Abstract
Chlorpyrifos (CPF) is a widely used organophosphorus pesticide. Increasing evidence has shown that exposure to CPF in early life might induce neurodevelopmental disorders, but the pathogenesis remains uncertain. Synaptic plasticity plays a crucial role in neurodevelopment. This study aimed to investigate the effect of CPF on synaptic plasticity in hippocampal neurons and establish the cellular mechanism underlying these effects. Using CPF-exposed rat and primary hippocampal neurons model, we analyzed the impact of CPF on the synaptic morphology, the expression level of a presynaptic protein, a postsynaptic protein and ionotropic glutamate receptors (iGluRs), as well as the effects on the Wnt/β-catenin pathway. We found that the synapses were shortened, the spines were decreased, and the expression of synaptophysin (Syp), postsynaptic density-95 (PSD-95), GluN1, GluA1 and Wnt7a, as well as active β-catenin in primary hippocampal neurons was decreased. Our study suggests that CPF exposure induced dysregulation of synaptic plasticity in rat hippocampal neurons, which might provide novel information regarding the mechanism of CPF-induced neurodevelopmental disorders.
Introduction
CPF is an organophosphorus pesticide used globally. More than 200,000 tons of CPF were used in 2015, and the demand for this insecticide is expected to increase annually[Citation1]. Half-life of CPF in soil ranges from 10 to 120 d[Citation2] and greatly depends on the soil texture, pH value, organic matter content, application concentration and microorganisms in the soil[Citation3–6]. CPF degrades to 3,5,6-trichloro-2-pyridinol (TCP) naturally. TCP has been termed as a persistent metabolite by the U.S. EPA whose half-life ranges from 65 to 360 d in soil[Citation7]. Moreover, they have been found to be transported from their original place of use by runoff and geochemical cycles, which leads to the contamination of rivers, groundwater, soil and accumulation in the food chain through biomagnification[Citation2,Citation8]. Masí a et al. detected the concentration of CPF was up to 65.3 μg/kg in soil, sediment and sludge samples collected from the Spanish Túria River Basin[Citation9]. Bhandari et al. investigated that CPF and its metabolite TCP was one of the main pesticides in the soil, with a concentration range of 1.23–239 μg/kg[Citation10]. Through decades of tracking, the concentration of CPF has been found to reach 303.8 μg/L in seawater, rivers, groundwater and even rainwater in different countries [Citation11]. At the same time, in the Tuscany area of Italy, the concentration of CPF in the atmosphere of urban and rural areas ranges from 3 to 580 pg/m3 and 3 to 430 pg/m3, respectively[Citation12]. The residues of CPF in the environment pose a serious health threat to humans and non-target organisms, which should arouse public attention[Citation13,Citation14]. Moreover, CPF, in combination with other contaminants, is toxic to many organisms[Citation15].
We previously showed that neonatal rats experience increased anxiety and amnesia in adulthood after being exposed to CPF[Citation16]. CPF exposure was also found to induce long-term spatial memory deficits in rats[Citation17]. Recently, a relationship between chronic low-level CPF exposure and developmental disorders has been established[Citation18,Citation19]. However, the neurotoxic mechanisms associated with CPF-induced neurodevelopmental disorders are not clear.
Synapses are intercellular junctions specialized for information transfer from a presynaptic neuron to a postsynaptic cell[Citation20]. Synaptic transmission is affected by presynaptic vesicles containing neurotransmitters and the detection of these neurotransmitters by postsynaptic receptors. Synaptic plasticity refers to the ability of neurons to modify the strength of their connections and is an important neurophysiological process involved in brain networks development and reorganization after damage[Citation21]. Synaptic plasticity is also thought to be involved in the early development of neural circuitry, and accumulating evidence indicates that impairment of this process contributes to neurodevelopmental, learning and memory disorders[Citation22,Citation23]. The Wnt/β-catenin pathway is referred to as the classical Wnt signaling pathway and can regulate hippocampal plasticity, synaptic transmission and neurogenesis[Citation24]. This signaling pathway is implicated in a variety of human pathologies, including developmental disorders[Citation25,Citation26].
There is scarce information regarding the effects of CPF exposure on the synaptic plasticity. In this study, we aim to analyze the impact of CPF on the synaptic plasticity in rat hippocampal neurons. Herein, we hypothesized that exposure to CPF induces the morphological and molecular changes related to synaptic plasticity. And by evaluating the expression levels of critical proteins involved in the Wnt signaling pathway, we further explored the possible mechanism. A novel perspective regarding the mechanism of CPF-induced neurodevelopmental disorders might be provided.
Materials and methods
Animals and treatment
All Sprague-Dawley (SD) rats used in the experiment were purchased from Changsha Slac Animal Corporation (Hunan, China). The experiments were approved by the Medical Ethics Committee of the Third Xiangya Hospital and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Efforts were made to minimize animal suffering and to reduce the number of animals used. SD rats at postnatal day 11 weighing 17–20 g, regardless of sex, were used for this experiment.
CPF (purity 99.9%, Sigma, St. Louis, MO) was dissolved in dimethyl sulfoxide (DMSO; Sigma) at a concentration of 5 mg/mL and was subcutaneously injected into the abdomen at a dose of 1 mL/kg (5 mg/kg) daily from postnatal day 11 to 14. As in our previous study, this dose used did not result in weight loss, impaired viability or any systemic toxicity, including signs of cholinergic hyperstimulation[Citation27,Citation28]. Control animals were administered corresponding vehicle injections according to the same schedule.
Immunofluorescent and hematoxylin-eosin (HE) staining
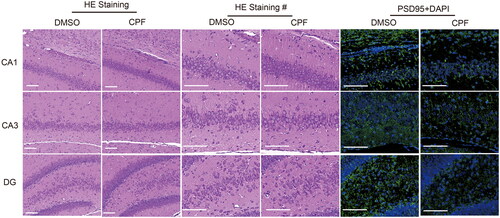
The brains of the rats were fixed in 4% paraformaldehyde overnight after perfusion with cold 0.1 M PBS (pH 7.4). For dehydration, the brains tissues were treated with graded ethanol. Then, the brain tissues were embedded with paraffin and cut into 5 μm-thick sections and stained with HE. HE staining were performed as previously described[Citation29,Citation30]. For immunofluorescent staining, the sections were pretreated with an antigen unmasking solution (Vector Laboratories, Burlingame, CA) and a spontaneous fluorescence-quenching agent before being incubated in 5% bovine serum albumin for 30 min at room temperature. Followed by incubation with anti-PSD-95 (1:100, Proteintech, Rosemont, IL) antibodies overnight at 4 °C, the sections were incubated with the secondary antibody (Servicebio, Wuhan, China) for 1 h at room temperature. Finally, DAPI was used to counterstain the nuclei. Images of the hippocampal CA1, CA3 and DG were acquired under a microscope (magnification 200×; Nikon, Tokyo, Japan) and merged using ImageJ software.
Primary cell culture, immunofluorescence, transfection and drug treatment
All SD rats used in the experiment were purchased from Changsha Slac Animal Corporation (Hunan, China). The experiments were approved by the Medical Ethics Committee of the Third Xiangya Hospital and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The culture method of primary hippocampal neurons was slightly modified based on a previously reported method[Citation31]. The hippocampus of E16–E18 rats was first isolated, and the surrounding vessels were removed. The isolated hippocampus was then digested and separated at 37 °C for 10 min with 0.25% trypsin (Gibco, Grand Island, NY) and 0.2% type I collagenase (Sigma) at a 1:1 ratio. The suspension was filtered via a 40 μm filter (Millipore, Billerica, MA) after adding DMEM/F12 (HyClone) with 10% fetal bovine serum (FBS, Gibco) supplementation before being centrifuged at 1,000 r/min for 5 min. The isolated cells were then stored in DMEM/F12 supplemented with 10% FBS, 1% Glutamax (Gibco), and 1% B-27 supplement (Invitrogen, Carlsbad, CA). According to the applications of the experiment, the cells were plated into different plates with different densities. The 96-well plates were used for subsequent CCK-8 experiments, and the 24-well plates were used for immunofluorescence staining and cell morphology observations. The cell density in each well was 2 × 105/mL. Moreover, 6-well plates were used to obtain cells for western blotting analysis, and the cell density in each well was 2 × 106/mL. Cells were cultured in an atmosphere of 95% humidified air and 5% CO2 at 37 °C. Four hours after plating, the culture medium changed to a neurobasal medium with 1% GlutaMax and 2% B27 supplementation, and this was changed every 3 d. The primary hippocampal neurons were mature at 6 d in vitro and identified by positive MAP2 expression using immunofluorescence techniques. The coverslips with the adhered cells were fixed with 4% paraformaldehyde for 15 min and then treated with 0.2% triton X-100 for 20 min at room temperature. Cells were incubated with an anti-MAP2 antibody (1:500, Abcam, Cambridge, UK) overnight at 4 °C after incubation with 5% bovine serum albumin (Geneview) for 30 min at room temperature. The next day, the cells were incubated with appropriate DyLight-labeled secondary antibodies (Jackson Immunoresearch, West Grove, PA) for 1 h at room temperature. Finally, DAPI (100 ng/mL, Sigma) was used to counterstain the nuclei. The cells were imaged with standard fluorescence microscopy (Olympus, Tokyo, Japan). ImageJ software (NIH, Bethesda, MD) was used to merge stained images.
To package the CTNNB1 lentivirus, the sequence containing CTNNB1 cDNA (NCBI reference sequence: NM_43540-1) was cloned into the Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin plasmid (Genechem, Shanghai, China). The titers of lentivirus particles were 5 × 108 U/mL. The hippocampal neurons were transfected with the lentivirus on the sixth day in vitro and used for drug treatment at 48 h post-viral transfection.
Subsequently, the neurons were treated with different concentrations (25, 50, 100 and 200 μM) of CPF for 6, 12, 24, 48 and 72 h, and the results were compared to those of the control cells treated with 0.1% DMSO[Citation31]. Immediately after the drug treatment, an inverted microscope was used to observe the cell morphology at 11 d in vitro. Moreover, a fluorescent inverted microscope was used to observe the dendritic spines at 14 d in vitro.
Cell viability assay
A CCK-8 assay was used to evaluate neuron viability immediately after the drug treatment (at 11 d in vitro). For this, 10 μL of CCK-8 reagent (Dojindo, Kumamoto, Japan) was added to the cells after incubation with CPF at different concentrations (0, 25, 50, 100 and 200 μM) for 6, 12, 24, 48 and 72 h. The absorbance at 450 nm was measured using an automated 96-well plate reader after the cells had been incubated for another 2 h. Then, we used the same method to measure the absorbance of the cells treated with CPF at a concentration of 50 μM for 0, 6, 12, 24, 48 and 72 h.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
RNA from the primary neurons on the eleventh day in vitro was extracted using the High Pure RNA Isolation Kit (Omega Bio-Tek, Inc., Norcross, GA) to synthesize cDNA with the RevertAidTM First Strand cDNA Synthesis Kit (Thermo Fisher Scientific Inc., Waltham, MA). Next, 2 μg extracted RNA in a 25 μL reaction system was utilized for the reverse transcription (RT) reaction with the following three-step incubation: 25 °C for 5 min, 42 °C for 60 min, and 75 °C for 15 min. The relative expression level of mRNA was then assessed using the SYBR Green Real-time PCR Master Mix Kit (Toyobo, Osaka, Japan). Samples were incubated at 95 °C for 60 s, followed by 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and cDNA extension at 72 °C for 30 s. The reaction was implemented using the LightCycler R 480II as an analyzer (Roche, Mannheim, Germany); GAPDH was used as a loading control. The genes were amplified using the following primers: TGGTAGTGCCTGTGATCGTGT (forward) and GGGGAGGGGGTCTTCAAACAA (reverse) for Syp, GTCACCCCTGCCCCATCATAA (forward) and GGTGTGTGAAAGACAGGGGAC (reverse) for PSD95, CCATCCTTCCTCCAGCCACTA (forward) and GAAATGTCGTGGGAGGGTGGT (reverse) for GluN1, and GGACAACTCAAGCGTCCAGAA (forward) and ACAGTAGCCCTCATAGCGGTC (reverse) for GluA1.
Western blot analysis
Proteins were isolated from the hippocampal neurons and were resolved with 10% SDS-PAGE gels on the eleventh day in vitro. After migration, they were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA) and blocked in tris-buffered saline containing 0.2% tween-20 and 5% skim milk powder for 1 h at room temperature. Then, the membrane was incubated overnight at 4 °C with the primary antibodies at specific dilutions as follows: anti-GluN1 (1:1,000, Abcam), anti-GluA1 (1 μg/mL, Abcam), anti-Syp (1:20,000, Abcam), anti-PSD-95-specific (1:1,000, Proteintech), anti-active-β-catenin (anti-ABC; 1:1,000, Millipore), and anti-Wnt7a (1:500, Proteintech). β-Tubulin (1:1,000, Cell Signaling Technology, Danvers, MA) was used as the gel loading control. After incubation with primary antibodies overnight at 4 °C, the blots were then incubated with a horseradish peroxidase-conjugated secondary antibody (1:3,000, Proteintech) for 1 h at room temperature. An enhanced chemiluminescence reagent was used to detect the proteins. Quantity One software was used for the analysis of band intensity.
Statistical analysis
Data were analyzed using the program Prism (GraphPad, Inc., San Diego, CA). Significant differences were identified using a two-way analysis of variance followed by an unpaired Student’s t-test. The results are expressed as the mean ± SEM from three independent experiments. The level of statistical significance was set to P< 0.05.
Results
Synaptic morphological changes in rat hippocampal neurons after CPF treatment
Through HE staining, we could clearly distinguish the zoomed hippocampal region of the rat and we could observe that the neuronal synapses in each region in the CPF-treated group were shortened (). Moreover, the expression level of PSD-95 in the CA1, CA3 and DG regions of the rat hippocampus decreased according to the immunofluorescence results ().
Synaptic morphological changes in primary hippocampal neurons after CPF treatment
The primary hippocampal neurons were mature at 6 d in vitro and were identified by positive MAP2 expression using immunofluorescence techniques (). CCK-8 assays revealed a concentration-dependent decrease in cell viability. When the concentration of CPF was 25 or 50 µM, regardless of the intervention time (6, 12, 24, 48 or 72 h), the cell viability of neurons did not obviously change compared with that in the control group (P> 0.05; ). However, when the concentration of CPF reached 100 μM or more, the cell viability began to decrease significantly (P< 0.05; ). Moreover, no significant time-dependent change in cell viability was observed after the CPF treatment (50 μM; P> 0.05; ).
Figure 2. CPF exposure induces structural changes of synapses in primary hippocampal neurons. a) Positive expression of MAP2 was used to identify primary hippocampal neurons (green: MAP2; Blue: DAPI, Scale bar: 25 μm). b) Representative bright-field images of primary hippocampal neurons treated with CPF (50 μM) for different durations (6, 12, 24, 48 and 72 h). Scale bar: 50 μm. Boxed areas on the top right corner represent the magnification indicated by an arrow in the respective image. c) CCK-8 quantification of the survival of primary hippocampal neurons treated with different concentrations of CPF (25, 50, 100 and 200 μM) in each treatment duration group (6, 12, 24, 48 and 72 h). d) Under CPF treatment (50 μM) for different durations (6, 12, 24, 48 and 72 h), cell viability was determined using CCK-8 assay (*P < 0.05; **P < 0.01). e) Representative bright-field images of dendritic spines as treated with CPF (50 μM) in 24 h (Scale bar: 10 μm). f) Bars represent the density of dendritic spines/10 μm without treatment and after CPF treatment. Results are expressed as density of dendritic spines (mean ± s.e.m). Each segment of 10 μm was considered one sample. The samples were obtained from different culture preparations, and at least six neurons were analyzed in each experimental group. Statistical analysis was carried out using one-way ANOVA (*P < 0.05).
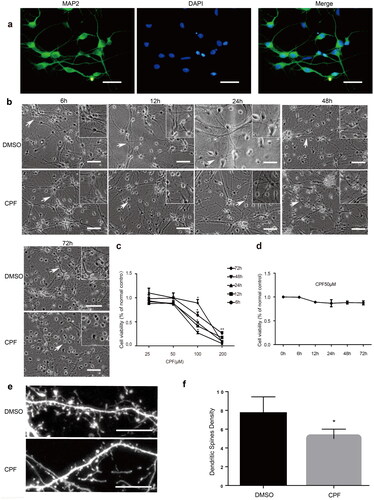
Primary hippocampal neurons were treated with 50 μM CPF for different times. Microscopic observations revealed no obvious change in the number of neurons. The cell body was still large and full, with a halo around it, and the network between synapses could be seen. However, compared with that in the control group, the cell body was slightly shrunken, the synapse was shortened, and the intersynaptic network was relatively sparse. Moreover, the change was more significant as the exposure time increased (). Further, through microscopic observations, we could also see that the number of dendritic spines in the primary hippocampal neurons at 14 d in vitro decreased after CPF treatment (.
CPF Exposure affects the expression level of presynaptic and postsynaptic vesicles in primary hippocampal neurons
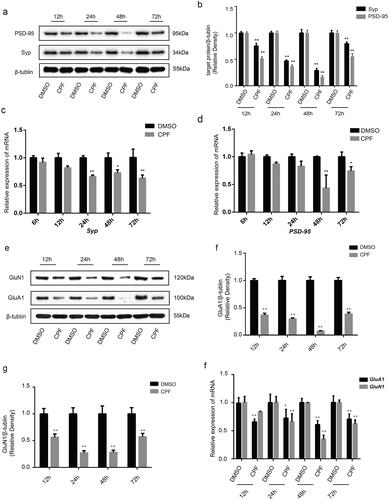
Protein levels of Syp and PSD-95 were lower in cells exposed to CPF for 12–72 h than in the corresponding controls (). The expression levels of the proteins gradually decreased between 12 and 48 h of CPF treatment, reaching their lowest point at 48 h, which was followed by an increase at 72 h. Further, the mRNA levels of Syp and PSD-95 were lower in cells exposed to CPF than in the corresponding controls ().
Figure 3. CPF affects the expression of synaptical vesicles and iGluRs in primary hippocampal neurons. a) Western blot analysis demonstrates levels of Syp and PSD-95 in Primary hippocampal neurons of each group. β-tubulin was used as an internal gel loading control. b) Densitometry of the Syp and PSD-95 bands are correlated with the β-tubulin band (n = 3/group; **P < 0.01). c) Quantitative PCR analysis of Syp mRNA levels in primary hippocampal neurons of each group (n = 3/group; *P < 0.05; **P < 0.01). d) Quantitative PCR analysis of PSD-95 mRNA levels in primary hippocampal neurons of each group (n = 3/group; *P < 0.05; **P < 0.01). e) Western blot analysis demonstrates levels of GluA1 and GluN1 in Primary hippocampal neurons of each group. β-tubulin was used as an internal gel loading control. f and g) Densitometry of the GluA1 and GluN1 bands are correlated with the β-tubulin band, respectively (n = 3/group; **P < 0.01). H: Quantitative PCR analysis of GluA1 and GluN1 mRNA levels in primary hippocampal neurons of each group (n = 3/group; *P < 0.05; **P < 0.01).
CPF exposure affects the expression level of iGluRs in primary hippocampal neurons
We also analyzed the expression levels of GluN1 and GluA1 in the primary hippocampal neurons. We found that the protein levels of GluN1 and GluA1 was lower in cells exposed to CPF for 12–72 than in corresponding controls (). The expression levels of the proteins gradually decreased between 12 and 48 h of CPF treatment, reaching their lowest point at 48 h, which was followed by an increase at 72 h. Similarly, GluN1 and GluA1 mRNA levels in the cells decreased significantly after the CPF treatment ().
CPF induces downregulation of wnt/β-catenin signaling in hippocampal neurons
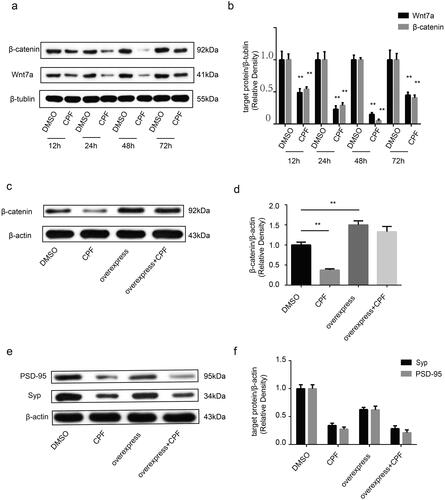
This study showed that Wnt7a and active-β-catenin expression decreased upon treatment with CPF (50 μM) within the range of 12–72 h, as compared to levels in corresponding controls (). Similar to these results, the Wnt7a and active-β-catenin expression levels gradually decreased between 12 and 48 h of CPF treatment, reaching their lowest point at 48 h, which was followed by an increase at 72 h. To verify that the Wnt/β-catenin signaling is involved in the effect of CPF on synaptic plasticity, we overexpressed β-catenin (). However, β-catenin overexpression did not compensate for the CPF-induced reduction in the expression levels of the pre- and postsynaptic proteins studied ().
Figure 4. CPF affects the expression of Wnt7a and active-β-catenin in primary hippocampal neurons, but an overexpression of β-catenin did not improve the Syp and PSD-95 protein levels caused by CPF. a) Western blot analysis demonstrates levels of Wnt7a and active-β-catenin in Primary hippocampal neurons of each group. β-tubulin was used as an internal gel loading control. b) Densitometry of the Wnt7a and active-β-catenin bands are correlated with the β-tubulin band (n = 3/group; **P < 0.01). c) β-catenin protein expression was determined by western blot in primary hippocampal neurons of each group (CPF: 50 μM, 48 h; overexpress: overexpress β-catenin). β-actin was used as an internal gel loading control. d) Densitometry of the β-catenin bands are correlated with the β-actin band (n = 3/group; **P < 0.01). e) Syp and PSD-95 protein expression was determined by western blot in primary hippocampal neurons of each group. β-actin was used as an internal gel loading control. f) Densitometry of the Syp and PSD-95 bands are correlated with the β-actin band (n = 3/group; *P < 0.05).
Discussion
Despite increasing concerns related to developmental neurotoxicity associated with exposure to CPF, this agent remains one of the most widely used organophosphate pesticides. In this study, we analyzed the effect of CPF on the morphological and molecular indices of synaptic plasticity in hippocampal neurons. We also tried to determine whether the changes in synaptic plasticity caused by CPF are mediated by the Wnt signaling pathway. We demonstrated that the synapses were shortened both in vivo and in vitro in hippocampal neurons after exposure to CPF. The cell numbers and viability of primary hippocampal neurons were unchanged upon treatment with ≤50 μM CPF. Therefore, this concentration (50 μM) was used in all subsequent experiments in vitro to evaluate the risks associated with this exposure level.
We also found that the number of dendritic spines decreased upon treatment with 50 μM CPF.
Dendritic spines are small, thin, specialized protrusions originating from neuronal dendrites primarily localized in the excitatory synapses[Citation32]. Alterations in the number and morphology of dendritic spines are frequently associated with synaptic development, maintenance and plasticity. At the morphological level, the shrinkage or loss of spines is associated with dysregulation on synaptic plasticity[Citation33].
Moreover, we found that CPF decreased the expression of Syp, PSD-95, GluN1 and GluA1 in primary hippocampal neurons. Syp and PSD-95 are the most widely used experimental protein markers of synaptic plasticity in the brain[Citation34–36]. Moreover, both Syp and PSD-95 are associated with neurodevelopmental diseases. Reduced Syp expression was found in a mouse model of attention deficit hyperactivity disorder (ADHD)[Citation37]. Recently, genomic studies have linked PSD-95 dysfunction to neurodevelopmental disorders such as autism spectrum disorder (ASD) and intellectual disorder[Citation38]. N-methyl-D-aspartate receptors (NMDARs) and AMPA-type glutamate receptors (AMPARs) are the primary iGluRs in the hippocampus. NMDARs are tetrameric protein complexes, typically comprising two subunits GluN1 and GluN2[Citation39]. In the central nervous system, GluN1 is ubiquitous. Based on the four AMPAR subunits, GluA1–4, heterotetramers are formed via the association of two dimers. GluA1/GluA2 is one of the most frequently observed dimers in the mammalian brain[Citation40]. NMDARs and AMPARs play an important role in synaptic plasticity[Citation41] and neuropathologies[Citation42] relevant to learning and memory. Moreover, NMDAR dysfunction has been linked to developmental disorders including ADHD [Citation43,Citation44] and ASD [Citation45]. Overall, this study suggested that CPF exposure affects morphological and molecular indices of synaptic plasticity in hippocampal neurons. These results might explain the mechanism through which CPF is associated with these neurological disorders.
The expression of Wnt/β-catenin signaling components was decreased in primary hippocampal neurons. Wnt signaling was recently shown to be involved in the remodeling of pre- and postsynaptic regions, thus regulating synaptic function and plasticity, which are critical for memory and learning[Citation46]. The Wnt/β-catenin pathway is referred to as the classical Wnt signaling pathway and can control hippocampal plasticity, synaptic transmission and neurogenesis[Citation47]. Wnt-7a stimulates dendritic spine morphogenesis and also attributes to synapse formation and function of hippocampal neurons[Citation48,Citation49]. β-Catenin proteins are present at high levels during synaptogenesis and localize both pre- and post-synaptically[Citation24]. Wnt signaling pathways are implicated in a variety of human pathologies, including developmental defects[Citation25,Citation26]. Our study suggests that CPF exposure decreases the expression of Wnt7a and β-catenin, consistent with our previous conclusion that CPF exposure might affect synaptic plasticity. However, when we overexpressed β-catenin, Syp and PSD-95 expression was not increased. This indicates that the decline in GluN1, GluA1, PSD-95 and Syp expression caused by CPF is related to Wnt/β-catenin signaling in an indirect manner. Many factors could thus be involved in regulating GluN1, GluA1, PSD-95 and Syp expression, and the molecular mechanisms involved require further investigation.
Conclusion
In summary, we found that the exposure of rat hippocampal neurons to CPF affects the morphological structure of synapses, downregulates the expression of presynaptic and postsynaptic proteins and iGluRs, and affects the Wnt7a/β-catenin pathway. This study indicated that the exposure to CPF induces dysregulation of synaptic plasticity in rat hippocampal neurons, which provides novel information regarding the mechanism of CPF-induced neurodevelopmental disorders. Further studies are needed to examine the potential mechanisms underlying the effects of CPF on synaptic plasticity, which might offer us new directions of treatment development for CPF-induced neurodevelopmental disorders.
Ethics approval
The experiments were approved by the Medical Ethics Committee of the Third Xiangya Hospital.
Consent for publication
All the authors agreed to submit the manuscript.
Author contributions
WZ and LZ designed this research. WZ, CZ, PW, GW performed the experiments. WZ, YD, HD, and JT analyzed the data. WZ, and LZ wrote the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Acknowledgements
The authors are grateful to the staff from the Medical Center Laboratory of the Third Xiangya Hospital for their excellent technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data will available from corresponding author, with reasonable request.
Additional information
Funding
References
- Elizabeth, M. J.; Jisha, M. S. Chlorpyrifos: Pollution and Remediation. Environ. Chem. Lett. 2015, 13, 269–291. DOI: 10.1007/s10311-015-0513-7.
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the Microbial Degradation and Catalytic Mechanisms of Chlorpyrifos. Environ. Res. 2021, 194, 110660. DOI: 10.1016/j.envres.2020.110660.
- Baez, M. E.; Espinoza, J.; Fuentes, E. Degradation Kinetics of Chlorpyrifos and Diazinon in Volcanic and Non-Volcanic Soils: Influence of Cyclodextrins. Environ. Sci. Pollut. Res. Int. 2018, 25, 25020–25035. DOI: 10.1007/s11356-018-2559-0.
- Amin, M.; Raza Gurmani, A.; Rafique, M.; Ullah Khan, S.; Mehmood, A.; Muhammad, D.; Hussain Syed, J. Investigating the Degradation Behavior of Cypermethrin (CYP) and Chlorpyrifos (CPP) in Peach Orchard Soils Using Organic/Inorganic Amendments. Saudi J. Biol. Sci. 2021, 28, 5890–5896. DOI: 10.1016/j.sjbs.2021.06.035.
- Farhan, M.; Ahmad, M.; Kanwal, A.; Butt, Z. A.; Khan, Q. F.; Raza, S. A.; Qayyum, H.; Wahid, A. Biodegradation of Chlorpyrifos Using Isolates from Contaminated Agricultural Soil, Its Kinetic Studies. Sci. Rep. 2021, 11, 10320. DOI: 10.1038/s41598-021-88264-x.
- Hadibarata, T.; Kristanti, R. A.; Bilal, M.; Yilmaz, M.; Sathishkumar, P. Biodegradation mechanism of Chlorpyrifos by Halophilic Bacterium Hortaea sp. B15. Chemosphere 2023, 312, 137260. DOI: 10.1016/j.chemosphere.2022.137260.
- Nandhini, A. R.; Harshiny, M.; Gummadi, S. N. Chlorpyrifos in Environment and Food: A Critical Review of Detection Methods and Degradation Pathways. Environ. Sci. Process Impact. 2021, 23, 1255–1277. DOI: 10.1039/d1em00178g.
- Dar, M. A.; Kaushik, G.; Villarreal-Chiu, J. F. Pollution status and Bioremediation of Chlorpyrifos in Environmental Matrices by the Application of Bacterial Communities: A Review. J. Environ. Manage 2019, 239, 124–136. DOI: 10.1016/j.jenvman.2019.03.048.
- Masiá, A.; Vásquez, K.; Campo, J.; Picó, Y. Assessment of Two Extraction Methods to Determine Pesticides in Soils, Sediments and Sludges. Application to the Turia River Basin. J. Chromatogr. A 2015, 1378, 19–31. DOI: 10.1016/j.chroma.2014.11.079.
- Bhandari, G.; Atreya, K.; Scheepers, P. T. J.; Geissen, V. Concentration and Distribution of Pesticide Residues in Soil: Non-Dietary Human Health Risk Assessment. Chemosphere 2020, 253, 126594. DOI: 10.1016/j.chemosphere.2020.126594.
- Sumon, K. A.; Rashid, H.; Peeters, E. T. H. M.; Bosma, R. H.; Van den Brink, P. J. Environmental Monitoring and Risk Assessment of Organophosphate Pesticides in Aquatic Ecosystems of North-West Bangladesh. Chemosphere 2018, 206, 92–100. DOI: 10.1016/j.chemosphere.2018.04.167.
- Estellano, V. H.; Pozo, K.; Efstathiou, C.; Pozo, K.; Corsolini, S.; Focardi, S. Assessing Levels and Seasonal Variations of Current-Use Pesticides (CUPs) in the Tuscan Atmosphere, Italy, Using Polyurethane Foam Disks (PUF) Passive Air Samplers. Environ. Pollut. 2015, 205, 52–59. DOI: 10.1016/j.envpol.2015.05.002.
- Arreguin-Rebolledo, U.; Páez-Osuna, F.; Betancourt-Lozano, M.; Rico-Martínez, R. Multi-and Transgenerational Synergistic Effects of Glyphosate and Chlorpyrifos at Environmentally Relevant Concentrations in the Estuarine Rotifer Proales Similis. Environ. Pollut. 2023, 318, 120708. DOI: 10.1016/j.envpol.2022.120708.
- Minassa, V. S.; Aitken, A. V.; Hott, S. C.; de Sousa, G. J.; Batista, T. J.; Gonçalves, R. d C. R.; Coitinho, J. B.; Paton, J. F. R.; Beijamini, V.; Bissoli, N. S.; et al. Intermittent Exposure to Chlorpyrifos Results in Cardiac Hypertrophy and Oxidative Stress in Rats. Toxicology 2022, 482, 153357. DOI: 10.1016/j.tox.2022.153357.
- Ju, H.; Yang, X.; Osman, R.; Geissen, V. Effects of Microplastics and Chlorpyrifos on Earthworms (Lumbricus Terrestris) and Their Biogenic Transport in Sandy Soil. Environ. Pollut. 2023, 316, 120483. DOI: 10.1016/j.envpol.2022.120483.
- Zhang, J.; Zhao, L. L.; Hu, Z. P.; Zhou, J.; Deng, L.; Gu, F.;Dai, H. M.; Huang, M. Effects of Low-Dose Chlorpyrifos Exposure on Dopaminergic Neurons in the Midbrain Substantia Nigra and Neural Behavioral Development in Neonatal Rats. Zhongguo Dang Dai Er Ke Za Zhi 2011, 13, 989–994. URL: http://www.zgddek.com/EN/Y2011/V13/I12/989.
- Wang, P.; Dai, H.; Zhang, C.; Tian, J.; Deng, Y.; Zhao, M.; Zhao, M.; Bing, G.; Zhao, L. Evaluation of the Effects of Chlorpyrifos Combined with Lipopolysaccharide Stress on Neuroinflammation and Spatial Memory in Neonatal Rats. Toxicology 2018, 410, 106–115. DOI: 10.1016/j.tox.2018.09.008.
- Fortenberry, G. Z.; Meeker, J. D.; Sánchez, B. N.; Barr, D. B.; Panuwet, P.; Bellinger, D.; Schnaas, L.; Solano-González, M.; Ettinger, A. S.; Hernandez-Avila, M.; et al. Urinary 3,5,6-Trichloro-2-Pyridinol (TCPY) in Pregnant Women from Mexico City: Distribution, Temporal Variability, and Relationship with Child Attention and Hyperactivity. Int J Hyg Environ Health 2014, 217, 405–412. DOI: 10.1016/j.ijheh.2013.07.018.
- Rauh, V.; Arunajadai, S.; Horton, M.; Perera, F.; Hoepner, L.; Barr, D. B.; Whyatt, R. Seven-Year Neurodevelopmental Scores and Prenatal Exposure to Chlorpyrifos, a Common Agricultural Pesticide. Environ. Health Perspect. 2011, 119, 1196–1201. DOI: 10.1289/ehp.1003160.
- Sudhof, T. C. Towards an Understanding of Synapse Formation. Neuron 2018, 100, 276–293. DOI: 10.1016/j.neuron.2018.09.040.
- Stampanoni, B. M.; Iezzi, E.; Gilio, L.; Centonze, D.; Buttari, F. Synaptic Plasticity Shapes Brain Connectivity: Implications for Network Topology. Int. J. Mol. Sci. 2019, 20, 6193. DOI: 10.3390/ijms20246193.
- Magee, J. C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. DOI: 10.1146/annurev-neuro-090919-022842.
- Ismail, F. Y.; Fatemi, A.; Johnston, M. V. Cerebral Plasticity: Windows of Opportunity in the Developing Brain. Eur. J. Paediatr. Neurol. 2017, 21, 23–48. DOI: 10.1016/j.ejpn.2016.07.007.
- Zwamborn, R. A. J.; Snijders, C.; An, N.; Thomson, A.; Rutten, B. P. F.; de Nijs, L. Wnt Signaling in the Hippocampus in Relation to Neurogenesis, Neuroplasticity, Stress and Epigenetics. Prog. Mol. Biol. Transl. Sci. 2018, 158, 129–157. DOI: 10.1016/bs.pmbts.2018.04.005.
- Kumar, M.; Camlin, N. J.; Holt, J. E.; Teixeira, J. M.; McLaughlin, E. A.; Tanwar, P. S. Germ cell Specific Overactivation of WNT/Betacatenin Signalling Has No Effect on Folliculogenesis but Causes Fertility Defects Due to Abnormal Foetal Development. Sci. Rep. 2016, 6, 27273. DOI: 10.1038/srep27273.
- Inestrosa, N. C.; Varela-Nallar, L. Wnt signalling in Neuronal Differentiation and Development. Cell Tissue Res. 2015, 359, 215–223. DOI: 10.1007/s00441-014-1996-4.
- Tian, J.; Dai, H.; Deng, Y.; Zhang, J.; Li, Y.; Zhou, J.; Zhao, M.; Zhao, M.; Zhang, C.; Zhang, Y.; et al. The effect of HMGB1 on Sub-Toxic Chlorpyrifos Exposure-Induced Neuroinflammation in Amygdala of Neonatal Rats. Toxicology 2015, 338, 95–103. DOI: 10.1016/j.tox.2015.10.010.
- Zhang, J.; Dai, H.; Deng, Y.; Tian, J.; Zhang, C.; Hu, Z.; Bing, G.; Zhao, L. Neonatal chlorpyrifos Exposure Induces Loss of Dopaminergic Neurons in Young Adult Rats. Toxicology 2015, 336, 17–25. DOI: 10.1016/j.tox.2015.07.014.
- Cardiff, R. D.; Miller, C. H.; Munn, R. J. Manual Hematoxylin and Eosin Staining of Mouse Tissue Sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. DOI: 10.1101/pdb.prot073411.
- Feldman, A. T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. Methods Mol. Biol. 2014, 1180, 31–43. DOI: 10.1007/978-1-4939-1050-2_3.
- Zhang, C.; Deng, Y.; Dai, H.; Zhou, W.; Tian, J.; Bing, G.; Zhao, L. Effects of Dimethyl Sulfoxide on the Morphology and Viability of Primary Cultured Neurons and Astrocytes. Brain Res. Bull. 2017, 128, 34–39. DOI: 10.1016/j.brainresbull.2016.11.004.
- Chidambaram, S. B.; Rathipriya, A. G.; Bolla, S. R.; Bhat, A.; Ray, B.; Mahalakshmi, A. M.; Manivasagam, T.; Thenmozhi, A. J.; Essa, M. M.; Guillemin, G. J.; et al. Dendritic Spines: Revisiting the Physiological Role. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 92, 161–193. DOI: 10.1016/j.pnpbp.2019.01.005.
- Amtul, Z; Atta-Ur-Rahman . Neural Plasticity and Memory: Molecular Mechanism. Rev. Neurosci. 2015, 26, 253–268. DOI: 10.1515/revneuro-2014-0075.
- Han, K.; Kim, E. Synaptic Adhesion Molecules and PSD-95. Prog. Neurobiol. 2008, 84, 263–283. DOI: 10.1016/j.pneurobio.2007.10.011.
- Matt, L.; Kim, K.; Chowdhury, D.; Hell, J. W. Role of Palmitoylation of Postsynaptic Proteins in Promoting Synaptic Plasticity. Front. Mol. Neurosci. 2019, 12, 8. DOI: 10.3389/fnmol.2019.00008.
- Liu, Y.; Zhang, Y.; Zheng, X.; Fang, T.; Yang, X.; Luo, X.; Guo, A.; Newell, K. A.; Huang, X.-F.; Yu, Y. Galantamine improves Cognition, Hippocampal Inflammation, and Synaptic Plasticity Impairments Induced by Lipopolysaccharide in Mice. J. Neuroinflammation. 2018, 15, 112. DOI: 10.1186/s12974-018-1141-5.
- Sorokina, A. M.; Saul, M.; Goncalves, T. M.; Gogola, J. V.; Majdak, P.; Rodriguez-Zas, S. L.; Rhodes, J. S. Striatal transcriptome of a Mouse Model of ADHD Reveals a Pattern of Synaptic Remodeling. PLoS One 2018, 13, e201553. DOI: 10.1371/journal.pone.0201553.
- Coley, A. A.; Gao, W. J. PSD95: A Synaptic Protein Implicated in Schizophrenia or Autism? Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018, 82, 187–194. DOI: 10.1016/j.pnpbp.2017.11.016.
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA Receptor Subunit Diversity: Impact on Receptor Properties, Synaptic Plasticity and Disease. Nat. Rev. Neurosci. 2013, 14, 383–400. DOI: 10.1038/nrn3504.
- Miladinovic, T.; Nashed, M. G.; Singh, G. Overview of Glutamatergic Dysregulation in Central Pathologies. Biomolecules 2015, 5, 3112–3141. DOI: 10.3390/biom5043112.
- Huganir, R. L.; Nicoll, R. A. AMPARs and Synaptic Plasticity: The Last 25 Years. Neuron 2013, 80, 704–717. DOI: 10.1016/j.neuron.2013.10.025.
- Lau, C. G.; Zukin, R. S. NMDA receptor Trafficking in Synaptic Plasticity and Neuropsychiatric Disorders. Nat Rev Neurosci 2007, 8, 413–426. DOI: 10.1038/nrn2153.
- Dorval, K. M.; Wigg, K. G.; Crosbie, J.; Tannock, R.; Kennedy, J. L.; Ickowicz, A.; Pathare, T.; Malone, M.; Schachar, R.; Barr, C. L. Association of the Glutamate Receptor Subunit Gene GRIN2B with Attention-Deficit/Hyperactivity Disorder. Genes Brain Behav. 2007, 6, 444–452. DOI: 10.1111/j.1601-183X.2006.00273.x.
- Jensen, P. S. Review: Methylphenidate and Psychosocial Treatments Either Alone or in Combination Reduce ADHD Symptoms. Evid Based Ment. Health 2009, 12, 18. DOI: 10.1136/ebmh.12.1.18.
- Duffney, L. J.; Zhong, P.; Wei, J.; Matas, E.; Cheng, J.; Qin, L.; Ma, K.; Dietz, D. M.; Kajiwara, Y.; Buxbaum, J. D.; Yan, Z. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015, 11, 1400–1413. DOI: 10.1016/j.celrep.2015.04.064.
- Oliva, C. A.; Vargas, J. Y.; Inestrosa, N. C. Wnts in Adult Brain: From Synaptic Plasticity to Cognitive Deficiencies. Front. Cell Neurosci. 2013, 7, 224. DOI: 10.3389/fncel.2013.00224.
- Narvaes, R. F.; Furini, C. Role of Wnt Signaling in Synaptic Plasticity and Memory. Neurobiol. Learn Mem. 2022, 187, 107558. DOI: 10.1016/j.nlm.2021.107558.
- Ramos-Fernández, E.; Tapia-Rojas, C.; Ramírez, V. T.; Inestrosa, N. C. Wnt-7a Stimulates Dendritic Spine Morphogenesis and PSD-95 Expression through Canonical Signaling. Mol. Neurobiol. 2019, 56, 1870–1882. DOI: 10.1007/s12035-018-1162-1.
- Lan, L.; Wang, W.; Huang, Y.; Bu, X.; Zhao, C. Roles of Wnt7a in Embryo Development, Tissue Homeostasis, and Human Diseases. J. Cell Biochem. 2019, 120, 18588–18598. DOI: 10.1002/jcb.29217.