and mammalian fossils are extremely rare (Bell et al. Citation2019a). The discovery of four dentary fragments at a single location is unique in our experience, and is almost certainly the result of breakage during the mining process of the original, more complete specimen. The only other fossils recovered from this site were plant scraps, unionid bivalves and a megaraptorid theropod tooth. All four fragments were collected from approximately 10 m3 of tailings and all share the distinctive translucent ‘amber’ coloured opal, fine preservation of external and internal details, and a very large mandibular canal.
The posterior-most three fragments share molar alveoli that are similar in depth and overall morphology. Two of the fragments (AM F132596 and AM F132597) can be unequivocally fitted together, the break between them being sharp and unabraded. AM F132598 cannot be fitted with these, being separated by a missing segment of the dentary below the m3. A larger gap separates AM F132599 from AM F132598, but the appearance of the opalization, the alveoli, and the proportions of the mandibular canal between this piece and AM F132598 are consistent with them being part of the same dentary.
Opalios splendens is the second monotreme known (after Teinolophos trusleri: Rich et al. Citation2016) to possess five molars. Archer et al. (Citation1993) argued that Dryolestida is the outgroup to Monotremata. Many dryolestoids have five or more molars, implying that this state may be plesiomorphic for Monotremata. Luo et al. (Citation2001), however, countered that southern hemisphere tribosphenids, which have three molars, form a clade with monotremes, implying that the presence of five molars may be a secondary reversion.
There is no morphological overlap between the only known specimen (a P4) of Stirtodon elizabethae (which was tentatively referred to Teinolophidae by Flannery et al. Citation2022a) and O. splendens, thus direct comparisons are not possible. But the P4 of S. elizabethae is far larger, broader and more robust than any lower premolar of O. splendens. It is highly likely that S. elizabethae was a very large monotreme (Flannery et al. Citation2022a), and far larger than O. splendens. Opalios splendens, however, represents a larger animal than T. trusleri, the only other teinolophid known. Teinolophos trusleri is unique among monotremes in retaining a well-developed Meckelian groove (Flannery et al. Citation2022a). Both taxa have five molars, with diastemata between most premolars (see Rich et al. Citation2016, Flannery et al. Citation2022a). As noted above, though, O. splendens differs from T. trusleri in lacking a diastema between p3 and p2.
Kollikodontids have highly specialized bunodont molars and a relatively narrow mandibular canal (Flannery et al. Citation2022a). Steropodontids have relatively tall, narrow dentaries with a well-developed ascending ramus (Flannery et al. Citation2022a). Both steropodontids and kollidodontids have a lower molar number (three and four, respectively), and in both the posterior-most molar is markedly reduced in size relative to the anterior ones (Flannery et al. Citation2022a). While m5 is smaller than m4 in O. splendens, the size difference is less than that seen between the posterior and penultimate molars in these two other families ().
Comparisons with other ornithorhynchoids
Opalios splendens is larger than Dharragarra aurora gen. et sp. nov., being similar in size to Ornithorhynchus anatinus (dentary length ∼76 mm versus 73.4–94.0 mm based on a sample of 13 O. anatinus individuals from the AM collections). The most striking differences between O. splendens and D. aurora are, in the latter, the absence of m4–5, the reduction of m3, the absence of diastemata between the premolars, and the reduced size of p1. Partial dentaries of the ornithorhynchids Obdurodon insignis Woodburne & Tedford, 1975, and Obdurodon dicksoni Archer, Jenkins, Murray, Hand & Godthelp, 1992, were described in detail by Musser & Archer (Citation1998), Archer et al. (Citation1992) and Archer et al. (Citation1992). Obdurodon insignis, O. dicksoni and O. anatinus are unique amongst monotremes in possessing a deep masseteric foramen and an internal coronoid process on the dentary (Archer et al. Citation1992). Obdurodon dicksoni is unique amongst crown ornithorhynchids in possessing a prominent ascending ramus (Archer et al. Citation1992); however, this is anteroposteriorly shorter than that of O. splendens. It also has a prominent dentary angle (Archer et al. Citation1992), a feature that is absent in O. splendens. Obdurodon insignis, by comparison, had a lower ascending ramus and seems to have lacked, or had only a rudimentary, dentary angle (Musser & Archer Citation1998).
The horizontal ramus of the dentary in O. anatinus is sinuous in shape when viewed laterally, the anterior (‘bill-covered’) section being sharply deflected ventrally, and the region posterior to the posterior horny masticatory pad is deflected dorsally. The dentary is also strongly sinuous in the labiobuccal plane, the section of the dentary bearing the anterior horny pad being deflected labially. In O. splendens the mandibular symphysis, which is shallow but distinct, likely extended to the anterior tip of the dentary, while in O. anatinus it terminates around 15–20 mm posterior to the dentary tip, allowing the dentaries to separate and flare laterally to support the bill. Only the posterior part of the dentary is known for the species of Obdurodon (Archer et al. Citation1992, 2012, Musser & Archer Citation1998), but what is preserved is similar to that of O. anatinus in having the region posterior to the molars being deflected dorsally. By contrast, the horizontal ramus of the dentary in O. splendens is almost straight in the dorsoventral and labiobuccal planes. The modest lateral broadening of the dentary anteriorly suggests that if a bill were present, it was far narrower than in O. anatinus. In the species of Obdurodon and O. anatinus, the articular facet is positioned high above the horizontal ramus and is transversely broad (Archer et al. Citation1992, Musser & Archer Citation1998). In O. anatinus there is no ascending ramus. In O. splendens, the articular facet is located below the level of the tooth crowns, is small, anteroposteriorly elongated, and a modest ascending ramus is present. In O. anatinus, a foramen is present on the ventral surface of the dentary near its tip. Two branches of the mandibular canal continue anteriorly to the dental foramen in O. splendens, indicating that additional foramina existed anterior to the preserved part of the dentary (). The larger of the two branches is positioned ventrally, suggesting that, as in O. anatinus, a foramen exited the dentary on its ventral surface (). The anterior end of the dentary is flattened and spatula-like in O. anatinus, while in O. splendens it is thicker and narrower.
Potential synapomorphies demonstrating a relationship of O. splendens with other Ornithorhynchoidea include: reduction of the ascending ramus of the dentary; presence of a deep hemispherical fossette for an enlarged masseter; torsion of the horizontal ramus of the dentary; and horizontal flattening of the anterior part of the dentary. However, O. splendens also possesses character states present in tachyglossids, including: an anteroposteriorly elongated articular facet of the dentary; and relatively narrow rostrum. This character state combination is evident in either crown tachyglossids or ornithorhynchids, but not both, indicating that O. slendens is likely placed outside of the crown group. The presence of five molars with premolars separated by diastemata are possibly plesiomorphic features otherwise seen only in T. trusleri (Rich et al. Citation2016, Flannery et al. Citation2022a), suggesting that ornithorhynchoids may derive directly from ancestors with a teinolophid-like morphology.
Family ?ORNITHORHYNCHIDAE Gray, Citation1825
Dharragarra aurora gen. et sp. nov.
()
2013, (b) (occlusal view, left dentary, steropodontid, Musser, p. 8.
2022a, gen. nov. ?Ornithorhynchidae (stem), Flannery et al., p. 5.
Diagnosis
Differs from teinolophids in lacking a Meckelian groove, possessing dentary torsion and three molars, and in lacking diastemata between the premolars. Differs from kollikodontids in possessing an enlarged mandibular canal, in possessing three rather than four molars and in possessing dentary torsion. Differs from Steropodontids in possessing dentary torsion, having a dorsoventrally flattened dentary, and a more reduced posterior molar. Differs from Opalios splendens in having three molars rather than five, and in lacking diastemata between p1 and p2. Amongst ornithorhynchoids, Dharragarra aurora differs from the species of Obdurodon and Ornithorhynchus in having molars with two roots rather than more than two; from Patagorhynchus pascuali in being larger; from Monotrematum sudamericanum in being smaller.
Etymology
The genus name derives from ‘Dharragarra’, meaning platypus in the Gamilaraay, Yuwaalaraay and Yuwaalayaay languages (Ash et al. Citation2003). The species name is Latin for ‘dawn’.
Holotype
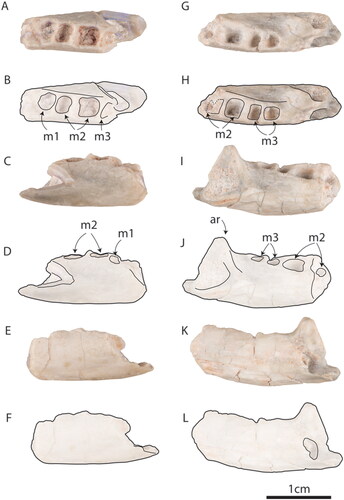
AM F97263, a partial left horizontal ramus with roots for m3, p1, p3 and p4, and alveoli for m1–2 and p3 ().
Type locality, unit and age
AM F97263 originated on the Coocoran Opal Field. Finch Clay facies, Wallangulla Sandstone Member, Griman Creek Formation; Cenomanian (Upper Cretaceous), 100.2–96.6 Ma (Bell et al. Citation2019b).
Description
AM F97263 is preserved in opaque, dark grey ‘potch’ and consists of the horizontal ramus from the posterior end of the molar row to the posterior root of p1 (). Its length is 32.6 mm. No tooth crowns are preserved. An alveolus retaining a root indicates the presence an m3 () which is much reduced relative to the more anterior molars. The root of m3 suggests that the tooth was similar in size to that in species of Obdurodon and juvenile Ornithorhynchus anatinus (Flannery et al. Citation2022a). The alveoli of m2 indicate that it was smaller than m1, and that its posterior moiety was larger than the anterior one (). The alveoli for m1 show that its anterior moiety was markedly smaller than the posterior one. The roots and alveoli of the premolars suggest that the molar–premolar boundary was clearly differentiated, as in all monotremes where that region is known (Flannery Citation2022a). All premolars were narrower than the molars. Both roots of p4 are preserved, indicating that it was smaller than the p3 (). The roots of p3 indicate that it was the largest premolar, and that its crown was narrow. The roots of p2 are similar in size and morphology to those of p4. A much smaller root, which is preserved at the anterior margin of the holotype, is identified as probably the posterior root of p1. A subtle depression, positioned on the lingual surface of the dentary below m2–3, may be a remnant of a Meckelian or myohyloid groove (). The dental canal is large in diameter and limits the depth of the dental alveoli towards the anterior margin of the preserved section of the jaw, making it doubtful that any teeth, except possibly remnant or procumbent ones, were present anterior to the p1. There are no diastemata separating any of the teeth in the preserved part of the dentary, apart from a very small gap between p3 and p4 (). A very large dental foramen exits the labial side of dentary ventral to the alveoli of p3 (). This is slightly posterior to its position in O. splendens (where it exits below p1). This apparent difference, however, may be due to breakage of the buccal wall of the foramen. An enlarged dental canal continues anterior to this point, suggesting that a second, more anteriorly positioned foramen was present.
The preserved part of the horizontal ramus is nearly straight in both the buccolingual and dorsoventral planes (). There is, however, some torsion of the dentary, which is broken off at p1, at which point the horizontal ramus is twisted through 55°. It is likely that the degree of torsion increased anteriorly, but this is impossible to substantiate due to breakage. The preserved fragment is heavily abraded, and there is no indication of a mandibular symphysis.
Remarks
We refer Dharragarra aurora to ?Ornithorhynchidae because, like Monotrematum sudamericanum, the species of Obdurodon and Ornithorhynchus anatinus (juveniles only) it has only three lower molars, the posterior-most of which is highly reduced (see Flannery Citation2022a). Because it is Cenomanian in age, D. aurora likely predates the split between tachyglossids and ornithorhynchids (Phillips et al. Citation2009, Zhou et al. Citation2021). It may thus be a stem ornithorynchid (sensu Flannery et al. Citation2022a). All such possible stem ornithorhynchids are classified as ?Ornithorhynchidae in this work.
No direct comparisons with Sundrius ziegleri Rich, Flannery, Evans, White, Ziegler, Maguire, Poropat, Trusler & Vickers-Rich, Citation2020 are possible. The lower premolars of D. aurora are, however, far smaller, less robust and broader than the P4 of S. ziegleri. Teinolophos trusleri is far smaller than D. aurora, and has a distinct Meckelian groove which is absent (or much less marked) in D. aurora. As in T. trusleri, however, the p3 is larger than the p4 (see Rich et al. Citation2016, Flannery et al. Citation2022a).
Comparisons with other ornithorhynchoids
Dharragarra aurora contrasts with Opalios splendens in its reduced molar number and lack of diastemata between the premolars. It is difficult to compare the degree of dental torsion in these taxa, but marked torsion exists in O. splendens (ca 90°), while in D. aurora around 55° of torsion is evident at the point where the dentary is broken. Dharragarra aurora shares an enlarged dental canal, a prominent dental foramen and torsion of the horizontal ramus of the dentary as potential synapomorphies with other (non-tachyglossid) ornithorhynchoids. The presence of three lower molars with the posterior-most being reduced in size and likely single rooted, are shared with the species of Obdurodon, Ornithorhynchus anatinus, and probably Monotrematum sudamericanum (Flannery et al. Citation2022a). We consider the presence of a highly reduced m3 to be a unique synapomorphy uniting this group.
The only other Late Cretaceous ornithorhynchoid is Patagorhynchus pascuali Chimento, Agnolín, Manabe, Tsuihiji, Rich, Vickers-Rich & Novas, Citation2023, from the early Maastrichtian deposits of Patagonia (Chimiento et al. 2023). The holotype of this taxon was identified as an m2 by Chimiento et al. (2023). However, its anterior moiety is markedly narrower than the posterior one, and the partial alveolus anterior to the molar is too small to support a molar root, but is consistent in size with that of the posterior alveolus of a premolar (see Chimiento et al. 2023). These features are consistent with the single preserved molar being an m1 rather than an m2—a designation adopted here.
Because P. pascuali is known from a single molar with an adhering dentary fragment, and D. aurora is known only from a near complete horizontal ramus of a left dentary that lacks molar crowns, few direct morphological comparisons can be made between these taxa. The m1 of P. pascuali is double-rooted as is that of D. aurora. The m1 also has shallow molar roots and a smaller anterior moiety than the posterior moiety. The p4 is likewise markedly smaller and narrower than the m1, although the m1 of P. pascuali is 5.8 mm in length (see Chimiento et al. 2023), while the alveolar length of the m1 in D. aurora is 7.2 mm. As tooth crown length is usually greater than alveolar length, it is likely that D. aurora was somewhat larger than P. pascuali. In addition, the chronostratigraphical and palaeogeographical gap between P. pascuali and D. aurora spanning ∼22 Ma from southern South America to Eastern Australia prompts us to classify these two taxa as separate genera.
Similarly, few direct comparisons can be made with M. sudamericanum. Pascual et al. (Citation2002) report that M. sudamericanum probably had three lower molars, with m3 being reduced in size, and m1 and m2 double-rooted—features all shared with D. aurora. By contrast, the species of Obdurodon and O. anatinus have multi-rooted m1 and m2 (Flannery et al. Citation2022a). In Obdurodon dicksoni the P4 is larger than P3, and it seems likely that the lower premolars had a similar size relationship (Archer et al. Citation1992). This is in marked contrast with D. aurora, where the p3 is larger than the p4. Furthermore, a very short diastema separates P3 and P4 in O. dicksoni, and there are no teeth anterior to P3 (Archer et al. Citation1992). On the other hand, D. aurora lacks diastemata between premolars and p1–2 are undoubtedly present.
Only juvenile O. anatinus have teeth: M1–2, an upper premolar and m1–3 (m3 being highly reduced: Flannery et al. Citation2022a). Dharragarra aurora shares the same lower molar formula, but has four lower premolars while O. anatinus lacks lower premolars. The preserved segment of the horizontal ramus of D. aurora is nearly linear in both the buccolingual and dorsoventral planes, which is in stark contrast with the equivalent segment in O. anatinus, where the posterior portion is deflected dorsally, and distal portion deflected ventrally. In D. aurora, the dentary is broken off at p1, a point at which the degree of torsion is 55°. In O. anatinus, torsion of the dentary commences around one third of the distance posteriorly from its anterior tip, resulting in a twisting of the long axis of the dentary (in cross-section) so that the lingual segment is twisted anticlockwise through around 90°. It is possible that a similar degree of torsion existed in D. aurora.
STEROPODONTIDAE Archer, Flannery, Ritchie & Molnar, Citation1985
Steropodon galmani Archer, Flannery, Ritchie & Molnar, Citation1985
()
Referred material
AM F161197, a partial right dentary with the base of the ascending ramus and large dental canal, hosting alveoli for m3, the posterior alveolus of the m2, and the posterior surface of the anterior alveolus of m2. AM F97262, a right dentary fragment preserving the anterior wall of anterior alveolus of the m3, both alveoli of m2, the posterior alveolus and part of the anterior alveolus of the m1.
Type locality, unit and age
AM F161197 was recovered from a tailing heap at Tank Five (29.406629°S, 147.779965°E), ∼5 km from Olga’s Dam. AM F97262 derives from the T Bone rush, Coocoran Opal Field. The holotype of Steropodon galmani (AM F66763) was found in one of the older opal fields near Lightning Ridge, ∼35–40 km from Tank Five. AM F161197 and AM F97262 are therefore the first records of S. galmani from the Coocoran Opal Fields. Finch Clay facies, Wallangulla Sandstone Member, Griman Creek Formation; Cenomanian (Upper Cretaceous), 100.2–96.6 Ma (Bell et al. Citation2019b).
Description
AM F161197 closely resembles the holotype of Steropodon galmani (AM F66763) in size and morphology. However, AM F161197 preserves the dentary further posteriorly than does AM F66763 (). AM F161197 is preserved in opaque, whiteish ‘potch’ and exhibits surface weathering equivalent to level WS2 on the 6-stage scale of Behrensmeyer (Citation1978). Unusually, this includes desiccation cracks, which are also often observable on unionid bivalve shells from the Finch Clay facies (ETS pers. obs.). The molar alveoli of AM F161197 are approximately square in dorsal profile, and the alveoli for the m3 indicate that it was smaller than the m2, as in AM F66763. The preserved segment of the ascending ramus rises at an angle of ∼70°. Judging from its preserved portion, the ascending ramus of AM F161197 was prominent and robust. The masseteric sulcus is moderately deep, suggesting a well-developed masseteric musculature. The posterior molar is situated well anterior to the ascending ramus, with a broad valley separating it from the ascending ramus. The opening of the mandibular canal is 3.2 mm in diameter (). Posteriorly, the dentary fragment terminates in a break. There is no indication of a dentary angle, although if commencing well posteriorly, it could have been broken away. A slight depression on the lingual side of the horizontal ramus posterior to the m3 might be a remnant of a Meckelian or myohyloid groove.
AM F97262 provides almost no additional information, as it replicates the morphology of AM F66763 and AM F161197. The diameter of the mandibular canal at the posterior break of the dentary is 2.9 mm. There is a slight depression on the lingual surface that may be a remnant of a Meckelian or myohyloid groove. The horizontal ramus is shallower than in AM F66763 and AM F161197, and may be a result of intraspecific variability.
Remarks
AM F161197 and AM F97262 add considerably to knowledge of the Meckelian groove in Steropodon galmani. AM F66763 is widely considered to have possessed a Meckelian groove (e.g., Luo Citation2007, Luo et al, Citation2001, Citation2002, Citation2007, Citation2017, Kielan-Jaworowska et al. Citation2004, Rougier et al. Citation2007, Wible et al. Citation2009), but recent re-examination has revealed post-mortem damage to the dentary above the Meckelian groove suggesting that this feature is actually a crack (see Flannery et al. Citation2022a). Notably, AM F161197 and AM F97262 both lack corresponding structures, although they do possess corresponding shallow depressions on the lingual sides of their dentaries.
Steropodon galmani differs from ornithorhynchoids in possessing a narrow and deep horizontal ramus of the dentary, and a prominent ascending ramus that in addition to its tribosphenic-like (but not tribosphenic) lower molars (see Flannery et al. Citation2022b), could suggest that S. galmani was a terrestrial omnivore.
?STEROPODONTIDAE
Parvopalus clytiei gen. et sp. nov.
()
Fig. 5. AM F161198, dentary of Parvopalus clytiei gen. et sp. nov. in A, B, occlusal, C, D, buccal and E, F, lingual views. Maf = mandibular foramen. Ar = ascending ramus.
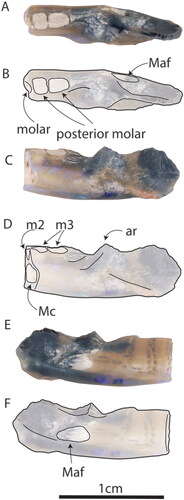
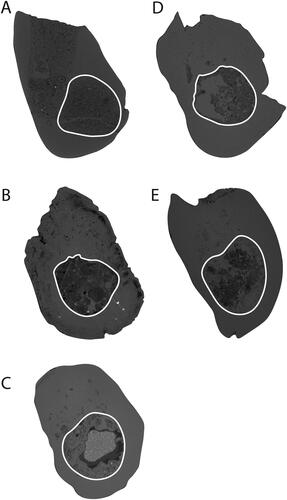
Fig. 6. Cross sections of the mandible scaled to similar sizes. A, Parvopalus clytiei (AM F161198) at the anterior of m3, B, Steropodon galmani (AM F161197) in between m2 and m3, C, Dharragarra aurora (AM F97263) in between m2 and m3, D, Steropodon galmani (AM F97262) in between m2 and m3, E, Opalios splendens (AM F132598) in between m1 and m2. The white line indicates the mandibular canal.
Diagnosis
Attributed to Monotremata on the basis of its enlarged mandibular canal and squared molar alveoli profile. Provisionally attributed to Steropodontidae based on its deep, narrow, horizontal ramus and prominent ascending ramus (rising at an angle of ∼70°); well-developed masseteric sulcus; and posterior molar positioned well anterior to the ascending ramus. Parvopalus clytiei is one of the smallest-known monotremes with the exception of Teinolophos trusleri (molar alveolar length of 3.2 mm versus molar tooth lengths of 1.1–1.7 mm: Flannery et al. Citation2022a). Differs from Steropodon galmani in being much smaller; possessing a more prominent ventral margin of the masseteric sulcus; and posterior alveoli of the posterior-most molar being larger than the anterior one indicating no reduction in posterior molar size. Differs from teinolophids in lacking a Meckelian groove, and from ornithorhynchids and kollikodontids in having a deeper, narrower dentary.
Etymology
Genus name is Latin for ‘small opal’. Species is named for Clytie Smith (Lightning Ridge), who has recovered many opal fossils at Lightning Ridge.
Holotype
AM F161198, partial left dentary, preserving the posteroventral margin of the ascending ramus to the posterior alveoli of the penultimate molar.
Type locality, unit and age
Found in 1999 in a tailings heap at Olga’s Dam (29.464407°S, 147.777180°E), ∼5 km south of Tank Five on the Coocoran Opal Fields. AM F161198 originated from a small field known as CC’s, which is near Smith’s Opal Field. Finch Clay facies, Wallangulla Sandstone Member, Griman Creek Formation; Cenomanian (Upper Cretaceous), 100.2–96.6 Ma (Bell et al. Citation2019b).
Description
AM F161198 has alveoli for the posterior-most molar (alveolar l = 3.2 mm) and the rear surface of the posterior alveolus of the penultimate molar (). The alveoli penetrate the dentary to the level of the dental canal, passing the upper part of the canal on its buccal side (). The mandibular foramen is moderately enlarged (1.6 mm in diameter) and the dental canal is large, being 2 mm in diameter below the posterior of the most anterior molar alveoli. The mandibular foramen is located lingual to the anterior margin of the ascending ramus about half-way between the dorsal and ventral margins of the horizontal ramus. There is no dentary angle. The base of the ascending ramus is stout, and similar in form to that of Steropodon galmani (see Archer et al. Citation1992). The remnant of a prominent ridge at the base of the masseteric sulcus is truncated by a break (). The alveoli of the posterior-most molar are located well anterior to the anterior edge of the ascending ramus, as in S. galmani (see Archer et al. Citation1992). The prominent mandibular canal enters the horizontal ramus immediately ventral to the leading edge of the ascending ramus.
Remarks
Parvopalus clytiei is the smallest known mammal fossil from the Lightning Ridge faunal assemblage. The largest molar of the tribosphenidan Bishops whitmorei Rich, Flannery, Trusler, Kool, van Klaveren & Vickers-Rich, 2001 (m2) is 1.8 mm long, and this species has an estimated bodyweight of ∼40 g (Flannery et al. Citation2022b). The alveoli for the roots of the posterior molar of P. clytiei are 3.2 mm in length. Mammalian molar crowns are usually longer than the molar alveoli, thus the posterior molar of P. clytiei is likely to have been almost twice as long as the largest molar of B. whitmorei. The dentary of P. clytiei is deep, unlike the dorsoventrally flattened dentaries of ornithorhynchids (see Flannery et al. Citation2022a).
Discussion
The geologically oldest documented monotreme taxon, Teinolophos trusleri, from the uppermost Barremian–lowermost Aptian ‘Wonthaggi Formation’ of the Bass Coast in southeastern Australia, suggests that monotremes originated in high-palaeolatitude environments of Australo-Antarctic Eastern Gondwana during or before the Early Cretaceous (Flannery et al. Citation2022a). By the early-Late Cretaceous (Cenomanian, around 30 Ma later), the groups had clearly diversified in multiple lineages represented by Kollikodontidae, Steropodontidae, Teinolophidae and Ornithorhynchoidea. In the early Maastrichtian, at least one monotreme lineage (Patagorhynchus pascuali attributed to ?Ornithorhynchidae) had reached South America (Chimiento et al. 2023). It therefore seems possible that the Turonian altithermal could have facilitated a trans-Antarctic migration of monotremes coincident with other biotic elements, such as angiosperms (Cantrill & Poole Citation2002).
The fossil record of ornithorhynchids stem spans an interval of some 40 Ma from the Cenomanian to Palaeogene, and is sourced entirely from southern polar habitats (Flannery et al. Citation2022a, Chimiento et al. 2023). A chronostratigraphical hiatus in occurrences then occurs from the early Paleocene (∼64 Ma) to late Oligocene (26 Ma) (see Woodburne et al. Citation1994). The crown ornithorhynchids Obdurodon spp. and Ornithorhynchus anatinus, which occur from the Oligocene to today, share a number of synapomorphies including: molars with more than two roots; reduction or loss of the ascending ramus; and presence of an internal coronoid process on the dentary (see Flannery et al. Citation2022a). These are unique to crown group ornithorhynchids, which notably all occurred in higher latitude settings extending up to 15°S (Carrick et al. Citation2008).
Edentulous ornithorhynchids, represented solely by O. anatinus, are known only from the Pleistocene to today, while toothed ornithorhynchids are known from the Oligo-Miocene. A limb fragment attributed to Ornithorhynchus (AMF 162429) by Rich et al. Citation1991, from the Pliocene Bow Local Fauna of New South Wales (Musser Citation2013) may represent either Ornithorhynchus or Obdurodon, or some other taxon. The holotype of Ornithorhynchus agilis De Vis, Citation1885, a junior synonym of O. anatinus (Archer et al. Citation1978), is an anterior dentary section lacking teeth from Pleistocene deposits at King Creek near Pilton on the Darling Downs in Queensland. It is the oldest evidence for an edentulous ornithorhynchid. If tooth loss in ornithorhynchids occurred in the Plio-Pleistocene, the lower molar row of ornithorhynchids remained largely unchanged for ∼95 Ma, with the m3 being present but highly reduced, and the anterior molars being low-crowned. The advantages of tooth loss in Ornithorhynchus are uncertain, but could be linked to dispersal of the Australo-New Guinean Water-rat (or Rakali) Hydromys chrysogaster Geoffroy, 1804. This large (ca 1 kg) aquatic, carnivorous rodent is of equivalent size to O. anatinus. The hydromyin clade originated in New Guinea around 8.5 Ma, with migration of the H. chrysogaster lineage to Australia sometime during the Pleistocene (Roycroft et al. Citation2022). The diet of H. chrysogaster includes fish, freshwater mussels, parastacid crayfish, spiders and insects (Woollard et al. Citation1978). Ornithorhynchus anatinus alternatively feeds on softer-bodied prey, such as worms, insect larvae and freshwater crustaceans (Bethge Citation2002). We therefore consider it plausible that competition between the ancestors of O. anatinus and H. chrysogaster may have trophically displaced harder-bodied prey from the diet of ornithorhynchids. Softer food items are more easily processed with horny pads rather than a battery of molars.
The higher-level mammalian clade diversification occurred during the Jurassic, and a trend for the development of larger body masses was evident by the Cretaceous (Close et al. Citation2015). All mid-Cretaceous and later monotremes are relatively large, the earliest being Kryoryctes cadburyi Pridmore, Rich, Vickers-Rich & Gambaryan Citation2005 and Sundrius ziegleri from the lower Albian Eumeralla Formation of Victoria (Pridmore et al. Citation2005, Rich et al. Citation2020). Coeval tribosphenidan mammals are otherwise comparatively smaller-bodied (Flannery et al. Citation2022b), and we suspect might have been present within the Lightning Ridge faunal assemblage but have not yet been detected because of size-related sampling biases. Whatever the case, the only fossil mammals thus far recovered from the Cenomanian deposits of Australia are monotremes, with 10 craniodental fragments () and three isolated vertebrae (see Smith Citation1999, p. 97) recovered from the Finch Clay facies to date.
Table 2. Number of known specimens per monotreme taxon, Lightning Ridge local fauna.
The Cenomanian Mata Armarilla Formation of Argentina is stratigraphically contemporaneous with the Finch Clay facies of the Griman Creek Formation, and was laid down at similar palaeolatitudes (56.5°S: Martin et al. Citation2022). The corresponding Mata Armarilla Formation fossil mammal assemblage includes only seven isolated dental fragments, yet these reflect a far more diverse higher-level clade fauna comprising ?Docodonta, Tribosphenida, Meridiolestida and Dryolestida (Martin et al. Citation2022). The docodontan and meridiolestidan are larger-bodied, and similar in size to the monotreme remains recovered from the Finch Clay facies. The absence of comparable lineage abundance, if not representatives of the gondwanatherian, docodontan and meridiolestidan clades in the Cretaceous of Australia, is therefore striking, and should be expected given the number of specimens now recovered at Lightning Ridge. Indeed, even Early Cretaceous mammal assemblages from high-palaeolatitudes in the Northern Hemisphere have higher relative clade diversity. For example, the Hauterivian–Barremian Ilek Formation in Siberia (∼54.2°N palaeolatitude) has yielded up to 38 reported mammal fossils representing potentially five order-level groups including Symmetrodonta, ?Tinodontidae (basal to Symmetrodonta), Multituberculata, Eutriconodonta, and Docodonta (Averianov et al. Citation2005, Lopatin et al. Citation2005, Citation2009). Likewise, the famous lower Aptian Jehol Biota from the Yixian and Jiufotang formations in China has produced 18 species allocated to four clades: Triconodonta, Multituberculata, Symmetrodonta and Eutheria (Bi et al. Citation2018, Zhou Citation2014, Mao et al. Citation2021, Wang et al. Citation2022). High southern palaeolatitude climatic and geographical isolation might explain the unusually low clade-level taxonomic diversity in the Finch Clay facies at Lightning Ridge, with these barriers preventing dispersal across the Eastern Antarctic landmass. It is therefore significant that monotremes were an apparently endemic southern polar group, as suggested by their geologically earliest radiations from the Barremian to Paleocene (Flannery et al. Citation2022a).
In closing, a substantial Turonian-to-Eocene chronostratigraphical gap still exists in our understanding of monotreme evolution, from which not a single monotreme fossil has yet been discovered. As a consequence, it is unclear whether a diverse monotreme fauna survived the end-Cretaceous mass extinction event, and subsequently persisted (and perhaps ecologically radiated) prior to the arrival of marsupials in Australia by ∼54 Ma (Godthelp et al. Citation1992). Filling this mysterious interval of monotreme diversity and adaptive development should be a primary focus for research in the future.
Conclusions
The co-occurrence of six morphologically distinct fossil mammal taxa (three named herein) in the Lightning Ridge faunal assemblage from the Finch Clay facies of the Griman Creek Formation provides the first indication that Australia was home to a hitherto cryptic diversity of monotremes during the mid-Cretaceous (as predicted by Darlington Citation1957).
The newly described Opalios splendens is annectant between a teinolophid-like ancestral monotreme morphotype and more derived ornithorhynchoids.
Dharragarra aurora demonstrates that the lower molar formula typical of Cenozoic ornithorhynchids was established by the Cenomanian.
New specimens of Steropodon galmani, along with the new small-bodied taxon, Parvopalus clytiei, expand current knowledge of morphological diversity in Cenomanian monotremes.
The loss of teeth in ornithorhynchids may have been related to ecological displacement caused by the arrival of aquatic rodents in Australia during the Pleistocene.
Acknowledgements
The AM facilitated this research, and Sandra Ingleby (AM) provided access to monotremes held in the institution’s mammal collections. Photography was provided by Abram Powell (AM), and CT scanning was undertaken by Thomas Peachey (AM). Clytie Smith and E.T.S. were instrumental in obtaining the fossil specimens from the Lightning Ridge opal fields. The reviewers and Editorial Board of Alcheringa provided constructive comments and improvements on our manuscript. Finally, we thank Auntie Brenda McBride for permission to use Yuwaalaraay, Yuwaalayaay and Gamilaraay names, and acknowledge the custodians of country in the Lightning Ridge district and pay our respects to Elders past, present and emerging.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/03115518.2024.2373100)
References
- Archer, M., Flannery, T.F., Ritchie, A. & Molnar, R.E., 1985. First Mesozoic mammal from Australia—an early Cretaceous monotreme. Nature 318, 363–366.
- Archer, M., Plane, M.D. & Pledge, N.S., 1978. Additional evidence for interpreting the Miocene Obdurodon insignis to be a fossil platypus (Ornithorhynchidae, Monotremata), and a reconsideration of the status of Ornithorhynchus agilis De Vis, 1888. The Australian Zoologist 20, 9–28.
- Archer, M., Plane, M.D. & Pledge, N.S., 1992. Additional Evidence for interpreting the Miocene Obdurodon insignis Woodburne & Tedford, 1975 to be a fossil platypus (Ornithorhynchidae: Monotremata) and a reconsideration of the status of Ornithorhynchus agilis De Vis, 1885. In: Augee, M. (ed.). Monotreme Biology. Royal Zoological Society of Nsw, 9–27.
- Archer, M., Murray, P., Hand, S.J. & Godthelp, H., 1993. Reconsideration of monotreme relationships based on the skull and dentition of the Miocene Obdurodon dicksoni. Chapter 7. In Mammal Phylogeny. Szalay, F.S., Novacek, M.J., & McKenna, M. C. eds. Springer-Verlag, New York.
- Archer, M., Jenkins, F., Hand, S.J., Murray, P. & Godthelp, H., 1992. Description of the skull and non-vestigial dentition of a Miocene platypus Obdurodon dicksoni n. sp. from Riversleigh, Australia, and the problem of monotreme origins. In Augee, M.L., ed. Platypus and Echidnas. Royal Zoological Society of Nsw, Mossman, Nsw, 15–27.
- Ash, A, Giacon, J & Lissarague, A. (Eds), 2003. Gamilaraay, Yuwaalaraay, & Yuwaalayaay Dictionary. IAD Press, Alice Springs.
- Averianov, A.O., Skutschas, P.P., Lopatin, A.V., Leshchinskiy, S.V., Rezvyi, A.S. & Fayngertz, A.V., 2005. Early Cretaceous mammals from Bol’shoi Kemchug 3 locality in west Siberia, Russia. Russian Journal of Theriology 4, 1–12.
- Behrensmeyer, A.K., 1978. Taphonomic and ecological information from bone weathering. Paleobiology 4, 150–162.
- Bell, P.R., Burns, M. & Smith, E.T., 2018. A probable ankylosaurian (Donisauria, Thyreophora) from the early Cretaceous of New South Wales, Australia. Alcheringa 42, 1–5.
- Bell, P.R., Brougham, T., Herne, M.C., Frauenfelder, T. & Smith, E.T., 2019a. Fostoria dhimbangunmal, gen. et sp. nov., a new iguanodontian (Dinosauria, Ornithopoda) from the mid-Cretaceous of Lightning Ridge, New South Wales, Australia. Journal of Vertebrate Paleontology 39, e1564757.
- Bell, P.R., Fanti, F., Hart, L.J., Milan, L.A., Craven, S.J., Birch, S.A. & Smith, E., 2019b. Revised geology, age, and vertebrate diversity of the dinosaur-bearing Griman Creek Formation (Cenomanian), Lightning Ridge, New South Wales, Australia. Palaeogeography, Palaeoclimatology, Palaeoecology 514, 655–671.
- Bell, P.R., Herne, M.C., Brougham, T. & Smith, E.T., 2018. Ornithopod diversity in the Griman Creek Formation (Cenomanian), New South Wales, Australia. Peerj. 6, e6008.
- Bethge, P., 2002. Energetics and foraging behaviour of the platypus Ornithorhynchus anatinus. Ph D. Thesis, University of Tasmania. Posted 2023.
- Bi, S., Zheng, X., Wang, X., Cignetti, N.E., Yang, S. & Wible, J.R., 2018. An Early Cretaceous eutherian and the placental–marsupial dichotomy. Nature 558, 390–395.
- Blumenbach, J.F., 1800. Über das Schnabelthier (Ornithorhynchus paradoxus) ein neuentdecktes Geschlecht von Säugthieren des fünften Welttheils. Magazin für den Neuesten Zustand der Naturkunde 2, 205–214.
- Bonaparte, C.L.J.L., 1837. or 1838. Synopsis vertebratorums systematis. Nuovi annali delle scienze naturali. Bologna, 2, 105–133.
- Brougham, T., Smith, E.T. & Bell, P.R., 2017. Isolated teeth of Anhangueria (Pterosauria: Pterodactyloidea) from the Lower Cretaceous of Lightning Ridge, New South Wales, Australia. Peerj. 5, e3256.
- Brougham, T., Smith, E.T. & Bell, P.R., 2019. New theropod (Tetanurae: Avetheropoda) material from the ‘mid’-Cretaceous Griman Greek Formation at Lightning Ridge, New South Wales, Australia. Royal Society Open Science 6, 180826.
- Carrick, F.N., Grant, T.R. & Temple-Smith, P.D., 2008. Platypus. In Van Dyck, S. and Strahan, R., eds., The Mammals of Australia, 3rd ed. Reed New Holland Press, Sydney, 32–35.
- Cantrill, D.J. & Poole, I.P., 2002. Cretaceous patterns of floristic change in the Antarctic Peninsula. Geological Society, London, Special Publications 194, 141–152.
- Chimento, N.R., Agnolín, F.L., Manabe, M., Tsuihiji, T., Rich, T.H., Vickers-Rich, P. & Novas, F.E., 2023. First monotreme from the Late Cretaceous of South America. Communications Biology 6, 146.
- Close, R.A., Friedman, M., Lloyd, G.T. & Benson, R.B.J., 2015. Evidence for a mid-Jurassic adaptive radiation in mammals. Current Biology 25, 2137–2142.
- Darlington, P.J., 1957. Zoogeography: The Geographical Distribution of Animals. John Wiley & Sons, New York, 675 pp.
- De Vis, C., 1885. In anon. The Daily Observer 549, 2.
- Flannery, T.F., Archer, M., Rich, T.H. & Jones, R., 1995. A new family of monotremes from the Cretaceous of Australia. Nature 377, 418–420.
- Flannery, T.F., Rich, T.H., Vickers-Rich, P., Ziegler, T., Veatch, E.G. & Helgen, K.M., 2022a. A review of monotreme (Monotremata) evolution. Alcheringa 45, 1–18.
- Flannery, T.F., Rich, T.H., Vickers-Rich, P., Veatch, E.G. & Helgen, K.M., 2022b. The Gondwanan Origin of Tribosphenida (Mammalia). Alcheringa 46, 1–14.
- Frauenfelder, T.G., Campione, N.E., Smith, E.T. & Bell, P.R., 2021. Diversity and palaeoecology of Australia’s southern-most sauropods, Griman Creek Formation (Cenomanian), New South Wales, Australia. Lethaia 54, 354–367.
- Gill, T., 1877. Vertebrate zoology. In: Baird, S.F. (Ed.), Annual Record of Science and Industry for 1876. Harper and Brothers, New York, 171–172.
- Godthelp, H., Archer, M., Cifelli, R., Hand, S.J. & Gilkeson, C.F., 1992. Earliest known Australian Tertiary mammal fauna. Nature 356, 514–516.
- Gray, J.E., 1825. An outline of an attempt at the disposition of the Mammalia into tribes and families with a list of the genera apparently appertaining to each tribe. Annals of Philosophy (n.s.) 10, 337–344.
- Hart, L.J., 2020. Taxonomic clarifications concerning the crocodyliform genus Isisfordia. Peerj. 8, e8630.
- Hart, L., Bell, P., Smith, E., Mitchell, D., Brougham, T. & Salisbury, S., 2021. A probable skeleton of Isisfordia (Crocodyliformes) and additional crocodyliform remains from the Griman Creek Formation (Cenomanian, New South Wales, Australia). Journal of Paleontology 95, 351–366.
- Illiger, C., 1811. Prodromus systematis mammalium et avium additis terminis zoographicis utriusque classis, eorumque versione germanica Berolini. Selfeld, Germany.
- Kear, B.P., 2003. Cretaceous marine reptiles of Australia: a review of taxonomy and distribution. Cretaceous Research 24, 277–303.
- Kear, B.P., 2006. Plesiosaur remains from Cretaceous high-latitude non-marine deposits in southeastern Australia. Journal of Vertebrate Paleontology 26, 196–199
- Kear, B.P., 2016. Cretaceous marine amniotes of Australia: perspectives on a decade of new research. Memoirs of Museum Victoria 74, 17–28.
- Kear, B.P. & Hamilton-Bruce, R.J., 2011. Dinosaurs in Australia. Mesozoic Life from the Southern Continent. CSIRO Publishing, Melbourne, 190 p.
- Kearney, M. & Clark, J.M., 2003. Problems due to missing data in phylogenetic analyses including fossils: a critical review. Journal of Vertebrate Palaeontology 23, 263–274
- Kemp, A. & Berrell, R., 2020. A new species of fossil lungfish (Osteichthyes: Dipnoi) from the Cretaceous of Australia. Journal of Vertebrate Paleontology 40, e1822369.
- Kielan-Jaworowska, Z., Cifelli, R.L. & Luo, Z.-X., 2004. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. Columbia University Press, New York, 700 pp.
- Kitchener, J.L., Campione, N.E., Smith, E.T. & Bell, P.R., 2019. High-latitude neonate and perinate ornithopods from the mid-Cretaceous of southeastern Australia. Scientific Reports 9, 19600.
- Linnaeus, C., 1758. Systema naturae per regia troa matirae. Secundum classes, ordines, genera, species cum characteribus, differentiis synonymis, locis. Editio decima, reformata. Stockholm, Laurenti Salvii 1, 824 pp.
- Lopatin, A.V., Maschenko, A.M., Averianov, A.O., Skutschas, P.P., Rezvyi, A.S. & Leshchinskiy, S.V., 2005. Early Cretaceous mammals from Siberia. 1 Tinodontidae. Paleontological Journal 39, 523–534.
- Lopatin, A.V., Averianov, A.O., Maschenko, A.M. & Leshchinskiy, S.V., 2009. Early Cretaceous Mammals of Western Siberia: 2. Tegotheriidae. Paleontological Journal 43, 453–462.
- Luo, Z.-X., 2007. Transformation and diversification in early mammalian evolution. Nature 450, 1011–1019.
- Luo, Z.-X., Chen, P., Li, G. & Chen, M., 2007. A new eutriconodont mammal and evolutionary development in early mammals. Nature 446, 288–293.
- Luo, Z.-X., Cifelli, R.L. & Kielan-Jaworowska, Z., 2001. Dual origin of tribosphenic mammals. Nature 409, 53–57.
- Luo, Z.-X., Kielan-Jaworowska, Z. & Cifelli, R.L., 2002. In quest for a phylogeny of Mesozoic mammals. Acta Palaeontologica Polonica 47, 1–78.
- Luo, Z.-X., Meng, Q.-J., Grossnickle, D.M., Liu, D., Neander, A.I., Zhang, Y.-G. & Ji, Q., 2017. New evidence for mammaliaform ear evolution and feeding adaptation in a Jurassic ecosystem. Nature 548, 326–329.
- Mao, F., Zhang, C., Liu, C. & Meng, J., 2021. Fossoriality and evolutionary development in two Cretaceous mammaliamorphs. Nature 592, 577–582.
- Martin, T., Goin, F.J., Schultz, J.A. & Gelfo, J.N., 2022. Early Late Cretaceous mammals from southern Patagonia, Santa Cruz Province, Argentina. Cretaceous Research 133, 105127–105127.
- Musser, A.M., 2013. Classification and evolution of the monotremes. In Neurobiology of Monotremes. Brain Evolution in Our Distant Mammalian Cousins. Ashwell, K., ed., CSIRO Publishing, Melbourne, 1–17.
- Musser, A. & Archer, M., 1998. New information about the skull and dentary of the Miocene platypus Obdurodon dicksoni, and a discussion of ornithorhynchid relationships. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 353, 1063–1079.
- Pascual, R., Goin, F.J., Balarino, L. & Udrizar Sauthier, D.E., 2002. New data on the Paleocene monotreme Monotrematum sudamericanum, and the convergent evolution of triangulate molars. Acta Palaeontologica Polonica 47, 487–492.
- Phillips, M.J., Bennett, T.H. & Lee, M.S.Y., 2009. Molecules, morphology, and ecology indicate a recent, amphibious ancestry for echidnas. Proceedings of the National Academy of Sciences 106, 17089–17094.
- Poropat, S.F., Bell, P.R., Hart, L.J., Salisbury, S.W. & Kear, B.P., 2023. An annotated checklist of Australian Mesozoic tetrapods. Alcheringa 47, 129–205.
- Pridmore, P.A., Rich, T.H., Vickers-Rich, P. & Gambaryan, P.P., 2005. A tachyglossid-like humerus from the Early Cretaceous of south-eastern Australia. Journal of Mammalian Evolution 12, 359–378.
- Rich, T.H., Archer, M., Hand, S.J., Godthelp, H., Muirhead, J., Pledge, N.S., Lundelius, E.L., Jr Flannery, T.F., Rich, L.S.V., Woodburne, M.O., Case, J.A., Whitelaw, M.J., Tedford, R.H., Kemp, A., Turnbull, W.D. & Rich, P.V., 1991. Australian Mesozoic and Tertiary mammal localities. In Vertebrate Palaeontology in Australasia. Vickers-Rich, P., Monaghan, J.M., Baird, R.F. & Rich, T.H., eds. Pioneer Design Studio and Monash University Publications Committee, Melbourne, 1005–1058.
- Rich, T.H., Vickers-Rich, P., Constantine, A., Flannery, T.F., Kool, L. & VAN Klaveren, N., 1999. Early Cretaceous mammals from Flat Rocks, Victoria, Australia. Records of the Queen Victoria Museum 106, 1–34.
- Rich, T.H., Hopson, J.A., Gill, P.G., Trusler, P., Rogers-Davidson, S., Morton, S., Cifelli, R.L., Pickering, D., Kool, L., Siu, K. & others. 2016. The Mandible and Dentition of the Early Cretaceous Monotreme Teinolophos trusleri. Alcheringa 40, 475–501.
- Rich, T.H., Flannery, T.F., Evans, A.R., White, M., Ziegler, T., Maguire, A., Poropat, S., Trusler, P. & Vickers-Rich, P., 2020. Multiple hypotheses about two mammalian upper dentitions from the Early Cretaceous of Australia. Alcheringa 44, 528–536.
- Rich, T.H., Flannery, T.F. & Vickers-Rich, P., 2020. Evidence for a remarkably large toothed monotreme from the Early Cretaceous of Lightning Ridge, NSW, Australia. In Prasad, G.V. & Patnaik, R., eds. Biological Consequences of Plate Tectonics: New Perspectives on Post-Gondwana and Break-up—a Tribute to Ashok Sahni, Vertebrate Paleobiology and Paleoanthropology. Springer International, Cham, Switzerland, 77–81.
- Rougier, G.W., Martinelli, A.G., Forasiepi, A.M. & Novacek, M.J., 2007. New Jurassic mammals from Patagonia, Argentina: A reappraisal of australosphenidan morphology and interrelationships. American Museum Novitates 3566, 1–54
- Roycroft, E., Fabre, P.H., Macdonald, A.J., Moritz, C., Moussalli, A. & Rowe, K.C., 2022. New Guinea uplift opens an ecological opportunity across a continent. Current Biology 32, 4215–4224.e3.
- Shaw, G., 1799. The Naturalist’s Miscellany. 10 (118),F.P. Nodder & Co., London.
- Smith, E.T., 2010. Early Cretaceous chelids from Lightning Ridge, New South Wales. Alcheringa 34, 375–384.
- Smith, E.T. & Kear, B.P., 2013. Spoochelys ormondea gen. et sp. nov., an archaic meiolaniid-like turtle from the Early Cretaceous of Lightning Ridge, Australia. Morphology and evolution of turtles, 121–146.
- Smith, E., 1999. Black Opal Fossils of Lightning Ridge. Kangaroo Press East Roseville, New South Wales, Australia, 112 pp.
- Wang, H.-B., Hoffman, C., Wang, D.C. & Wang, Y.-Q., 2022. New mammal from the Lower Cretaceous Jehol Biota and implications for eutherian evolution. Philosophical Transactions of the Royal Society 377.
- Wible, J.R., Rougier, G.W., Novacek, M.J. & Asher, R.J., 2009. The eutherian mammal Maelestes gobiensis from the Late Cretaceous of Mongolia and the phylogeny of Cretaceous Eutheria. Bulletin of the American Museum of Natural History 2009, 1–123.
- Woollard, P., Vestjens, W.J.M. & Maclean, L., 1978. The ecology of the eastern water rat Hydromys chrysogaster at Griffith NSW: food and feeding habits. Wildlife Research 5, 59–73.
- Woodburne, M.O., Macfadden, B.J., Case, J.A., Springer, M.S., Pledge, N.S., Power, J.D., Woodburne, J.M. & Springer, K.B., 1994. Land mammal biostratigraphy and magnetostratigraphy of the Etadunna Formation (Late Oligocene) of South Australia. Journal of Vertebrate Paleontology 13, 483–515.
- Zhou, Z., 2014. The Jehol Biota, an Early Cretaceous terrestrial Lagerstätte: new discoveries and implications. National Science Review 1, 543–559.
- Zhou, Y., Shearwin-Whyatt, L., Li, J., Song, Z., Hayakawa, T., Stevens, D., Fenelon, J.C., Peel, E., Cheng, Y., Pajpach, F., Bradley, N., Suzuki, H., Nikaido, M., Damas, J., Daish, T., Perry, T., Zhu, Z., Geng, Y., Rhie, A., Sims, Y., Wood, J., Haase, B., Mountcastle, J., Fedrigo, O., Li, Q., Yang, H., Wang, J., Johnston, S.D., Phillippy, A.M., Howe, K., Jarvis, E.D., Ryder, O.A., Kaessmann, H., Donnelly, P., Korlach, J., Lewin, H.A., Graves, J., Belov, K., Renfree, M.B., Grutzner, F., Zhou, Q. & Zhang, G., 2021. Platypus and echidna genomes reveal mammalian biology and evolution. Nature 592, 756–762.