Abstract
Gut microbiota is known as unique collection of microorganisms (including bacteria, archaea, eukaryotes and viruses) that exist in a complex environment of the gut. Recently, this has become one of the most popular areas of research in medicine because this plays not only an important role in disease development, but gut microbiota also influences drug pharmacokinetics. These alterations in drug pharmacokinetic pathways and drug concentration in plasma and blood often lead to an increase in the incidence of toxicological events in patients. This review aims to present current knowledge of the most commonly used drugs in clinical practice and their dynamic interplay with the host’s gut microbiota as well as the mechanisms underlying these metabolic processes and the consequent effect on their therapeutic efficacy and safety. These new findings set a foundation for the development of personalized treatments specific to each metabolism, maximizing drugs’ therapeutic effects and minimizing the side effects because they are one of the major limiting factors in treating patients.
1. Introduction
The human gut was not considered an important organ in the human body in the past, except for its well-known roles in digestion and absorption of food and drugs. Recently, this 'forgotten' organ has become an uprising star of many studies (Wilson and Nicholson Citation2017).
The intestine of human being is home to a large population of microorganisms and it has almost 100 trillion inhabitants. It largely outnumbers all other communities of microbes connected with the body’s surface (Schupack et al. Citation2022). Therefore, the gut microbiota can be portrayed as a unique and special organ that has many different functions (Yang et al. Citation2022). Namely, the human organism is in harmony with a countless number of symbiotic bacteria that are a constitutive part of the gut microbiota (Adak and Khan Citation2019). These microorganisms create an environment rich in nutrients needed for their own survival. In return, they make extremely important contributions to vitally important processes for human health, such as the proper function of the immune system and the metabolism of nutrients, drugs and different neurotransmitters (Rinninella et al. Citation2019).
For a longer period of time, the main problem for scientists was the lack of adequate techniques for the identification of gut composition. One of the main methods being used was the culturing of samples but it had many limitations such as a small number of bacterial species that were isolated and observed (Li and Hu Citation2019). In recent years, the standard technology used in the studies was 16S rRNA sequencing. It is usually extracted from feces and further investigated with polymerase chain reaction (PCR). Nowadays, metagenomic sequencing (MGS) has become the new standard for microbiota evaluation (Li and Hu Citation2019). Many studies have shown that the pharmacokinetic process and the bioavailability of different drugs are often in a complex and dynamic dialogue with gut microbiota. This has an important effect on the efficacy of these drugs, as well as on the occurrence of different unwanted toxicological events (Zhang et al. Citation2021). Therefore, gut microbiota is recognized as one of the crucial environmental factors influencing the metabolism of drugs and xenobiotics as well as the inter-individual variations of drug effects (Koppel et al. Citation2017; Wilson and Nicholson Citation2017; Adak and Khan Citation2019; Adak and Khan Citation2019).
The four fundamental processes of pharmacokinetics that are under the possible influence of microbiota are absorption, distribution, metabolism and excretion (ADME). These are separate yet, in many aspects, interrelated processes that occur between the administration and elimination of drugs from the body (Ioannidis Citation2019). One of the most common ways of drug administration is oral administration. It is usually preferred by patients because it is more convenient, comfortable and easy to comply with medication counseling. Ideally, after oral administration, the drug would be completely and quickly absorbed from the gut and it would affect only therapeuticly targeted cells. However, in the real world this scenario is not commonly noted. After oral ingestion, drugs start their journey through the digestive tract and are initially faced with trillions of bacteria that actively produce and secrete important metabolic enzymes such as oxidase, reductase and transferase. These enzymes have the ability to alter the pharmaceutical compounds of drugs, resulting in a change of their pharmacokinetic properties and half-life. Therefore, the intestinal microbiota can transform these drugs, even before the first-pass effect through the liver (Lee et al. Citation2019). However, there are still many unknowns effects of the gut microbiota on orally administered drugs (Xie et al. Citation2020).
The rediscovery of the impact that the gut has on disease development such as cancer, neurodegenerative diseases and inflammatory diseases of the bowel as well as its ability to alter drug pharmacokinetics has led to a renewed interest in this research area (Wilson and Nicholson Citation2017). The bioavailability of some drugs (such as acetaminophen, amlodipine, tamoxifen, metronidazole etc.) as well as their efficacy and toxicity are affected by bacterial enzymes in the gut (Xie et al. Citation2020). Additionally, this relationship is bidirectional because drugs also can alter the composition of bacterial genera in the gut (Winston and Theriot Citation2020). Efforts to discover and research the impact of gut microbiota on human health have increased dramatically and the important effects of this organ are the focus of many different studies. Therefore, this study aims to portray the changes noted in the pharmacokinetics of commonly used drugs that occur by the action of different enzymes produced by the gut microbiota. The current knowledge and new findings in this area are discussed further in the review as well as the possible implementations of these findings in everyday clinical practice.
2. Dialogue between gut microbiota and its environment
The gut microbiota is a quite dynamic surrounding that is susceptible to important inter- and intra-individual variations. The composition of microbiota community in intestines is tightly connected to many different environmental factors, nutrition, intake of drugs, organism’s age and genetics of the human hosts. However, different environmental stressors such as heat, noise, cold, intensive physical activity and disruption of the circadian cycle can alter the protective function of gut-blood barrier and healthy bacterial genera in the gut. For example, after exposure to these stress factors, the reduction of beneficial bacteria such as Bacteroidetes was noted. On the other hand, pro-inflammatory bacterial genera such as Firmicutes showed an increased abundance. Consequently, different metabolic disorders occur such as insulin intolerance and hyperlipidemia.
Additionally, it was shown that the disruption of circadian rhythm and chronic psychological stress could alter the composition of bacterial genera in the gut such as the reduction of Lactobacillus that is well-known for their beneficial effects (Hills et al. Citation2019). The combination of all of these factors leads to increased inflammation and gut dysbiosis as shown in . Some recent studies (Karl et al. Citation2018; Hills et al. Citation2019; Gacesa et al. Citation2022) noted that there is a strong link between the weight and calculated body mass index of patients, their cholesterol levels, nutrition, insulin tolerance and the microbiota. A recently conducted study (Gacesa et al. Citation2022) on Dutch population consisting of three generations of Dutch families, aimed to show a profile of gut microbiota in the population. The study found important correlations between the environment and gut composition. It showed that patient surrounding is the main factor shaping the microbiota in humans and that both physical and psychological factors affect its composition (Gacesa et al. Citation2022).
Figure 1. Environmental stressors effecting the gut microbiota composition (Karl et al. Citation2018).
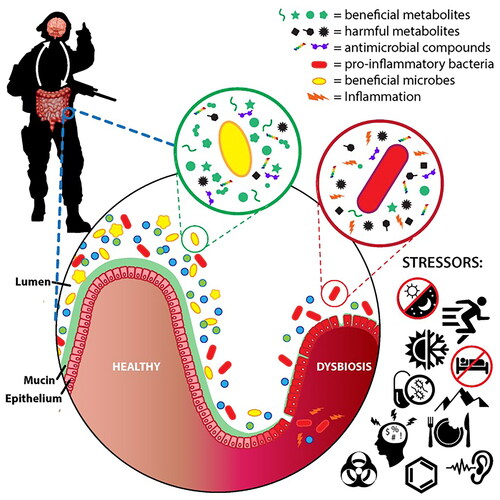
Changes in non-living environment and climate (such as acute plateau hypoxia) can effect the collection and quantity of intestinal flora (Xie et al. Citation2020). Although there are many factors, inside and outside of the host organism (endogenous and exogenous factors) such as age, genetics and climate that have been discovered as the potential changer of the gut community. Furthermore, a patient’s diet is recognized as an extremely important determinant of its structure and function (Nakov and Velikova Citation2020). There are many differences between the intestinal microbiota of patients who are predominantly on Western diet and the microbiota of patients who mostly eat food rich in fiber such as the food represented in the Mediterranean diet (Gomaa Citation2020). Namely, the alterations in the structure of gut microbiota were noted in different subjects in the study conducted by Gomaa (Gomaa Citation2020). Statistical analysis showed that these alterations were closely connected to the differences in the subject’s diet and nutrition habits. Therefore, a particular diet and consumption of specific types of foods such as fibers, promotes the growth and reproduction of specific bacterial strains, consequently bringing a change in fermentation metabolism. It also directly effects intestinal acidity and basicity in the gut (pH levels) that could be responsible for the development of pathogenic flora (Gomaa Citation2020). As it is already well known, most drugs are weak acids and bases and have specific and optimal pH and pKa for their solubility and bioavailability. It means that each change in the host’s intestine pH could effect the solubility and bioavailability of the drugs that could directly effect the ADME process. Many authors discussed options, how to improve the bioavailability of drugs and the pharmaceutical industry is searching for practical solutions last 30 years. However, pH and pKa are one of the easiest solutions (Taniguchi et al. Citation2014).
3. Effects of gut microbiota on drugs undergoing liver metabolism
The complex dialogue occurring between the liver and gut microbiota enzymes, bile acid metabolism and drug metabolism is still being researched. Namely, different studies discovered that bile acids, after they are excreted into the bile undergo a process of being metabolized by gut microbiota. Afterwards, the bile acids are reabsorbed into the blood that is the final step in the process and marked as the enterohepatic cycle of bile acids (Zimmermann et al. Citation2019). There are many metabolizing enzymes involved in different processes that are happening in mucosa of the small intestine but the most valuable ones are cytochromes P450 (CYPs). They are particularly important enzymes, which are responsible for the major part of the first phase reactions that take place while administered drugs are being metabolized (Hu et al. Citation2022). A large number of drug interactions that are mediated through the activation or inhibition of CYP enzymes, especially CYP3A have been initially assumed to be expressed exclusively in liver but the research showed that they are also being expressed at the level of the gut (Zhang et al. Citation2018).
Generally, in healthy organism and physiological conditions, the gut microbiota could modulate the proteome as well as the set of RNA transcripts. Transcriptome and collection of metabolites called metabolome in the liver mainly by down-regulating CYP-mediated xenobiotic metabolism and changing the hepatic expression of genes. After the secretion of bile acids from the liver to the bile, their enterohepatic cycle starts. The bile acids are reabsorbed in the ileum, where they interact with the farnesoid X receptor (FXR). FXR is placed in the gut and could detect an increased concentration of bile acids intracellularly. This onsets the production of different growth factors, such as fibroblast growth factor 19 that enters the portal blood circulation and interacts with fibroblast growth factor 4 (Dempsey and Cui Citation2019). Consequently, downregulation of CYP7A1 and a decrease in the production of new primary bile acids could be noted as shown in . Interestingly, it was shown that the expression of FXR is effected by changes in microbiota composition. The lower levels of Lactobacillus genera lead to a lower production of an enzyme called bile salt hydrolase (BHS). This increased the levels of tauro-β-muricholic acid (MCA) that is the potent antagonist of FXR, consequently leading to the development of insulin resistance, obesity and fatty liver disease. Therefore, the FXR has a protective role in the organism and it is possible to enhance its effects by altering the gut microbiota and bacterial genera that are included in its expression (Dempsey and Cui Citation2019).
Figure 2. The enterohepatic pathway of primary bile acids and its effect on CYP enzymes (Bozward et al. Citation2021).
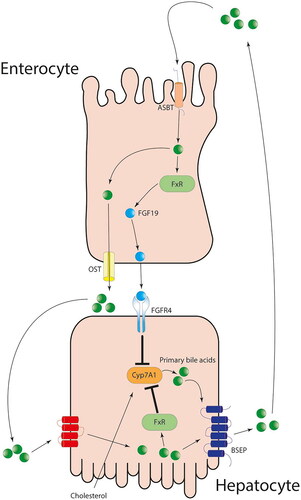
Furthermore, Björkholm et al. (Citation2009) showed that the microbiota has an important effect on the expression of different genes in mice. Namely, key genes are described as the genes that regulate the metabolic function of liver. It was noted that they are being more expressed (mostly chimeric antigen receptor) in germ-free mice compared with mice that have been conventionally raised and that are pathogen-free (SPF). These changes in the gene expressions are most likely a consequence of the gut microbiota actions and its metabolism of endogenous molecules (Bozward et al. Citation2021). Moreover, Claus et al. (Citation2011) used germ-free mice to portray the important effects of microbial activity in the creation of metabolic phenotype in the gut of axenic (germ-free) mice. They examined the effects that exposure to a typical microbial environment of the gut of mice has on axenic mice. It was noted that the levels of CYP3A11, CYP2C29 and CYP8B1 in the liver were significantly less expressed in the germ-free mice.
After 20 days of the gut being colonized by bacteria and the bacteria adapting to its surrounding, an increase in CYP2E1 and CYP2D9 was noted. More importantly, there was no reduction in the levels of CYP3A11, CYP2C29 and CYP8B1 (Dempsey and Cui Citation2019). Selwyn et al. (Citation2015) also used RNA sequencing on the feces collected from germ-free mice to try to portray the effect of bacteria in the gut on the regulation of the expression of a large number of genes that effect drug metabolism and processing. Germ-free mice showed a 51% increase in the expression of messenger RNA responsible for coding the CYP1A2. Interestingly, the production of mRNA that codes CYP4A14 increased by 202%. In addition, there was an up-regulation in some of the important bile acids transporters. Therefore, the research showed that intestinal bacteria have an important role in the differences that occurred in the individual response to drugs and the metabolism of bile acids (Selwyn et al. Citation2015). Ishii et al. (Citation2012) examined how enteric bacteria change and regulate CYP expression. The authors compared CYP3A mRNA expression levels in GF and SPF mice and the results showed the higher activity and expression of CYP3A protein and the higher activity of CYP3A in SPF mice than in GF mice. This confirms that the bacteria that is a part of the gut microbiota community, increase CYP3A expression in the liver (Ishii et al. Citation2012).
One of the most commonly used antibiotics, effective for anaerobic infections is metronidazole. It eliminates bacteria from the organism by acting as a 'Trojan horse' – metronidazole enters the bacterial cell as a prodrug and is being transformed into deadly metabolites. As many other drugs do, metronidazole goes through phases of liver transformation and undergoes the kidneys’ metabolism to be eliminated from the patient’s organism (Dingsdag and Hunter Citation2018). Yet, although being used in clinical practice for years, the exact metabolism of this drug is still not deciphered completely. Recent studies have shown that CYP2A6 is one of the main enzymes in the liver that allows metronidazole to release its potential as an antibiotic. It was also shown that gut microbiota can alter metronidazole metabolism by the action of CYPs placed in the gut itself (Dingsdag and Hunter Citation2018; Zemanová et al. Citation2021). For example, it was observed that metronidazole is being modified by enzymes produced by Clostridium perfringens. These enzymes modify the structure of the imidazole ring, consequently reducing the levels of metronidazole metabolites (Dingsdag and Hunter Citation2018). Moreover, Zemanová et al. (Citation2021) compared the effect of gut microbiota on the pharmacokinetics of metronidazole in observed populations. This experiment included control groups, which were germ-free (GF) and specific-pathogen-free (SPF) mice that were not applied with metronidazole. The experimental groups consisted of GF and SPF mice that have been followed after the application of metronidazole (n = 4). They were administered one intragastric dose (5 mg/kg) of this drug.
After examination, it was found that GF mice had significantly higher levels of metronidazole in their blood, almost 30% than SPF mice (Zemanová et al. Citation2021). In addition, the expression of mRNA that encodes CYP2A5/4 was increased after 24 h in SPF mice that is one of the possible reasons behind the enhancement of metronidazole effects. It was noted that the expression of transcription factor CAR that is included in the regulation of CYPs expression in the liver was increased in SPF mice. In GF mice, there was no measured increase in CAR expression. In addition, the study measured alterations in the expression of various CYPs such as CYP2B10. Interestingly, after metronidazole application in SPF mice, enzyme activity increased whereas their activity in GF mice was not affected. Further studies should confirm these findings and discover a way to enhance the effect of pharmacotherapy with metronidazole by alterations in the gut composition (Zemanová et al. Citation2021).
The dynamic dialogue between enzymes that metabolize drugs and the gut microbiota is well known. The activity of CYP3A expressed in the liver is presumed to be changed by gut microbiota. Therefore, bacteria that are homed in the gut should be under surveillance and monitored in order to better the safety profile of drugs and minimize the side effects (Togao et al. Citation2020). With new methods being widely used, such as culture and strain isolation, DNA extraction, PCR amplification, different quantitative PCR assays, and tandem mass spectrometry for metabolite qualitative analysis. More insight into the gut microbiota composition, its alterations and effects on the pharmacokinetics of drugs is being researched. The modulation of the gut microbiota composition is the future of precision medicine, although many more studies are required to gather enough knowledge to be able to create clinical guidelines as well as to produce drugs for the targeted alterations of the bacterial genera in the gut or at least to get information about expected individual reactions of patients to specific drug’s formulations. Additionally, future studies should use new emerging methods, called in silico methods that combine computer algorithms with existing knowledge about gut microbiota to recapitulate microbial and host dynamic interplay. Furthermore, supervised machine learning can predict outcomes of possible interventions as well as to screen different drugs for their possible alterations by the gut microbiota. This optimizes drug development and leads to the production of better formulations and dose optimization for patients (Togao et al. Citation2020).
4. Relationship of gut microbiota and drugs pharmacokinetics
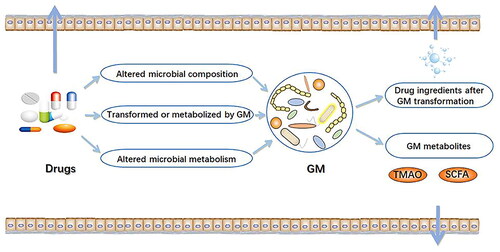
Many studies have been conducted in the last two decades attempting to prove the interplay between gut microbiota and drug pharmacokinetics as shown in . Four basic processes researched in the area of pharmacokinetics are drug absorption, their distribution in the organism followed by drug metabolism. The final step is drug elimination or excretion. This dialogue between four complex pharmacokinetic processes has an important role in the successful clinical outcome after the treatment with the observed drug in the organism as well as in the occurrence of drug potential side effects. As it is known, the same drug can show positive and toxicological effects. The most important differing factor is the used dosage that is highly influenced by the dynamic of basic pharmacokinetic processes (Chen et al. Citation2021).
Table 1. A brief overview of studies researching gut microbiota and drug pharmacokinetics.
Moreover, Zimmermann-Kogadeeva et al. (Citation2020) observed changes in drug kinetics in two groups of animals. The first group of gnotobiotic mice was germ-free and the second group had unaltered microbiota. The study showed a significant reduction of drug in the large intestine in the second group confirming the hypothesis that gut microbiota has the power to alter drug pharmacokinetics. It is important to highlight that this process goes bidirectional, meaning that different drugs could alter the bacteria genera in the gut. On the other hand, gut microbiota could also interfere with drug metabolism and, both indirectly and directly, alter their structure and function in the organism as shown in . One of the metabolites that gut microbiota produces from dietary choline, marked as Trimethylamine-N-oxide (TMAO), is associated with major cardiovascular events. In addition, the gut enhances the production of some beneficial molecules through food fermentation and their release into the circulation, such as short chain fatty acids (SCFA). These molecules have strong anti-inflammatory properties and they help to maintain the integrity of the intestinal barrier (Zimmermann-Kogadeeva et al. Citation2020).
Figure 3. Pharmacokinetics of drugs effected by gut microbiota (Chen et al. Citation2021).
4.1. Gut microbiota interplay with drugs used in cardiology
Haiser et al. (Citation2013) studied the effect of gut Actinobacterium Eggerthella lenta on the inactivation of a drug often used in cardiology - digoxin. Digoxin is classified as a cardiac glycoside and was used even in the Roman Empire. It is produced from the foxglove plant and is then mostly used for the treatment of congestive heart failure. This pharmacokinetic study was conducted on gnotobiotic mice and showed an increased intake of dietary proteins can alter the composition of the gut microbiota. Additionally, it reduces in vivo the metabolism of digoxin, consequently significantly altering drug concentrations in the serum and urine. It is considered the possible cause of the individual alterations of digoxin doses noted in the patient’s blood and serum that are based on the individual diversity and composition of the gut microbiota. (Haiser et al. Citation2013; Tuteja and Ferguson Citation2019). It paves the way for the possibility of using the gut microbiota as a tool for reducing digoxin toxicity through its microbiota manipulation (Lu et al. Citation2014; Li et al. Citation2016).
Matuskova et al. (Citation2014) investigated whether the probiotic E. coli strain Nissle 1917 influences the pharmacokinetics of the concomitantly taken antiarrhythmic drug amiodarone and showed increased drug absorption caused by this probiotic by tracking several pharmacokinetic parameters. Furthermore, gnotobiotic mice experiments revealed that increased protein intake can limit microbial drug inactivation (Haiser et al. Citation2014). This should be thoroughly investigated given the narrow therapeutic range and possible toxicity of amiodarone (Noh et al. Citation2017). Amlodipine is classified as a calcium channel blocker and it is often prescribed for the treatment of hypertension (Tuteja and Ferguson Citation2019). Yoo et al. (Citation2016) conducted a study in which rat feces has been incubated with amlodipine to investigate the effect of enzymes produced by gut microbiota on amlodipine’s metabolism and pharmacokinetics.
By using spectrophotometric techniques, Kim et al. (Citation2016) analyzed and identified the exact chemical molecules that are amlodipine metabolites, produced by the action of gut microbiota-produced enzymes such as M1. Interestingly, M1 is also known as an important metabolite formed in the liver by metabolic enzymes placed in hepatic cells. The pharmacokinetics of amlodipine was compared between two groups. The first group was formed by control rats, which have not been exposed to antibiotics that alter the microbiota composition. The second group consisted of rats that have been administered ampicillin. It was noticed that antibiotics, when ingested orally increased the plasma levels of amlodipine, the parent drug (Kim et al. Citation2016). Therefore, alterations in gut microbiota composition by taking antibiotics concomitantly with antihypertensive drugs result in changes in the level of antihypertensive drugs. Therefore, their efficacy in blood pressure control is interrupted (Kim et al. Citation2016).
Statins are widely prescribed drugs and have been used for years, however yet the considerable individual variation in therapeutic response remains one of the main problems in statins therapy. Genetics is only one small part of this variability (Yoo et al. Citation2014). Lovastatin and simvastatin can be taken as an example. Yoo et al. (Citation2014) investigated on rats how gut microbiota involved in lovastatin metabolism, particularly focusing on its biotransformation to one of the important lovastatin metabolites, the active hydroxy-acid metabolite (M8). The authors compared how the profile of lovastatin and its metabolites concentration, especially M8 changes in plasma, after the oral administration of this drug. Two groups of animals were used. The control group of rats has not been administered with antibiotics. On the other hand, the experimental group of rats have been treated with antibiotics such as ampicillin or they have been treated with a mixture of antibiotics, containing erythromycin, cefadroxil and oxytetracycline. Pharmacokinetic analyses showed an experimental group of rats had significantly lower levels of systemic exposure to lovastatin metabolite M8 as opposed to the control group of rats. The resulting pharmacokinetic parameters suggested that the formation of lovastatin active metabolite M8 is effected by the enzymes produced by gut microbiota (Yoo et al. Citation2014).
4.2. Gut microbiota interplay with drugs used in neurology and psychiatry
The metabolism of gut microbiome can affect not only locally acting drugs but also the efficacy of the drugs targeting distant organ systems (Weersma et al. Citation2020). One example is levodopa, a drug primarily used as a therapy option for Parkinson’s disease. In the recent decade, different studies (Narożańska et al. Citation2014; Maini Rekdal et al. Citation2019) have shown that enzymes called microbial decarboxylases have the ability to metabolize levodopa, consequently changing its plasma values. These enzymes are produced by the activity of bacteria in human gut. Therefore, the authors proposed that noted variations in microbial activity in the gut and the production of their enzymes are a contributing factors to the wide range of responses to levodopa in patients as well as the lower efficacy of levodopa and the development of its adverse side effects (Narożańska et al. Citation2014). In addition, many researchers have attempted to establish a relationship between Helicobacter pylori (HP) and its products with changes in the pharmacokinetics of levodopa by comparing H. pylori-positive and H. pylori-negative patients (Pierantozzi et al. Citation2006; Adamiak et al. Citation2010).
These studies have shown that the infection with HP prolonged the onset time of levodopa action and its beneficial effects on the motor and nonmotor symptoms of Parkinson disease, as opposed to the onset of its action in patients that do not have HP infections. Additionally, it was shown that the 'on-time’, which is the time period when levodopa effectively controls the symptoms of Parkinson’s disease was shorter when compared to PD patients who did not have HP infection. These data suggest that HP prevents the absorption of levodopa in PD patients, however, the exact mechanism is still not deciphered and further studies are needed to investigate this phenomenon. After administering antibiotic treatment to PD patients to eradicate HP, the 'onset’ time decreased and the 'on-time’ increased when compared to the pretreatment values. Therefore, by the use of pharmacokinetic analysis, it was proven that HP eradication increases the efficacy of levodopa treatment in patients with Parkinson’s disease (Lee et al. Citation2021).
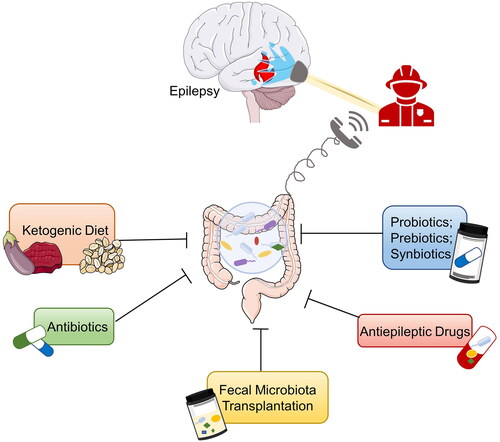
Epilepsy is one of the most emerging health problems in the modern world and quite often, the standard therapy protocols that are being used in clinical practice do not have the effect that is expected. In addition, one of the main issues is the development of drug-resistant epilepsy. Recent studies proposed that the gut microbiota composition, affected by an unhealthy diet is one of the important reasons for increased resistance to anti-epileptic drugs. A group of scientists evaluated feces samples taken from a group of patients that have epilepsy and respond to antiepileptic drugs. These feces samples were compared to the feces samples taken from the group of patients that do not respond to the therapy. Statistical analysis showed that the composition of the gut microbiota was different in both groups. Patients who responded to the treatment had a relative abundance of Bacteroides finegoldii and Bifidobacterium sp., as opposed to the patients did not respond to the therapy and had a relative abundance of Negativicutes sp. (Whang et al. Citation2019). This is the potential marker for detecting the group of patients that will not respond well to the antiepileptic drug therapy as shown in .
Figure 4. Potential therapies for epilepsy based on gut microbiota (Ding et al. Citation2021).
4.3. Gut microbiota interplay with drugs used in diabetology
Given the emerging role of gut microbiome in overweight patients with diabetes mellitus, mostly type 2, the dynamic dialogue between gut microbiome and drugs used as antidiabetics remains of immediate research interest (Aoki et al. Citation2017). Aoki et al. (Citation2017) investigated how the probiotic Bifidobacterium lactis effects the efficacy of metabolic syndrome treatment in mice. In addition, the underlying mechanisms of probiotics involved in the effects of antidiabetic therapy were observed. Results of this study suggested that Bifidobacterium lactis, a commonly used proliferative and viable probiotic, enhances the effects of drugs in treating metabolic syndrome and diabetes mellitus. Further, an improved glucose tolerance was noticed in mice. Moreover, the accumulation of visceral fat was lowered through changes in the composition of bacteria consisting in the gut microbiota and the elevations in the levels of short-chain fatty acids were noted (Aoki et al. Citation2017).
Liraglutide is a second and third line of drugs used in the treatment of patients diagnosed with type 2 diabetes, especially when the control of glycemia was not satisfactory after administration of metformin. Liraglutide acts as a glucagon-like peptide-1 agonist (Shyangdan et al. Citation2011). Zhao et al. (Citation2018) investigated the potential ability of liraglutide to modulate the composition of bacteria in the gut. 16S ribosomal RNA gene sequencing was used to detect alterations in the gut microbiota. The results showed that liraglutide, through modulation of the structure of the gut microbiota such as an increase in Bacteroidetes and a decrease in Firmicutes, altered glucose and lipid metabolism. Therefore, the authors proved that liraglutide lowers the body weight of the rats who were overweight and the rats who were simultaneously overweight and had diabetes (Zhao et al. Citation2018). Similarly, Tilg and Moschen (Citation2014) placed great emphasis on the specific member of the microbiota, Akkermansia muciniphila because the decreased levels of this bacteria were described in the gut of patients with diabetes. In addition, when administered to murine, Akkermansia muciniphila expresses antidiabetic effects (Tilg and Moschen Citation2014).
4.4. Gut microbiota interplay with drugs used in pain treatment
Kim et al. (Citation2016) investigated pharmacological interactions between antibiotics and acetylsalicylic acid, a widely used analgesic and antipyretic. By using a technique called tail-bleeding assay, the authors evaluated how the antithrombotic activity of acetylsalicylic acid altered after the antibiotic treatment. It was found that ampicillin administration significantly lengthened bleeding time in rats that were also administered acetylsalicylic acid. In order to discover possible alterations in gut microbiota, the researchers performed medium culture and pyrosequencing techniques. Analysis of rat fecal samples in these assays indicated that ampicillin treatment resulted in a disturbance in the amounts and compositional profile of gut microbiota. The number and variety of gut microbiota were reduced. When it comes to the phylum level, therapy with ampicillin leads to a significant increase in Proteobacteria and a significant decrease in Firmicutes. In addition, the authors indicated that therapy with ampicillin significantly reduces the levels of acetylsalicylic acid. This was metabolized by the activity of the gut microbiota for 67% of observed rats (Kim et al. Citation2016). Zhang et al. (Citation2019) monitored the interaction between amoxicillin and acetylsalicylic acid in observed rats. The study results showed that amoxicillin alters the pharmacokinetics of salicylic acid that is an active product of the metabolism of acetylsalicylic acid. This also slowed down the metabolism of acetylsalicylic acid and dynamic pharmacokinetic alterations of this drug, occurring because of the altered activity of the gut microbiota (Zhang et al. Citation2019). All of the above suggests that concomitant administration of antibiotics and acetylsalicylic acid results in changes in the safety profile and efficacy of acetylsalicylic acid, mostly due to the composition alterations of gut flora.
The consequences of action of gut microbiota enzymes on the pharmacokinetics of acetaminophen have been questioned, especially considering acetaminophen is one of the most used over-the-counter drugs. Malfatti et al. (Citation2020) found that the administration of amoxicillin or the combination of ampicillin and neomycin in observed mice modify the bioavailability of acetaminophen marked with carbon. They administered orally a single dose of the drug (100 mg/kg) to two groups of animals. The control group of rats that have not received any antibiotics had the three most prevalent phyla: Proteobacteria, Actinobacteria and Bacteroidetes. The group who received antibiotics had only phyla Trypanosomatidae and Proteobacteria detected. In addition, the sulfate metabolite was significantly decreased in the urine of rats' group that have been administered antibiotics, when compared to the controls. On the opposite, the glucuronide metabolite was increased in the rats who received an antibiotic. Therefore, it is proposed that the change in the acetaminophen (APAP) metabolism is caused by abnormalities in the gut microbiota and the alterations of its composition caused by antibiotic use (Malfatti et al. Citation2020).
Gong et al. (Citation2018) investigated whether the gut microbiota modulates diurnal variations in acetaminophen-induced hepatotoxicity because acetaminophen-induced hepatotoxicity is more severe with nocturnal intake than with morning intake. Mice were separated into two groups; one group was given antibiotics that were non-absorbable continuously for 3 days, the other group was not administered any antibiotics. The goal of antibiotic administration in the first group was to deplete the gut microbiota and to investigate the effect of gut microbiota and its enzymes on acetaminophen (APAP) induced diurnal variation of hepatotoxicity. The toxic dose (300 mg/kg) of APAP was orally administered when the light is on that was the start of the resting period (ZT0) and then when the light is off that was a start of an active period (ZT12). It was shown that APAP did not induce hepatotoxic events when the light was on but significant liver damage occurred when the light was off after the same dose of APAP was administered (Tilg and Moschen Citation2014).
To clarify the effect of gut microbiota on liver injury started by APAP, the authors transplanted fecal microbiota to observed mice. The fecal content was first collected from mice in both phases. The next step was to orally administer fecal content to mice recipients who were previously administered antibiotics that depleted their gut microbiota. By using the 16S RNA sequencing technique, it was noted that clusters overlapped between the ZT0 donor and recipient. The clusters also overlapped between the ZT12 donor and ZT12 recipient. These findings suggested that fecal microbiota between donor and recipient was similar. Interestingly, the livers of mice that received ZT12 fecal contents displayed a significantly larger area of necrosis, when compared to the necrosis in the liver of mice that received ZT0 content after APAP was given in the ZT0 phase. In addition, the level of a gut microbial metabolite called 1-phenyl-1,2-propanedione (PPD) that also enhanced liver damage caused by APAP administration (In vivo and in vitro) was significantly higher at the ZT12 phase than at the ZT0 phase. They found that yeast fungi called Saccharomyces cerevisiae could be used to ameliorate the PPD effects and the decrease in liver damage induced by APAP at ZT12 (Gong et al. Citation2018).
4.5. Gut microbiota interplay with drugs used in oncology
Irinotecan, also called CPT-11 is an anti-cancer chemotherapeutic drug mostly used intravenously to treat colon cancer. The side effect of irinotecan is severe diarrhea caused by the action of symbiotic enzymes called b-glucuronidases, produced from bacteria in the gut that activates this drug into its active metabolic form. These enzymes are mostly responsible for irinotecan reactivation in the gut and the development of side effects such as severe diarrhea that are mitigated or avoided by the use of their inhibitors. The bacteria involved are Clostridium ramosum, Escherichia coli and Bacteroides vulgatus. Wallace et al. (Citation2010) found that oral intake of inhibitor of bacterial enzymes such as microbial b-glucuronidases, protected mice from CPT-11-induced toxicity.
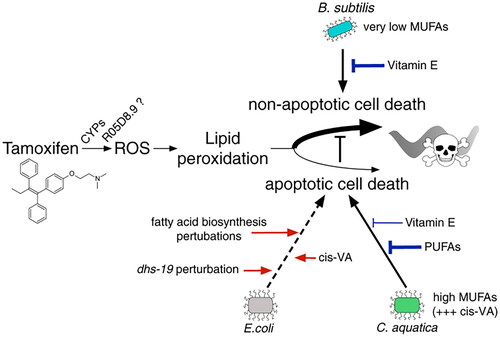
In addition, the results noticed in the study conducted by Wang at al. (Wang et al. Citation2019) that the administration of Escherichia coli Nissle 1917 had protective effects on intestinal injury induced by irinotecan. The key findings were that irinotecan treatment altered the diversity of gut microbiota composition. This drug increased the Proteobacteria relative abundance, compared with its abundance in control group. Therefore, Escherichia coli Nissle application improved the gut microbiota dysbiosis (Wang et al. Citation2019). In recent years, there were many studies (Diot et al. Citation2021) that have been developing a model system by using the nematode Caenorhabditis elegans to assess the response of the animals to different drugs such as tamoxifen. It belongs to the class of drugs called SERMs, selective estrogen receptor modulators that is the standard treatment for breast cancers positive for estrogen receptors together with chemotherapy and radiation therapy. The authors of this study (Diot et al. Citation2021) changed the diet of larval stage animals with one of the three following bacteria: C.aquatica, B. subtilis or E. coli. After the tamoxifen administration to C.elegans, it was proven that tamoxifen toxicity increased in animals fed by C.aquatica and B. subtilis that showed a delay in development.
On the other hand, there was little, if any effect on the animals fed by E. coli, which showed that a bacterial diet can modify tamoxifen toxicity. The genetic screens were conducted on those bacterial species and the results indicated that it is not E. coli that affects this drug but it is C.aquatica. Two detected types of C.aquatica increased and two decreased tamoxifen toxicity most likely by changing the tamoxifen transport and the amount of tamoxifen being taken up by the C. elegans. In addition, B. subtilis that is present in many available probiotics on the market was shown to induce the non-apoptotic mechanisms of cells death after tamoxifen applications than C.aquatica and E. coli by activating apoptotic pathways as shown in . One of the emerging new treatments in oncology is the use of immune checkpoint inhibitors (ICI) because its administration starts the powerful cascade-induced activation of the T-lymphocytes and it boosts the immune system to fight against cancer.
Figure 5. The interplay between Tamoxifen and effect of different bacterial species on its toxicity (Diot et al. Citation2021).
A recent study (Derosa et al. Citation2018) has shown that gut microbiota is one of the factors affecting outcomes of this therapy. It was shown that the patients with renal cell carcinoma (RCC) and patients with non-small-cell lung cancer (NSCLC), who were receiving anti-programmed cell death ligand-1 monoclonal antibody (mAb) monotherapy and prior to that antibiotics for 30 days had shorter progression-free survival and shorter overall survival (Derosa et al. Citation2018). Furthermore, Routy et al. (Citation2018) showed that the monoclonal antibodies that attack PD-1 (Programmed cell death protein) are effected by dysbiosis of gut microbiota. They conducted a study on mice with proven sarcoma and RET melanoma who were treated with PD-1 antibody or its combination with CTLA-4 monoclonal antibody. Firstly, mice were in an environment without pathogen and one group received a 14 days course of 3 antibiotics (colistin, streptomycin and ampicillin) while the control group did not receive any drugs.
The results showed that mice who were receiving antibiotics had lower survival time and the effect of ICI was not as good as in the control group. The authors also studied the effect of gut microbiota change caused by taking antibiotics in patients with urothelial carcinoma and non-small-cell lung carcinoma, who were treated with PD-1 immunotherapy. The results indicated that group of patients who were taking antibiotics had overall survival shorter than the control group of patients who were not treated with antibiotics. Statistical analysis indicated that the use of antibiotics is a predictive factor for patients the development of resistance to immunotherapy with PD-1 monoclonal antibodies (Routy et al. Citation2018). Matson et al. (Citation2018) studied the reasons behind noticeable variability in therapeutic response of individuals fighting metastatic melanoma who are being administered anti-PD-1 drugs. They collected fecal samples from the patients before they received anti-PD-1 therapy, in order to find an association between the composition of their gut microbiota and the response to the treatment. The study showed that the gut microbiota can moderate the clinical impact of therapy with anti-PD-1 drugs. It was also noted that the patients who responded better to this treatment had more present three bacterial species: Collinsella aerofaciens, Bifidobacterium longum and Enterococcus faecium. These data could be used in the future for applying probiotics that contain these bacteria as the enhancer of the anti-PD-1 therapy effect (Whang et al. Citation2019).
5. Conclusion
Every human organism is a universe yet to be deciphered and one of the many unknowns is gut microbiota. Studies showed that gut microbiota is an important reason for different individual reactions to orally administered drugs such as lovastatin and acetaminophen. There are many different metabolic pathways that gut microbiota uses to influence drug pharmacokinetics such as digoxin, amiodarone, amlodipine, liraglutide etc. Although many studies that have been conducted on mice and rats showed that the gut effects a large number of previously mentioned clinically important and frequently used drugs, there is a small number of studies that confirm these findings on humans. Therefore, present knowledge is still limited. In future studies, it should be precisely determined how microorganisms effect each drug in vivo in human organisms and the exact underlying pharmacokinetic pathways. This would set a foundation for the development of personalized medicine, unlock the doors for manipulation of gut microbiota and set a foundation for building better pharmacokinetic models that can quantitatively predict microbiota contributions to systemic drug exposure.
Acknowledgement
The authors are grateful for the financial support from the International Society of Engineering Science and Technology (ISEST) UK.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adak A, Khan MR. 2019. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 76(3):473–493.
- Adamiak U, Kaldonska M, Klodowska-Duda G, Wyska E, Safranow K, Bialecka M, Gawronska-Szklarz B. 2010. Pharmacokinetic-pharmacodynamic modeling of levodopa in patients with advanced Parkinson disease. Clin Neuropharmacol. 33(3):135–141.
- Aoki R, Kamikado K, Suda W, Takii H, Mikami Y, Suganuma N, Hattori M, Koga Y. 2017. A proliferative probiotic Bifidobacterium strain in the gut ameliorates progression of metabolic disorders via microbiota modulation and acetate elevation. Scient Rep. 7(1):1–10.
- Björkholm B, Bok CM, Lundin A, Rafter J, Hibberd ML, Pettersson S. 2009. Intestinal microbiota regulate xenobiotic metabolism in the liver. PloS One. 4(9):e6958.
- Bozward AG, Ronca V, Osei-Bordom D, Oo YH. 2021. Gut-liver immune traffic: deciphering immune-pathogenesis to underpin translational therapy. Front Immunol. 12:711217.
- Chen H-Q, Gong J-Y, Xing K, Liu M-Z, Luo J-Q. 2021. Pharmacomicrobiomics: exploiting the drug-microbiota interactions in antihypertensive treatment. Front Med. 8:742394.
- Claus SP, Ellero SL, Berger B, Krause L, Bruttin A, Molina J, Paris A, Want EJ, de Waziers I, Cloarec O, et al. 2011. Colonization-induced host-gut microbial metabolic interaction. MBio. 2(2):e00271-00210–e00210.
- Dempsey JL, Cui JY. 2019. Microbiome is a functional modifier of P450 drug metabolism. Curr Pharmacol Rep. 5(6):481–490.
- Derosa L, Hellmann MD, Spaziano M, Halpenny D, Fidelle M, Rizvi H, Long N, Plodkowski AJ, Arbour KC, Chaft JE, et al. 2018. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 29(6):1437–1444.
- Ding M, Lang Y, Shu H, Shao J, Cui L. 2021. Microbiota–gut–brain axis and epilepsy: a review on mechanisms and potential therapeutics. Front Immunol. 12:742449.
- Dingsdag SA, Hunter N. 2018. Metronidazole: an update on metabolism, structure–cytotoxicity and resistance mechanisms. J Antimicrob Chemother. 73(2):265–279.
- Diot C, García-González AP, Vieira AF, Walker M, Honeywell M, Doyle H, Rivera Y, Na H, Zhang H, Lee M. 2021. Bacteria modulate tamoxifen-induced death via host fatty acid metabolism. bioRxiv.
- Gacesa R, Kurilshikov A, Vich Vila A, Sinha T, Klaassen MAY, Bolte LA, Andreu-Sánchez S, Chen L, Collij V, Hu S, et al. 2022. Environmental factors shaping the gut microbiome in a Dutch population. Nature. 604(7907):732–739.
- Gomaa EZ. 2020. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 113(12):2019–2040.
- Gong S, Lan T, Zeng L, Luo H, Yang X, Li N, Chen X, Liu Z, Li R, Win S, et al. 2018. Gut microbiota mediates diurnal variation of acetaminophen induced acute liver injury in mice. J Hepatol. 69(1):51–59.
- Haiser HJ, Gootenberg DB, Chatman K, Sirasani G, Balskus EP, Turnbaugh PJ. 2013. Predicting and manipulating cardiac drug inactivation by the human gut bacterium Eggerthella lenta. Science. 341(6143):295–298.
- Haiser HJ, Seim KL, Balskus EP, Turnbaugh PJ. 2014. Mechanistic insight into digoxin inactivation by Eggerthella lenta augments our understanding of its pharmacokinetics. Gut Microbes. 5(2):233–238.
- Hills RD, Jr., Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. 2019. Gut Microbiome: profound implications for diet and disease. Nutrients. 11(7):1613.
- Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, Zhang X, Weng Z, Lu Q, Jiao L. 2022. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nature Commun. 13(1):1–13.
- Ioannidis JPA. 2019. Reproducible pharmacokinetics. J Pharmacokinet Pharmacodyn. 46(2):111–116.
- Ishii M, Toda T, Ikarashi N, Ochiai W, Sugiyama K. 2012. Effects of intestinal flora on the expression of cytochrome P450 3A in the liver. Yakugaku Zasshi. 132(3):301–310.
- Karl JP, Hatch AM, Arcidiacono SM, Pearce SC, Pantoja-Feliciano IG, Doherty LA, Soares JW. 2018. Effects of psychological. Environmental and Physical Stressors on the Gut Microbiota. Front Microbiol. 9:2013.
- Kim IS, Yoo D-H, Jung I-H, Lim S, Jeong J-J, Kim K-A, Bae O-N, Yoo HH, Kim D-H. 2016. Reduced metabolic activity of gut microbiota by antibiotics can potentiate the antithrombotic effect of aspirin. Biochem Pharmacol. 122:72–79.
- Koppel N, Maini Rekdal V, Balskus EP. 2017. Chemical transformation of xenobiotics by the human gut microbiota. Science. 356(6344)
- Lee SH, Choi N, Sung JH. 2019. Pharmacokinetic and pharmacodynamic insights from microfluidic intestine-on-a-chip models. Expert Opin Drug Metab Toxicol. 15(12):1005–1019.
- Lee H, Lee S, Lee DH, Kim DW. 2021. A comparison of the gut microbiota among adult patients with drug-responsive and drug-resistant epilepsy: an exploratory study. Epilepsy Res. 172:106601.
- Li H, He J, Jia W. 2016. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin Drug Metab Toxicol. 12(1):31–40.
- Li J, Hu FB. 2019. Research digest: reshaping the gut microbiota. Lancet Diabetes Endocrinol. 7(9):671.
- Lu L, Wu Y, Zuo L, Luo X, Large PJ. 2014. Intestinal microbiome and digoxin inactivation: meal plan for digoxin users? World J Microbiol Biotechnol. 30(3):791–799.
- Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. 2019. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 364(6445):eaau6323.
- Malfatti MA, Kuhn EA, Murugesh DK, Mendez ME, Hum N, Thissen JB, Jaing CJ, Loots GG. 2020. Manipulation of the gut microbiome alters acetaminophen biodisposition in mice. Scientific Reports. 10(1):1–10.
- Matson V, Fessler J, Bao R, Chongsuwat T, Zha Y, Alegre M-L, Luke JJ, Gajewski TF. 2018. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 359(6371):104–108.
- Matuskova Z, Anzenbacherova E, Vecera R, Tlaskalova-Hogenova H, Kolar M, Anzenbacher P. 2014. Administration of a probiotic can change drug pharmacokinetics: effect of E. coli Nissle 1917 on amidarone absorption in rats. PloS One. 9(2):e87150.
- Nakov R, Velikova T. 2020. Chemical metabolism of xenobiotics by gut microbiota. Curr Drug Metab. 21(4):260–269.
- Narożańska E, Białecka M, Adamiak-Giera U, Gawrońska-Szklarz B, Sołtan W, Schinwelski M, Robowski P, Madaliński MH, Sławek J. 2014. Pharmacokinetics of levodopa in patients with Parkinson disease and motor fluctuations depending on the presence of Helicobacter pylori infection. Clin Neuropharmacol. 37(4):96–99.
- Noh K, Kang YR, Nepal MR, Shakya R, Kang MJ, Kang W, Lee S, Jeong HG, Jeong TC. 2017. Impact of gut microbiota on drug metabolism: an update for safe and effective use of drugs. Arch Pharm Res. 40(12):1345–1355.
- Pierantozzi M, Pietroiusti A, Brusa L, Galati S, Stefani A, Lunardi G, Fedele E, Sancesario G, Bernardi G, Bergamaschi A, et al. 2006. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 66(12):1824–1829.
- Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC. 2019. What is the healthy gut microbiota composition? a changing ecosystem across age, environment, diet, and diseases. Microorganisms. 7(1):14.
- Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C, Roberti MP, et al. 2018. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 359(6371):91–97.
- Schupack DA, Mars RAT, Voelker DH, Abeykoon JP, Kashyap PC. 2022. The promise of the gut microbiome as part of individualized treatment strategies. Nat Rev Gastroenterol Hepatol. 19(1):7–25.
- Selwyn FP, Cui JY, Klaassen CD. 2015. RNA-Seq quantification of hepatic drug processing genes in germ-free mice. Drug Metab Dispos. 43(10):1572–1580.
- Shyangdan D, Cummins E, Royle P, Waugh N. 2011. Liraglutide for the treatment of type 2 diabetes: a single technology appraisal. Health Technol Assess. 15(1):77–86.
- Taniguchi C, Kawabata Y, Wada K, Yamada S, Onoue S. 2014. Microenvironmental pH-modification to improve dissolution behavior and oral absorption for drugs with pH-dependent solubility. Expert Opin Drug Deliv. 11(4):505–516.
- Tilg H, Moschen AR. 2014. Microbiota and diabetes: an evolving relationship. Gut. 63(9):1513–1521.
- Togao M, Kawakami K, Otsuka J, Wagai G, Ohta‐Takada Y, Kado S. 2020. Effects of gut microbiota on in vivo metabolism and tissue accumulation of cytochrome P450 3A metabolized drug: midazolam. Biopharm Drug Dispos. 41(7):275–282.
- Tuteja S, Ferguson JF. 2019. Gut microbiome and response to cardiovascular drugs. Circ Genom Precis Med. 12(9):e002314.
- Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, Venkatesh M, Jobin C, Yeh L-A, Mani S, et al. 2010. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 330(6005):831–835.
- Wang Y, Sun L, Chen S, Guo S, Yue T, Hou Q, Feng M, Xu H, Liu Y, Wang P. 2019. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan–induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sciences. 231:116529.
- Weersma RK, Zhernakova A, Fu J. 2020. Interaction between drugs and the gut microbiome. Gut. 69(8):1510–1519.
- Whang A, Nagpal R, Yadav H. 2019. Bi-directional drug-microbiome interactions of anti-diabetics. EBioMedicine. 39:591–602.
- Wilson ID, Nicholson JK. 2017. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl Res. 179:204–222.
- Winston JA, Theriot CM. 2020. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 11(2):158–171.
- Xie Y, Hu F, Xiang D, Lu H, Li W, Zhao A, Huang L, Wang R. 2020. The metabolic effect of gut microbiota on drugs. Drug Metab Rev. 52(1):139–156.
- Yang L, Yuan TJ, Wan Y, Li WW, Liu C, Jiang S, Duan JA. 2022. Quorum sensing: a new perspective to reveal the interaction between gut microbiota and host. Future Microbiol. 17:293–309.
- Yoo D-H, Kim IS, Van Le TK, Jung I-H, Yoo HH, Kim D-H. 2014. Gut microbiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab Dispos. 42(9):1508–1513.
- Yoo HH, Kim IS, Yoo D-H, Kim D-H. 2016. Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction. J Hypertens. 34(1):156–162.
- Zemanová N, Lněničková K, Vavrečková M, Anzenbacherová E, Anzenbacher P, Zapletalová I, Hermanová P, Hudcovic T, Kozáková H, Jourová L. 2021. Gut microbiome affects the metabolism of metronidazole in mice through regulation of hepatic cytochromes P450 expression. PloS One. 16(11):e0259643.
- Zhang X, Han Y, Huang W, Jin M, Gao Z. 2021. The influence of the gut microbiota on the bioavailability of oral drugs. Acta Pharm Sin B. 11(7):1789–1812.
- Zhang J, Sun Y, Wang R, Zhang J. 2019. Gut microbiota-mediated drug-drug interaction between amoxicillin and aspirin. Scient Rep. 9(1):1–8.
- Zhang J, Zhang J, Wang R. 2018. Gut microbiota modulates drug pharmacokinetics. Drug Metab Rev. 50(3):357–368.
- Zhao L, Chen Y, Xia F, Abudukerimu B, Zhang W, Guo Y, Wang N, Lu Y. 2018. A glucagon-like peptide-1 receptor agonist lowers weight by modulating the structure of gut microbiota. Front Endocrinol. 9:233.
- Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. 2019. Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science. 363(6427):eaat9931.
- Zimmermann-Kogadeeva M, Zimmermann M, Goodman AL. 2020. Insights from pharmacokinetic models of host-microbiome drug metabolism. Gut Microbes. 11(3):587–596.
