Abstract
Toxicity of gamma irradiated mycotoxins aflatoxin B1 (AFB1) and ochratoxin A (OTA) was investigated in vitro. AFB1 and OTA stock solutions (50 mM, in methanol) were gamma irradiated (5 and 10 kGy) and non-irradiated and irradiated mycotoxins solutions were tested for cytotoxicity on Pk15, HepG2 and SH-SY5Y cell lines (MTT assay, 1–500 μM concentration range; 24 h exposure). Degradation of mycotoxin molecules was examined by liquid chromatography tandem mass spectrometry (HPLC-MS/MS). AFB1 and OTA radiolytic products were less toxic than the parent mycotoxins to all of the tested cell lines. Gamma irradiation even at 5 kGy had effect on AFB1 and OTA molecules however, this effect was dependent on chemical structure of mycotoxin. Since gamma irradiation at low dose reduced initial level of both mycotoxins, and gamma irradiated mycotoxins had lower toxicity in comparison to non-irradiated mycotoxins, it can be concluded that gamma irradiation could be used as decontamination method.
Introduction
Mycotoxins aflatoxin B1 (AFB1) and ochratoxin A (OTA) have been found as contaminants of various commodities all over the world.[Citation1–3] Recent review article reported AFB1 concentrations in foodstuffs of up to 42 and 90 μg/kg in Africa and Asia, respectively, and OTA of up to 9 μg/kg in Central and South America and Europe, and 15 μg/kg in Asia.[Citation3] Toxicity and carcinogenicity of AFB1 and OTA to experimental animals are well documented.[Citation4] Due to proven carcinogenicity to humans, International Agency for Research on Cancer (IARC) classified AFB1 as a Group 1 carcinogen,[Citation5] while OTA is classified into group 2B (possible human carcinogen).[Citation6] To protect human and animal health the governments around the world set specific regulations for acceptable concentrations of mycotoxins in food and feed. In the European Union maximum levels and guidance values for mycotoxins in food intended for human consumption have been set by Commission Regulation (EU) 1881/2006 and Commission Recommendation No 2013/165/EU.[Citation7,Citation8] According to them for AFB1 maximum level in all cereals and all products derived from cereals is set to 2.0 μg/kg, while for OTA maximum level in all products derived from unprocessed cereals, including processed cereal products and cereals intended for direct human consumption is set to 3.0 μg/kg.[Citation7]
To reduce mycotoxin contamination and consequently human and animal exposure to them, several studies tested gamma irradiation as decontamination method.[Citation9–14] Gamma irradiation is a physical method based on the ability of high-energy photons generated by radiation of 60Co to induce chemical changes of target molecule by causing its degradation or remodelling. In mycotoxin molecules the most prone to the interaction with high-energy photons generated by radiation of 60Co are aromatic or heterocyclic rings. Studies so far indicate that gamma irradiation can reduce mycotoxin levels in various commodities. Gamma irradiation at low dose (up to 6 kGy) reduced for around 90% AFB1 level in fruit and red chillies samples,[Citation9,Citation12] and dose of 10 kGy in maze seed samples reduced AFB1 level for around 95%.[Citation14] Similarly, gamma irradiation at dose of 10 kGy almost completely degraded OTA in coffee beans,[Citation11] and same dose (10 kGy) in dry-cured meat products reduced OTA for around 22.5%.[Citation13]
Although studies indicate that gamma irradiation could be suitable decontamination method, data on toxicity of radiolytic products of mycotoxins (products formed by gamma irradiation of mycotoxins) are lacking. Therefore, the aim of this study was to test the toxicity of the parent mycotoxin and its radiolytic products on three cell lines, Pk15, HepG2 and SH-SY5Y in order to mimic different target organs (kidney, liver and brain). Additionally, degradation of mycotoxin molecules was monitored by HPLC-MS/MS.
Materials and methods
Gamma irradiation of mycotoxin stock solutions
Mycotoxins AFB1 and OTA (Sigma-Aldrich, St. Louis, MO, USA) stock solutions at 50 mM were prepared in methanol and aliquoted in amber glass vials. For each mycotoxin, one sample was not irradiated (non-irradiated sample, positive mycotoxin control) and two samples were subjected to gamma irradiation.
Mycotoxin stock solutions (in closed amber glass vials) were gamma irradiated in a panoramic 60Co source at the Radiation Chemistry and Dosimetry Laboratory, Ruđer Bošković Institute (Zagreb, Croatia). The radiation doses were 5 and 10 kGy and the dose rate was 140 Gy/min. The dose rate was established using an ethanol-chlorobenzene dosimetry system[Citation15] and calculated daily taking into account the radioactive decay of 60Co. The irradiation was performed at ambient temperature (about 18 °C) and ambient atmosphere of a gamma chamber.
HPLC-MS/MS analysis
HPLC-MS/MS analysis of non-irradiated and irradiated AFB1 and OTA stock solutions was performed using an HPLC (Infinity 1260, Agilent, Santa Clara, USA) coupled with a triple quadrupole mass detector (6410, Agilent, Santa Clara, USA). The mobile phase consisted of 0.1% formic acid (in water) and acetonitrile, and the injection volume was 1 μL. The mass spectrometry conditions were as follows: electrospray ionisation (ESI), positive polarity, fragmentor voltage 150 and 110 V for AFB1 and OTA, respectively, capillary voltage 6 kV (+), source temperature 350 °C, nebuliser 45 psi and gas flow 5 L/min. The mass spectrometer was operated in MS2 scan (full scan mode). Protonated molecular ions of AFB1 at m/z = 313 and OTA at m/z = 404 were monitored.
Cell viability
Cell viability was determined by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide (MTT assay; Sigma-Aldrich St. Louis, MO, USA), using porcine kidney epithelial (Pk15), human hepatoma (HepG2) and human neuroblastoma (SH-SY5Y) cells. Cells were seeded in 96-well tissue culture plates at a planting density of 8 × 104 cells/mL and maintained at 37 °C in a humidified atmosphere of 5% CO2 and 95% of air. Cells were treated with non-irradiated AFB1 or OTA (mycotoxin positive control) or with AFB1 or OTA irradiated at 5 or 10 kGy at final concentrations of 1–500 μM for 24 h. AFB1 and OTA stock solutions were prepared in methanol; by diluting stock solutions in PBS buffer, the final methanol concentration in the mycotoxin solutions for cell treatment was less than 1% and did not affect cell viability. In each experiment, a negative control (PBS-buffer treated) and an MTT-positive control were included. After the treatment, the cell medium was removed and cells incubated with MTT solution at a final concentration of 0.5 mg/mL. After incubation (3 h, at 37 °C in an atmosphere of 5% CO2 and 95% of air), formazan crystals were dissolved in dimethyl sulfoxide (DMSO) and absorbance was read at 570 nm on a multilabel plate reader (Victor™, PerkinElmer, Walthman, MA, USA).
Statistical analysis
The results of cell viability of two independent sets of experiments (each with minimum six replicates) are expressed as mean percentage (%) ± SD, compared to negative control (PBS-treated cells, set at 100%). The differences among treatments were tested by one-way analysis of variance (ANOVA) followed by a post hoc Tukey test using Statistica software version 10.0 (StatSoft Inc. 1984-2011, USA). The statistical significance was set at 95% (P = 0.05).
Results and discussion
Cytotoxicity of AFB1 and OTA
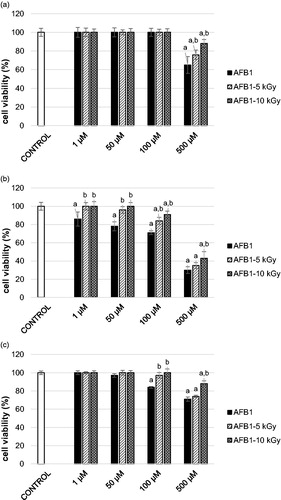
AFB1 was cytotoxic to all of the tested cell lines, SH-SY5Y, Pk15 and HepG2 (). These results are in agreement with previous studies that showed decreased cell survival after treatment with AFB1.[Citation16–19] Cytotoxicity of AFB1 to SH-SY5Y was recorded at 100 µM (reduction of cell viability for 16 ± 1.2%; P < 0.05, compared to negative control; ), while AFB1 was cytotoxic to Pk15 only at 500 μM (reduction of cell viability for 35 ± 8.9%; P < 0.05, compared to negative control; ). As expected, HepG2 cells were the most sensitive to AFB1 treatment. A decrease in HepG2 viability (by 14 ± 7.8%; P < 0.05, compared to negative control; ) was observed even after the lowest applied AFB1 concentration (1 μM). AFB1 is a well-documented hepatocarcinogen whose toxicity is mainly connected to its metabolic activation and genotoxic metabolite formation. Since AFB1 is predominantly metabolised in the liver, the human hepatoma HepG2 cell model is considered to be the most suitable system for testing its in vitro toxicity. This system represents liver cells and includes a wide spectrum of phase I and II enzyme activities enabling them to mimic metabolic conditions of xenobiotics in the living organism.[Citation20,Citation21] The results on cell viability observed in our study after treatment with AFB1 differ from studies by Costa et al.,[Citation22] Ghaderi et al.[Citation23] and Liu et al.[Citation24] This could be attributed to different cell viability tests, exposure conditions, culture medium as well as supplements added to the medium. However, the results are comparable to the study by Curcuera et al.[Citation25] in which using the same assay (MTT) similar IC50 on HepG2 cells for AFB1 was obtained (about 100 µM).
Figure 1. Cell viability of: (A) Pk15 cells, (B) HepG2 cells, and (C) SH-SY5Y cells after treatment with non-irradiated aflatoxin B1 (AFB1, 1–500 µM, 24 h; solid black columns) or gamma irradiated AFB1 at 5 or 10 kGy (1–500 µM, 24 h; AFB1-5 kGy, diagonally striped columns, AFB1-10 kGy, diagonally crossed columns); cell viability is assessed with MTT test. The results are expressed as mean percentage (%) ± standard deviation, compared to negative control (PBS-treated cells, set at 100%; solid white columns) of two independent set of experiments, each with six replicates; column represent mean value and error bar standard deviation. a - different from negative control (PBS-control); b - different from respective non-irradiated control (AFB1-treated). One-way ANOVA followed by Tukey test (P < 0.05).
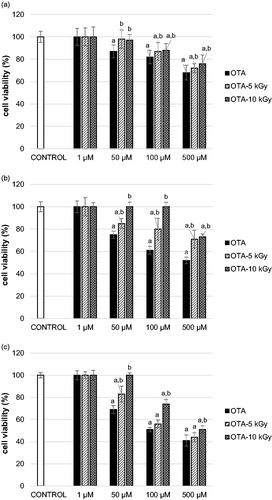
In this study OTA induced a decrease in cell viability to all of the tested cell lines. The cytotoxicity of OTA to Pk-15, HepG2 and SH-SY5Y was observed at a concentration of 50 μM (). However, a more pronounced toxic effect of OTA (at concentration 50 μM) was recorded on SH-SY5Y cells (reduction of cell viability for 31 ± 3.6%, P < 0.05, compared to negative control; ) and HepG2 cells (reduction of cell viability for 25 ± 3.1%; P < 0.05, compared to negative control; ), while kidney cells (Pk 15) seemed to be more resistant (reduction of cell viability for 13 ± 5.6%; P < 0.05, compared to negative control; ). These results are in agreement with Zhang et al.[Citation26] who observed on SH-SY5Y cells and primary neurons OTA toxicity at low concentrations (0.25 μM and 0.5 μM, respectively). Since their results were comparable to those obtained previously by Hundhausen et al.[Citation27] on hepatocytes, the authors concluded that neurons could be equally susceptible to OTA as hepatocytes. Moreover, the same authors, by comparing their results to study conducted on kidney tubulus cells[Citation28,Citation29] concluded that neurons may be even more susceptible to OTA than kidney cells as is observed in our study. Curcuera et al.[Citation25] and Gayathari et al.[Citation30] obtained a similar IC50 for OTA on HepG2 as in our study (360 and 210 μM, respectively). Additionally, as in our study Curcuera et al.[Citation25] found that AFB1 was more toxic to HepG2 than OTA.
Figure 2. Cell viability of: (A) Pk15 cells, (B) HepG2 cells, and (C) SH-SY5Y cells after treatment with non-irradiated ochratoxin A (OTA, 1–500 µM, 24 h; solid black columns) or irradiated OTA at 5 or 10 kGy (1–500 µM, 24 h; OTA-5 kGy, diagonally striped columns, OTA-10 kGy, diagonally crossed columns); cell viability is assessed with MTT assay. The results are expressed as mean percentage (%) ± standard deviation, compared to negative control (PBS-treated cells, set at 100%; solid white columns) of two independent set of experiments each with six replicates; column represent mean value and error bar standard deviation. (a) Different from negative control (PBS-control); (b) Different from respective non-irradiated mycotoxin control (OTA-treated). One-way ANOVA followed by Tukey test (significant at level P < 0.05).
Cytotoxicity of gamma irradiated AFB1 and OTA
Results on the cytotoxicity of gamma irradiated mycotoxins demonstrated that radiolytic products of gamma irradiated AFB1 and OTA in comparison to the parent mycotoxin were less toxic to all of the cell lines tested. In comparison to the parent molecules at the same concentration observed reduction in toxicity of gamma irradiated mycotoxins to Pk-15, HepG2 and SH-SY5Y cells was by around 20% (from 5 to 40%) (). For example, while non-irradiated AFB1 at 100 μM was cytotoxic to SH-SY5Y cells, AFB1 at the same concentration (100 μM) irradiated at 10 kGy was not toxic to SH-SY5Y cells, i.e. a complete reduction of AFB1 toxicity (reduction by around 16%; P < 0.05, compared to non-irradiated AFB1; ) was observed. These results clearly indicate that gamma irradiation induced formation of less toxic products than parent mycotoxin. In available literature there are only few studies on toxicity of gamma irradiated mycotoxins. In study of Kumar et al.,[Citation11] human intestinal epithelial (Int-407) cells were treated with gamma irradiated OTA (at 10 kGy) and by applying the same MTT assay an approximately 7-fold reduction of cytotoxicity of irradiated OTA is observed. In recent study cytotoxicity of gamma irradiated OTA (gamma irradiated to up to 10.3 kGy) was tested on HepG2 cells and 2-fold reduction of OTA cytotoxicity (assessed by neutral red assay) was observed.[Citation31] The difference in reduction of cytotoxicity of gamma irradiated OTA in above mentioned studies (7- and 2- fold) and in our study (only 20%) can be explained by the process of gamma irradiation. Gamma irradiation is more effective in reducing mycotoxin level when mycotoxin is present in lower concentration and when sample has higher moisture content.[Citation10,Citation32,Citation33] Although gamma irradiation of OTA for in vitro testing in the study of Kumar et al.[Citation11] and Calado et al.[Citation31] is not clearly described, it could be that they gamma irradiated OTA at lower concentration.
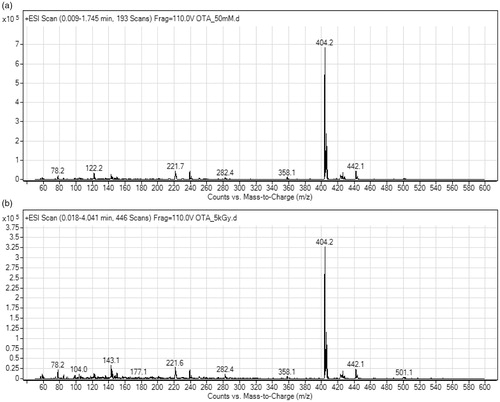
Results of HPLC-MS/MS analysis of non-irradiated and gamma irradiated mycotoxin solutions in our study revealed that gamma irradiation even at 5 kGy had impact on both, AFB1 and OTA. The signal intensity of AFB1 (AFB1 ion at 313.2 m/z) in AFB1 stock solution gamma irradiated at 5 kGy was reduced around 16 times compared to non-irradiated AFB1 stock solution (0.2 × 106 vs. 3.2 × 106; ). On the other hand, the signal intensity of OTA (OTA ion at 404.2 m/z) was reduced only twice (3.2 × 105 vs. 7.2 × 105; ). In the mass spectra of irradiated AFB1 solutions ions at 285.2, 345.2, 359.2, and 381.2 m/z were observed that could have been the product of AFB1 degradation or molecule remodelling. Ions pointing to molecule degradation or remodelling were not observed in the mass spectra of the irradiated OTA solution probably due to low concentration of these products reaching the instrument detection limit. The lower level of AFB1 and OTA detected in irradiated mycotoxin solutions confirmed that gamma irradiation has impact on AFB1 and OTA. Observed difference in the effect of gamma irradiation on AFB1 and OTA could be attributed to their chemical structure.
Figure 3. Mass spectra of aflatoxin B1 (AFB1) stock solutions (50 mM): (A) non-irradiated, and (B) irradiated at 5 kGy dose.
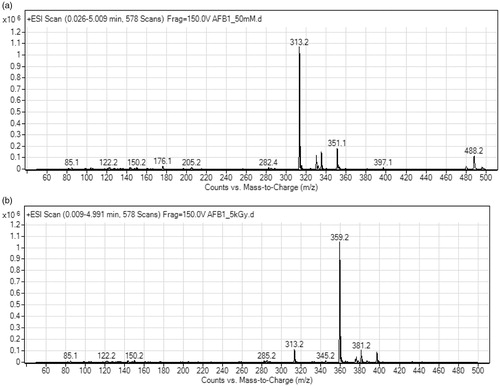
Figure 4. Mass spectra of ochratoxin A (OTA) stock solutions (50 mM): (A) non-irradiated, and (B) irradiated at 5 kGy dose.
HPLC-MS/MS analysis indicates that AFB1 molecule is more susceptible to degradation and remodelling induced by high-energy photons generated by 60Co than OTA. Different mycotoxin susceptibility to gamma irradiation observed in our study was previously reported by Mutler and Erkoc.[Citation34] They tested different aflatoxins and found that AFB1 was the most radiosensitive and aflatoxin B2 the most resistant to gamma irradiation. The high-energy photons generated by 60Co can affect double bounds in aromatic or heterocyclic rings in mycotoxin’s molecule. AFB1’s superior susceptibility to gamma irradiation could be attributed to feasible addition reaction on the 8,9 double bound in the furan ring initiated by the free radicals generated during gamma irradiation. This observation is confirmed by Wang et al.[Citation33] In study of Wang et al.[Citation33] AFB1 was gamma irradiated (up to 10 kGy) and by HPLC-MS/MS analysis seven radiolytic products of gamma irradiated AFB1 are identified and six of them were considered less toxic that AFB1. In that study the toxicity evaluation was based on the structure–activity relationship (SAR) analysis rather than on testing on live, in vitro model as in our study. The authors considered radiolytic product in which the addition reaction occurred on the double bond in the terminal furan ring less toxic than AFB1. According to quantitative SAR studies, the furofuran moiety of the AFB1 structure is essential for its toxicity and carcinogenicity. It is demonstrated that the presence of double bound in the terminal furan ring is important determinant of AFB1 acute and chronic effects in rats.[Citation33,Citation35,Citation36] Therefore, in our study the lower toxicity of AFB1 radiolytic products could be explained by lower unsaturation or the number of double-bonds in comparison to non-irradiated AFB1.
This study demonstrated that gamma irradiation (at 5 and 10 kGy) has an impact on both, AFB1 and OTA molecules. Radiolytic products formed by gamma irradiation of AFB1 and OTA are found to be less toxic to cells (Pk15, HepG2, SH-SY5Y) than the parent compounds (non-irradiated mycotoxins). Even low gamma irradiation dose (5 kGy) reduced AFB1 and OTA levels as detected by HPLC-MS/MS. However, AFB1 was more susceptible to gamma irradiation than OTA. AFB1 radiolytic products are probably results of addition reaction on double bound in the terminal furan ring. The mechanism of OTA degradation is more complex since its radiolytic products were not detected. Based on the results of studies examining impact of gamma irradiation on nutritional quality of food[Citation37–39] international food organisations (FAO/IAEA/WHO)[Citation40] concluded that gamma irradiation dose of up to 10 kGy has no effects on nutritional quality of food and has no adverse effect on human and animal health. Since in this study radiation up to 10 kGy was applied, gamma irradiation can be considered an effective and safe method for mycotoxin detoxification in the production of food and feed.
Acknowledgements
The authors wish to thank to Mr. Makso Herman for editing the manuscript.
References
- Reddy, K. R. N.; Salleh, B.; Saad, B.; Abbas, H. K.; Abel, C. A.; Shier, W. T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin. Rev. 2010, 29, 3–26.
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, K.; Nicolau, A.; Aprodu, I.; Puel, O.; Oswald, I. P. Current situation of mycotoxin contamination and co-occurrence in animal feed - focus on Europe. Toxins 2012, 4, 788–809. doi: 10.3390/toxins4100788
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. doi: 10.3390/toxins8020045
- International Programme on Chemical Safety, IPCS. Safety evaluation of certain mycotoxins in food. WHO Food Add Ser. 2001, 47, 103–415.
- International Agency for Research on Cancer, IARC. Some traditional herbal medicines, some mycotoxins, naphthalene, and styrene. IARC Monographs. Vol. 82, IARC, Lyon, 2002.
- International Agency for Research on Cancer, IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 56, IARC, Lyon, 1993.
- Commission Regulation (EU) 1881/2006.of 19 December 2006. setting maximum levels for certain contaminants in foodstuffs. Off. J. L 364:5–24.
- Commission Recommendation No. Off. J. L 2013, Vol. 91, 12–15. /165/EU of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products.
- Aziz, N. H.; Moussa, L. A. A. Influence of gamma-radiation on mycotoxin producing moulds and mycotoxins in fruits. Food Control 2002, 13, 281–288.
- Jalili, M.; Jinap, S.; Noranizan, M. A. Aflatoxins and ochratoxin a reduction in black and white pepper by gamma radiation. Rad. Phys. Chem. 2012, 81, 1786–1788.
- Kumar, S.; Kunwar, A.; Gautam, S.; Sharma, A. Inactivation of A. ochraceus spores and detoxification of ochratoxin a in coffee beans by gamma irradiation. J. Food Sci. 2012, 77, 44–51.
- Iqbal, S. Z.; Bhatti, I. A.; Asi, M. R.; Zuber, M.; Shahid, M.; Parveen, I. Effect of γ irradiation on fungal load and aflatoxins reduction in red chillies. Rad. Phys. Chem. 2013, 82, 80–84.
- Domijan, A.-M.; Pleadin, J.; Mihaljević, B.; Vahčić, N.; Frece, J.; Markov, K. Reduction of ochratoxin a in dry-cured meat products using gamma-irradiation. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess 2015, 32, 1185–1191.
- Markov, K.; Mihaljević, B.; Domijan, A.-M.; Pleadin, J.; Delaš, F.; Frece, J. Inactivation of aflatoxicogenic fungi and the reduction of aflatoxin B1 in vitro and in situ using gamma irradiation. Food Control 2015, 54, 79–85.
- Ražem, D.; Anđelić, L. J.; Dvornik, I. Consistency of ethanol-chlorobenzene dosimetry. Proceedings of IAEA Symposium on High-Dose Dosimetry. IAEA: Vienna, 1984; 143–156.
- Chan, H. T.; Chan, C.; Ho, J. W. Inhibition of glycyrrhizic acid on aflatoxin B1-induced cytotoxicity in hepatoma cells. Toxicology 2003, 188, 211–217.
- Lei, M. M.; Zhang, N.; Qi, D. In vitro investigation of individual and combined cytotoxic effects of aflatoxin B1 and other selected mycotoxins on the cell line porcine kidney 15. Exp. Toxicol. Pathol. 2013, 65, 1149–1157.
- Nones, J.; Nones, J.; Trentin, A. G. Flavonoid hesperidin protects neural crest cells from death caused by aflatoxin B(1). Cell Biol. Int. 2013, 37, 181–186.
- Sun, L. H.; Lei, M. Y.; Zhang, N. Y.; Gao, X.; Li, C.; Krumm, C. S.; Qi, D. S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon 2015, 95, 6–12.
- Cao, J.; Liu, Y.; Jia, L.; Jiang, L. P.; Geng, C. Y.; Yao, X. F.; Kong, Y.; Jiang, B. N.; Zhong, L. F. Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J. Agric. Food Chem. 2008, 56, 12059–12063.
- Ehrlich, V.; Darroudi, F.; Uhl, M.; Steinkellner, H.; Zsivkovits, M.; Knasmueller, S. Fumonisin B(1) is genotoxic in human derived hepatoma (HepG2) cells. Mutagenesis 2002, 17, 257–260.
- Costa, S.; Utan, A.; Speroni, E.; Cervellati, R.; Piva, G.; Prandini, A.; Guerra, M. C. Carnosic acid from rosemary extracts: A potential chemoprotective agent against Aflatoxin B1. An in vitro study. J. Appl. Toxicol. 2007, 27, 152–159.
- Ghaderi, M.; Allameh, A.; Soleimani, M.; Rastegar, H.; Ahmadi-Ashtiani, H. R. A comparison of DNA damage induced by aflatoxin B1 in hepatocyte-like cells, their progenitor mesenchymal stem cells and CD34+ cells isolated from umbilical cord blood. Mut. Res. Gen. Toxicol. Environ. Mutag. 2011, 719, 14–20.
- Liu, Y.; Du, M.; Zhang, G. Proapoptotic activity of Aflatoxin B1 and sterigmatocystin in HepG2 Cells. Toxicol. Report 2014, 1, 1076–1086.
- Corcuera, L. A.; Arbillaga, L.; Vettorazzi, A.; Azqueta, A.; López de Cerain, A. Ochratoxin a reduces Aflatoxin B1 induced DNA damage detected by the comet assay in Hep G2 cells. Food Chem. Toxicol. 2011, 49, 2883–2889.
- Zhang, X.; Boesch-Saadatmandi, C.; Lou, Y.; Wolffram, S.; Huebbe, P.; Rimbach, G. Ochratoxin a induces apoptosis in neuronal cells. Genes Nutr. 2009, 4, 41–48.
- Hundhausen, C.; Bosch-Saadatmandi, C.; Augustin, K.; Blank, R.; Wolffram, S.; Rimbach, G. Effect of vitamin E and polyphenols on Ochratoxin A-induced cytotoxicity in liver (HepG2) cells. J. Plant Physiol. 2005, 162, 818–822.
- Boesch-Saadatmandi, C.; Loboda, A.; Jozkowicz, A.; Huebbe, P.; Blank, R.; Wolffram, S.; Dulak, J.; Rimbach, G. Effect of ochratoxin A on redox-regulated transcription factors, antioxidant enzymes and glutathione-S-transferase in cultured kidney tubulus cells. Food Chem. Toxicol. 2008, 46, 2665–2671.
- Boesch-Saadatmandi, C.; Wagner, A. E.; Graeser, A. C.; Hundhausen, C.; Wolffram, S.; Rimbach, G. Ochratoxin a impairs Nrf2-dependent gene expression in porcine kidney tubulus cells. J. Anim. Physiol. Anim. Nutr. 2009, 93, 547–554.
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V. S.; Alshatwi, A. A.; Akbarsha, M. A. Hepatotoxic effect of Ochratoxin A and citrinin, alone and in combination, and protective effect of vitamin E: In vitro study in HepG2 cell. Food Chem. Toxicol. 2015, 83, 151–163.
- Calado, T.; Fernández-Cruz, M. L.; Cabo Verde, S.; Venâncio, A.; Abrunhosa, L. Gamma irradiation effects on Ochratoxin A: Degradation, cytotoxicity and application in food. Food Chem. 2018, 240, 463–471.
- Calado, T.; Venancio, A.; Abrunhosa, L. Irradiation for mould and mycotoxin control: A review. Comp Rev. Food Sci. Food Saf. 2014, 13, 1049–1061.
- Wang, F.; Xie, F.; Xue, X.; Wang, Z.; Fan, B.; Ha, Y. Structure elucidation and toxicity analyses of the radiolytic products of Aflatoxin B1 in methanol-water solution. J. Hazard Mat. 2011, 192, 1192–1202.
- Mutluer, B.; Erko, F. U. Effects of gamma irradiation on aflatoxins. Z Lebensm. Unters. Forch. 1987, 185, 398–401.
- Mishra, H. N.; Das, C. A review on biological control and metabolism of aflatoxin. Crit. Rev. Food Sci. Nutr. 2003, 43, 245–264.
- Wogan, G. N.; Edwards, G. S.; Newberne, P. M. Structure-activity relationships in toxicity and carcinogenicity of aflatoxins and analogues. Cancer Res. 1971, 31, 1936–1942.
- Aziz, N. H.; Souzan, R. M.; Azza, A. Effect of gamma-irradiation on the occurrence of pathogenic microorganisms and nutritive value of four principal cereal grains. App. Rad. Isotopes 2006, 64, 1555–1562.
- Taipina, M. S.; Garbelotti, M. L.; Lamardo, L. C. A.; Santos, J. S.; Rodas, M. A. B. The effect of gamma irradiation on the nutritional properties of sunflower whole grain cookies. Proc. Food Sci. 2011, 1, 1992–1996.
- Hamza, R. G.; Afifi, S.; Abdel-Ghaffar, A.-R. B.; Borai, I. H. Effect of gamma-irradiation or/and extrusion on the nutritional value of soy flour. Biochem. Anal. Biochem. 2012, 1, 118. doi: 10.4172/2161-1009.1000118
- World Health Organisation (WHO). High-dose irradiation: Wholesomeness of food irradiated with doses above 10 KGy. Joint FAO/IAEA/WHO study group. Technical Report Series, No. 890. 1999, Geneva.
