Abstract
This study investigated the effects of simvastatin, a lipid-regulating drug; irgarol, an antifouling biocide; and PBDE-47, a brominated flame retardant, on the estuarine fish, Fundulus heteroclitus. Sublethal effects (changes in glutathione (GSH), lipid peroxidation (LPx), acetylcholinesterase (AChE), and cholesterol (CHL) levels) and lethal effects (survival) were determined after individual exposure to the three compounds. There were no significant differences in GSH or CHL levels in fish exposed to any of the test compounds. LPx levels significantly decreased with increasing irgarol concentrations. AChE levels were significantly lower in fish exposed to simvastatin at the 1.25 mg/L concentration and significantly higher at the PBDE-47 concentration of 0.0125 mg/L. The LC50 values were 2.68, 3.22, and > 0.1 mg/L for simvastatin, irgarol and PBDE-47, respectively.
Introduction
Increased urbanization of southeastern US coastal areas has led to contamination of adjacent sediments and waters with a variety of compounds, including pharmaceuticals, pesticides, and industrial by-products.[ Citation 1 ] These compounds may have deleterious effects on estuarine biota.
Simvastatin, a lipid regulating drug sold under the trade name Zocor®, inhibits 3-hydroxy-3-methylglutaryl coenzyme A which lowers total and low-density lipoprotein cholesterol production.[ Citation 2 ] It has also been shown to decrease ubiquinone, which could result in impaired antioxidant protection.[ Citation 3 ] Irgarol 1051 is an herbicide ingredient in many marine antifouling paints used worldwide. It causes photosystem II inhibition like other triazine herbicides. PBDE-47 is one of a class of flame retardants (polybrominated diethyl ethers) found in many consumer products. It has been shown to accumulate in crustaceans, fishes and marine mammals and may interfere with endocrine system function.[ Citation 4 ]
The mummichog, Fundulus heteroclitus, inhabits estuaries from the Gulf of St. Lawrence south to northeastern Florida. These fish are quite abundant and density can range up to 6 fish/m2 in tidal creeks. They are ecologically significant as they are prey for commercially and recreationally important fishes in addition to aquatic birds. Mummichogs also have an important role in mosquito control.[ Citation 5 ]
The purpose of this research was to determine the lethal effects (survival) on Fundulus heteroclitus using the three contaminants simvastatin, irgarol, and PBDE-47; along with measuring sublethal effects using the biomarkers glutathione (GSH), lipid peroxidation (LPx), acetylcholinesterase (AChE), and cholesterol (CHL).
Materials and methods
Collection
Mummichogs (F. heteroclitus), at 45 – 75 mm in length, were collected from Cherry Point Creek (N 32°36′ 04.29″; W 080°11′07.01″), a tidal tributary of the North Edisto River Estuary, SC, USA. Fish were acclimated at least seven days in 76-L tanks at 25°C, 20 ppt salinity and 16-h light:8-h dark cycle and fed Tetramin® Fish Flakes.
Toxicity tests
To assess acute toxicity 96-h static renewal toxicity tests were performed with each compound. Tests were conducted in 4-L glass jars to which 3.5-L of test media was added per jar. There were three replicates per concentration with five fish in each jar. The nominal test concentrations of simvastatin were 0, 0.625, 1.25, 2.5 and 5.0 mg/L. The nominal test concentrations of irgarol were 0, 0.625, 1.25, 2.5 and 5.0 mg/L. The nominal test concentrations of PBDE-47 were 0, 0.0125, 0.025, 0.05 and 0.10 mg/L. PBDE-47 concentrations higher than 0.10 mg/L were not attempted due to its low water solubility ranging from 0.00146 to 0.0153 mg/L.[ Citation 6 ] The jars were placed in an environmental chamber set at 25°C with a 16-h light:8-h dark cycle and aerated. Fish were not fed during the tests. At each 24 h media change, mortality and water quality parameters [temperature (°C), pH, salinity (ppt), and dissolved oxygen (mg/L)] were recorded.
Biomarker tests
For the biomarker tests, separate 96-h exposures were performed at levels based on previous testing and under conditions as described for toxicity testing. The nominal test concentrations of simvastatin were control, 0.625, 1.25, 2.5 and 5.0 mg/L. The nominal test concentrations of irgarol were control, 0.3125, 0.625, 1.25, and 2.5 mg/L. The nominal test concentrations of PBDE-47 were control, 0.0125, 0.025, 0.05 and 0.10 mg/L. These tests were performed as described previously. At the end of each test, surviving fish were dissected and livers removed for CHL, GSH and LPx analysis and brains removed for AChE analysis. All samples were frozen after collection at −70°C until further analysis.
Glutathione (GSH) concentrations were assessed using the 5,5′-dithiosbis(2-nitrobenzoic acid)-oxidized glutathione (DTNB-GSSG) reductase recycling assay described in Ringwood et al.[ Citation 7 ] Data are expressed as GSH (nmol/g wet weight). Lipid peroxidation (LPx) was assessed using the thiobarbituric acid assay, as described in Ringwood et al.,[ Citation 7 ] to quantify malondialdehyde (MDA), a by-product of LPx. Data are expressed as MDA (nmol/g wet weight). Acetylcholinesterase (AChE) concentrations were assessed as described in Key and Fulton.[ Citation 8 ] Data are expressed as AChE (nmol/mg protein/min). Cholesterol (CHL) concentrations were assessed using the Amplex Red Cholesterol Kit (Molecular Probes A12216) as based on Hoguet and Key.[ Citation 9 ] Data are expressed as CHL (mg/mg tissue).
Chemical analysis
For all tests with the three compounds, the stock solutions were made in 100% pesticide grade acetone which was used as a carrier (0.1%). Acetone was added to the control groups equal to the amount of carrier solvent used for the tests.
Technical grade simvastatin (97% purity) was obtained from Sigma–Aldrich (St. Louis, MO). Analytical grade simvastatin (99.4% purity) was obtained from Fisher Scientific (Fairlawn, NJ). Stock solutions were quantified to be 101.8% of nominal for a 10,000 mg/L stock solution and 100.4% of nominal for a 100 mg/L stock solution.[ Citation 10 ]All nominal treatment concentrations were made from these stocks.
For all irgarol tests, technical grade Irgarol 1051 (98.8% purity) was obtained from Sigma-Aldrich (St. Louis, MO, USA). For chemical analysis, Irgarol 1051 standard was obtained from Ciba Specialty Chemicals Inc. (Tarrytown, NY, USA). The stock solution was quantified to be 92.6% of a nominal 10000 mg/L stock solution.[ Citation 11 ] All nominal treatment concentrations were made from this stock.
For all PBDE-47 tests, technical grade (99% purity) was obtained from Chem Service Inc. (West Chester, PA). Analytical grade PBDE-47 in isooctane (100 mg/L) was obtained from o2si smart solutions, LLC (Charleston, SC). The stock solution was quantified to be 103.8% of nominal for a 100 mg/L stock solution.[ Citation 12 ] All nominal treatment concentrations were made from this stock.
Statistics and analysis
Median lethal concentrations (LC50s) with 95% confidence intervals were determined using the trimmed Spearman-Karber method.[ Citation 13 ] For the mortality data, analysis of variance (ANOVA) with Dunnett's procedure for comparison to determine significant differences from the control response was performed to determine the Lowest Observed Effect Concentration (LOEC) and the No Observed Effect Concentration (NOEC).[ Citation 10 , Citation 11 , Citation 14 ] For comparisons within each biomarker assay, the data were analyzed with ANOVA. For the biomarker tests, a one-way ANOVA with Dunnett's (PROC GLM, SAS Institute Cary, NC, USA) procedure for multiple comparisons versus the control was used to detected significant differences between treatment groups. If ANOVA showed no significance, a monotonic trend analysis (isotonic regression) was performed on this data using Williams' test as described by Newman.[ Citation 15 ] For all statistical tests, alpha was set to 0.05 a priori.
Results and discussion
Average water quality parameters for the toxicity tests are shown in . Dissolved oxygen levels ranged from 5.91 to 6.57 mg/L, pH ranged from 7.44 to 7.67, temperature ranged from 22.02 to 24.08°C and salinity ranged from 19.62 to 21.39 ppt. These ranges were all within the acclimated conditions described previously and within natural mummichog field conditions.[ Citation 5 ]
Table 1 Average water quality parameters with standard deviations for the simvastatin, irgarol and PBDE-47 96-h toxicity tests.
The 96-h LC50 test results for all compounds are shown in . A 96-h LC50 could not be obtained for PBDE-47 as there was only 13.4% mortality in the highest exposure of 0.1 mg/L. As stated previously, further testing to obtain a 96-h LC50 in mummichogs was not attempted due to the compound's low water solubility. In other estuarine species, a grass shrimp (Palaemonetes pugio) 96-h LC50 was obtained at 0.078 mg/L.[ Citation 12 ] However, 50% mortality was not obtained with other estuarine crustaceans tested.[ Citation 16 , Citation 17 ] PBDE-47 is an important contaminant in terms of bioaccumulation in aquatic animals and has been found in surface waters at concentrations much lower than that of simvastatin and irgarol. In North America, PentaBDEs (of which PBDE-47 is a major constituent), have been found in surface waters at concentrations up to 0.00016 μ g/L.[ Citation 4 ] The main concern with PBDE exposure in aquatic animals is bioaccumulation.[ Citation 12 ]
Table 2 Toxicity values (mg/L) for mummichogs exposed to simvastatin, irgarol and PBDE-47 for 96-h.
Simvastatin has been measured in sewage treatment plant effluent and influent at levels of 0.001 and 0.004 μ g/L, respectively.[ Citation 18 ] Clearly, this is well below the LC50 and LOEC values for mummichogs (). Other published toxicity values in fish could not be found. When toxicity is compared to other organisms, the adult grass shrimp (Palaemonetes pugio) 96-h LC50 was above 10 mg/L, while the larval grass shrimp 96-h LC50 was 1.18 mg/L[ Citation 10 ] which was comparable to the mummichog value of 2.68 mg/L.
Irgarol has been measured in seawater worldwide at levels up to 4.2 μ g/L in Singapore[ Citation 19 ] to 1.03 μ g/L in the US.[ Citation 20 ] As with simvastatin, these levels are well below the LC50 and LOEC values for mummichogs (). The 96-h LC50 of 3.22 mg/L for mummichogs is comparable to other estuarine fish values of 1.58 mg/L for Menidia beryllina and 3.5 mg/L for Cyprinodon variegatus.[ Citation 20 ] Crustaceans, such as grass shrimp, had a similar 96-h LC50 value of 2.46 mg/L.[ Citation 11 ]
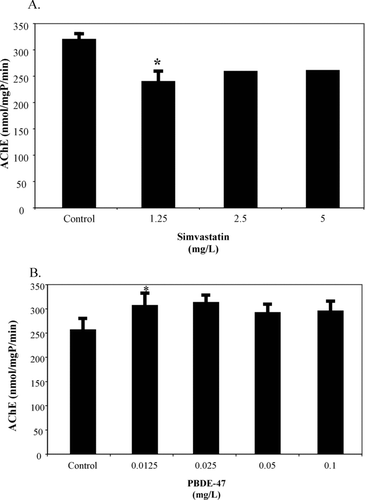
Biomarker results for simvastatin only yielded significant changes for AChE. All concentrations measured had AChE levels lower than the control, but only the lowest concentration of 1.25 mg/L was significantly reduced (p = 0.0193; ). While the NOEC for mortality was 1.25 mg/L, clearly sublethal effects were occurring. However, this was still well above published environmental concentrations (see above). While simvastatin can reduce the production of CHL, it did not occur in mummichogs at the levels used in this research. In fact, there was a slight increase from 0.005674 mg/mg tissue for the controls up to 0.007074 mg/mg tissue for the 5.0 mg/L exposure.
Fig. 1 Acetylcholinesterase (AChE) levels in mummichogs exposed to simvastatin (A.) and PBDE-47 (B.) for 96-h. ∗Significantly different from control value.
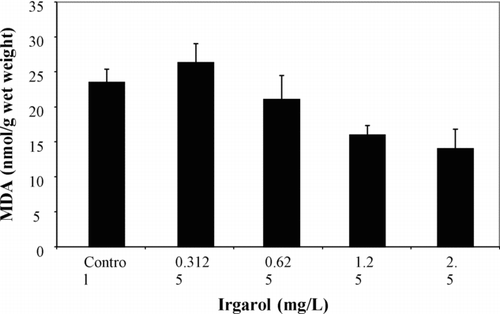
Irgarol biomarker results only showed significant changes with LPx levels (). After a peak in the lowest concentration of 0.313 mg/L, LPx levels then showed a significant downward trend (p = 0.0059). Sublethal effects were therefore occurring at levels 4 times lower than the NOEC of 1.25 mg/L but still well above reported environmental levels. Other biomarker levels were not significantly affected by irgarol exposures.
Fig. 2 Lipid peroxidase levels expressed as malondialdehyde (MDA) in mummichogs exposed to irgarol for 96-h. Williams' test indicated a downward significant trend from control to the highest concentration.
As with simvastatin, PBDE-47 biomarker results were only significant in the AChE assay. AChE levels in the lowest concentration of 0.0125 mg/L were significantly higher than controls (p = 0.0239; ). Significant sublethal effects occurred below the PBDE-47 NOEC value but were still well above environmental levels. For all compounds tested, biomarker data with no significant effects were not shown.
No one biomarker of the four tested emerged as a useful measure of simvastatin, irgarol or PBDE-47 exposure in mummichogs. Both the LC50s and the sublethal effects detected in simvastatin and irgarol exposures occurred at levels many times higher than that found in the environment. For PBDE-47, a better assay of exposure in mummichogs may be a measure of thyroid levels since this compound bioaccumulates. Contamination of the estuarine environment is an evolving occurrence due to the vast array of manmade chemical products available. While individually these three compounds may not appear to be toxic compared to environmental levels, it is the mixture of these contaminants with other anthropogenic compounds that should be investigated.
Acknowledgments
NOAA's National Ocean Service (NOS) does not approve, recommend, or endorse any proprietary product or material mentioned in this publication.
References
- Van Dolah , R. , Riekerk , G. , Bergquist , D. , Felber , J. , Chesnut , D. and Holland , A. 2007 . Estuarine habitat quality reflects urbanization at large spatial scales in South Carolina's coastal zone . Sci. Total. Environ. , 390 : 142 – 154 .
- Hess , D. and Fagan , S. 2001 . Pharmacology and clinical experience with simvastatin . Exp. Opin. Pharmacother. , 2 : 153 – 163 .
- Passi , S. , Stancato , A. , Aleo , E. , Dmitrieva , A. and Littarru , G. 2003 . Statins lower plasma and lymphocyte ubiquinol/ubiquinone without affecting other antioxidants and PUFA . BioFactors , 18 : 113 – 124 .
- Hale , R. , Alaee , M. , Manchester-Neesvig , J. , Stapleton , H. and Ikonomou , M. 2003 . Polybrominated diphenyl ether flame retardants in the North American environment . Environ. Intern. , 29 : 771 – 779 .
- Abraham , B. J. 1985 . Species profiles: life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic) - mummichog and striped killifish , Vicksburg, MS : Waterways Experiment Station . US Fish and Wildlife Service Biological Report 82 (11). US Army Corps of Engineers TR EL-82-4
- Palm , A. , Cousins , I. , Mackay , D. , Tysklind , M. , Metcalfe , C. and Alaee , M. 2002 . Assessing the environmental fate of chemicals of emerging concern: a case study of the polybrominated diphenyl ethers . Environ. Poll. , 117 : 195 – 213 .
- Ringwood , A. , Hoguet , J. , Keppler , C. , Geilazyn , M. , Ward , B. and Rourk , A. 2003 . Cellular biomarkers (lysosomal destabilization, glutathione and lipid peroxidation) in three common estuarine species: a methods handbook , Durham, NH : The Cooperative Institute for Coastal and Estuarine Environmental Technology .
- Key , P. and Fulton , M. 2002 . Characterization of cholinesterase activity in tissues of the grass shrimp (Palaemonetes pugio) . Pest. Biochem. Physiol. , 72 : 186 – 192 .
- Hoguet , J. and Key , P. 2007 . Activities of biomarkers in multiple life stages of the model crustacean, Palaemonetes pugio . J. Exp. Mar. Biol. Ecol. , 353 : 235 – 244 .
- Key , P. , Hoguet , J. , Reed , L. , Chung , W. and Fulton , M. 2008 . Effects of the statin antihyperlipidemic agent simvastatin on grass shrimp, Palaemonetes pugio . Environ. Toxicol. , 23 : 153 – 160 .
- Key , P. , Chung , K. , Hoguet , J. , Sapozhnikova , Y. and Fulton , M. 2008 . Effects of the anti-fouling herbicide Irgarol 1051 on two life stages of the grass shrimp, Palaemonetes pugio . J. Environ. Sci. Health , 43 ( B ) : 50 – 55 .
- Key , P. , Chung , K. , Hoguet , J. , Shaddrix , B. and Fulton , M. 2008 . Toxicity and physiological effects of brominated flame retardant PBDE-47 on two life stages of grass shrimp, Palaemonetes pugio . Sci Total Environ. , 399 : 28 – 32 .
- Hamilton , M. , Russo , R. and Thurston , R. 1977 . Trimmed Spearman-Karber method for estimating median lethal concentrations in toxicity bioassays . Environ. Sci. Tech. , 11 : 714 – 719 .
- Gelber , R. D. , Lavin , P. T. , Mehta , C. and Schoenfeld , D. 1985 . “ Statistical analysis ” . In Fundamentals of Aquatic Toxicology , Edited by: Rand , G. M. and Petrocelli , S. R. 110 – 123 . New York : Hemisphere Publishing Corporation .
- Newman , M. 1995 . Quantitative Methods in Aquatic Ecotoxicology , Boca Raton, FL : Lewis Publishers .
- Breitholtz , M. and Wollenberger , L. 2003 . Effects of three PBDEs on development, reproduction and population growth rate of the harpacticoid copepod Nitocra spinipes. Aquat . Toxicol. , 64 : 85 – 96 .
- Wollenberger , L. , Dinan , L. and Breitholtz , M. 2005 . Brominated flame retardants: activities in a crustacean development test and in an ecdysteroid screening assay . Environ. Toxicol. Chem. , 24 : 400 – 407 .
- Miao , X. and Metcalfe , C. 2003 . Determination of cholesterol-lowering statin drugs in aqueous samples using liquid chromatography-electrospray ionization tandem mass spectrometry . J. Chromat. A. , 998 : 133 – 141 .
- Konstantinou , I. and Albanis , T. 2004 . Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment; a review . Environ. Intern. , 30 : 235 – 248 .
- Hall , L. Jr. , Geddings , J. , Solomon , K. and Balcomb , R. 1999 . An ecological risk assessment for the use of Irgarol 1051 as an algaecide for antifoulant paints . Crit. Rev. Toxicol. , 29 : 367 – 437 .