Abstract
This paper introduces taxonomy of clinical uncertaintes and argues that the choice of scientific method should match the underlying level of uncertainty. Clinical trial is one of these methods aiming to resolve clinical uncertainties. Whenever possible these uncertainties should be quantified.
The paper further shows that the still ongoing debate about the usage of “equipoise” vs. “uncertainty principle” vs. “indifference” as an entry criterion to clinical trials actually refers to the question “whose uncertainty counts”. This question is intimately linked to the control of research agenda, which is not quantifiable and hence is not solvable to equal acceptability to all interested parties. The author finally shows that there is a predictable relation between [acknowledgement of] uncertainty (the moral principle) on which trials are based and the ultimate outcomes of clinical trials. That is, [acknowledgement of] uncertainty determines a pattern of success in medicine and drives clinical discoveries.
I. ACKNOWLEDGEMENT OF UNCERTAINTY: A COMMON DENOMINATOR AMONG THREE MAIN ETHICAL APPROACHES TO RESEARCH IN HUMANS
Progress in therapeutics and prevention has been feasible because of the willingness of people to enroll in clinical trials. This is also acknowledged in the Declaration of Helsinki, which states that “medical progress is based on research which ultimately must rest in part on experimentation involving human subjects” (CitationWorld Medical Association, 2000). Once the experiment is successfully completed, we are in a position to learn which treatment is superior. This knowledge, justified from the utilitarian perspective, however, will primarily help future patients who did not volunteer to be a participant in the experiment (CitationMiller & Rosenstein, 2003). The patients who actually participated in the trial may or may not benefit from the treatments being tested. Therefore, by asking current patients to sacrifice for the benefit of future patients (CitationMiller & Rosenstein, 2003), we are at risk of rescinding Kant's categorical imperative that we should treat others “as an end in itself, never merely as a means” (CitationKant, 1964), thus subjugating our duties to patients' best interest to the utilitarian goal for the good of others (CitationFoster, 2001). It is this tension between “duty to individuals” versus “societal value” of clinical research that has historically raised ethical concerns regarding the use of humans for testing of health care interventions (CitationHill, 1952).
Patients, however, are autonomous beings and via the process of providing their voluntary consent, are free to exercise their rights to participate in clinical trials. The purpose of seeking consent is to allow patients “to exercise what makes them essentially humans” (CitationFoster, 2001), rational human beings who have to deal with a mixture of conflicting and common interests. The other party in this interaction is a researcher, who as a representative of society's future patients, carries out his own interests, which may or may not correspond to the patients' best interest.
Is there a common denominator among these three main ethical theories, i.e., utilitarian, duty-oriented and right-based approaches to medical research on humans (CitationFoster, 2001)? The main view put forward here is that the science and ethics of clinical research is an epistemological problem revolving around our uncertainties about the relative effects of clinical interventions, which in the field of therapeutics always involves the issue of comparison (CitationDjulbegovic & Hozo, 2002). If there were no uncertainties, the ethical dilemma of research would have been largely non-existent: society would know which treatments are better, individuals would not be exposed to any known or unknown risks, and therefore there would be no choices to be made (CitationDjulbegovic, 2004). Indeed, from the perspective of society and individual, it is that uncertainty about unknown harms and the hope for benefits and how we construe our response to these uncertainties, that is at the intersection of science and ethics of clinical research (CitationDjulbegovic, 2001; CitationChalmers, 2004).
So, the entire scientific and ethical debate of clinical research can (and should) be recast from a position of the need to acknowledge and better characterize the existing uncertainties (say, about the relative effects of competing treatment alternatives, which is the focus of this article). It is not my objective in this article to delve into measurement of uncertainty and whether subjective (Bayesian) or objective (frequentist) approach to the probability calculus should be employed- many more competent authors than I have dealt with the theory of probability, which has been formally used to quantify uncertainties related to the events of interest to us. My own view is that depending on the situation, one may accept either approach, as long as the requirement of total relevant evidence to inform our judgments is maintained (see below).
However, my main point is that once the existing uncertainty is recognized and acknowledged, further steps will be facilitated in the form of the next logical question: how do we devise an effective resolution of the uncertainty which we have now formally accepted (CitationDjulbegovic, 2001)? Of course, a general answer lies in the acceptance of scientific method as the means to address articulated uncertainties (CitationHastie & Dawes, 2001). More specifically, the question can be formulated in terms of tailoring a specific scientific (clinical) method to a given level of (clinical) uncertainty. However, before we can propose which method is appropriate for a given uncertainty, we must first characterize the levels of uncertainties more definitively. Thus, the next logical requirement for science and ethics of clinical research is presented in terms of the need to develop a taxonomy of clinical research uncertainties.
II. TAXONOMY OF CLINICAL UNCERTAINTIES
Assessment of uncertainties can be qualitative and quantitative. The first and most important step in articulating uncertainties is a qualitative, or roughly a semi-quantitative exercise. Uncertainty can range from simply not having the factual confirmation of what is an otherwise sufficiently clear understanding [of treatment effects] to maximum uncertainty (CitationLilford & Djulbegovic, 2001). Wittgenstein in his musings “On Certainty” remarked: “The difference of ‘knowing’ and the concept of ‘being certain’ is not of any great importance, except when ‘I know’ is meant to mean ‘I cannot be wrong’.” (CitationWittgenstein, 1969)
In clinical medicine this concept often takes the form of expressions regarding a guess or hunch by researchers about their beliefs in the effects of treatments to be tested. This should be distinguished from a prediction that is rationally grounded by means of established scientific laws. The investigators' unarticulated experience, however, forms the basis of their beliefs, which is often sufficient for actions. This may culminate in formulation of a general research strategy suggesting how to answer the uncertainties that are in the process getting better articulated.
It appears that a more quantitative, rational decision-making model is preceded by the qualitative considerations in which intuition (hunches) serves to economize among a potentially huge number of (treatment) options (CitationHastie & Dawes, 2001). According to this view, researchers use their background knowledge and experience to quickly sort out among the available treatment alternatives and decide which ones remain viable for further testing. After this is done, the researchers may still retain their beliefs and hunches regarding which treatment is better, but now they have their uncertainties better articulated and may be willing to submit their hunches (i.e. uncertainties about their predictions regarding which is the better treatment option) to testing within a rational scientific framework.
Quantitatively, uncertainty is typically formulated in terms of
-
frequency (probability) of event,
-
assessments of credibility intervals around these estimates and
-
entropy, which relates to uncertainty about the choice (in our case, about one treatment vs. another) (CitationShannon & Waever, 1962).
According to information theory entropy, or maximum uncertainty occurs when the probability of various choices is equal (CitationShannon & Waever, 1962). Using this approach “equipoise” refers to maximum uncertainty regarding the choice of one treatment vs. another (CitationDjulbegovic, 2001). Understood this way equipoise has a very specific interpretation referring to “equal” beliefs, or equally distributed uncertainty about the relative effects of competing treatment alternatives. This notion of “equal” beliefs does not necessarily assume any precise quantitative distribution of treatment effects, but rather refers to the substantial uncertainty expressed more or less qualitatively in Wittgenstein's sense that researchers admit their hunches, predictions, guesses or beliefs may easily (roughly, equally likely) proved to be wrong after testing is performed.
It is important, however, to emphasize here that equipoise clearly implies articulated treatment alternatives about whose relative merits researchers are maximally uncertain. Therefore, equipoise represents a [specific] measure of uncertainty. The question is then which is the most appropriate method of clinical research to address this level of uncertainty and where can equipoise fit into the taxonomy of clinical uncertainties?
Attempts to characterize uncertainties in clinical medicine have been rather rudimentary and revolved around the usage of “equipoise” versus “uncertainty principle” versus “indifference” (see next section). As argued here, of crucial importance to advancing this debate is to develop taxonomy of uncertainties in clinical medicine. Courtney, Kirkland, and Court made a cogent case that our strategy under uncertainty will require different methods and analytical tools depending on “the levels of uncertainty” in which we find ourselves (CitationCourtney et al., 1999). I adopted their classification from the business world to the clinical environment and clinical trials methodology.
shows a proposed taxonomy of clinical uncertainty about expected (future) treatment effects. The underlying idea is that choice of scientific methodology and analytic technique should be tailored to the underlying uncertainty about treatment effects. One (methodological) size does not fit all (levels of uncertainties). When the effects of treatments are dramatic and recognizable, we do not need randomized controlled trials (RCTs) to demonstrate that a particular intervention is effective (CitationDjulbegovic, 2000). This is, for example, how penicillin, insulin, blood transfusion etc were introduced in medical practice. This is titled as “Clear-Enough Future” scenario in (we still can be wrong, but have a pretty clear picture what the future will look like).
FIGURE 1 Taxonomy of clinical uncertainties. The figure illustrates how choice of scientific methodology is tailored to underlying uncertainties about treatment effects. RCT-randomized controlled trial
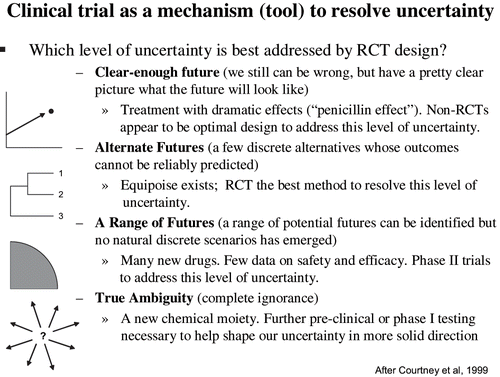
When estimated uncertainties about benefits and harms of competing treatment alternatives are high, however, we can never be sure if the observed effect were due to treatment(s) or because of other factors related to patient selection or other biases and random errors that may have been introduced in a particular study. Under these circumstances, the use of a RCT design represents not only the most rational method to resolve these uncertainties (see below), but is simply the most reliable means to address uncertainties about treatment effects (CitationSilverman & Chalmers, 2001).
RCTs represents a method of choice to resolve uncertainties when alternatives are clearly formulated and when it is estimated that there is about equal probability that one treatment is better than the other (i.e. when we are in equipoise). This scenario is known as “Alternate Futures”—a few discrete alternatives whose outcomes cannot be reliably predicted have emerged culminating in equipoise.
The third level of uncertainty is dubbed as a “Range of Futures.” There is a range of “potential futures” but no natural discrete scenarios have yet emerged. This is currently seen as many new drugs are being introduced into clinical arena, but with insufficient data on safety and efficacy. We typically require phase II trials to address this level of uncertainty.
The final category of uncertainty is known as “True Ambiguity (complete ignorance).” Although we are very uncertain about the value of a particular intervention, the state of existing knowledge does not allow commitment to any of potential treatment options. Therefore, in the sense of human clinical research, uncertainty (about the specific treatment choice) virtually does not exist. Typically this situation may be seen when a new chemical moiety is developed. Further inquiry in terms of pre-clinical or phase I testing is necessary to help shape our uncertainty in a more solid direction prior to undertaking further research in humans.
Uncertainties are not fixed categories. In fact, it is rather typical that uncertainty may shift or migrate toward one of the other over time. As additional knowledge about interventions is acquired through research, a scenario “range of futures” would typically solidify into “alternate future” with two or more alternatives culminating in equipoise which would in turn require a RCT as a method of choice to address newly articulated uncertainties. Viewed from this perspective, uncertainties that are addressed by phase I and phase II trials are not classified as “equipoise.” Patients and physicians in these situations may be uncertain if enrollment into phase II (or even phase I) trial will result in any benefit, but they do not believe that there is a clear alternative to a new promising, but unproven therapy. They may be uncertain, but they are not in equipoise. Hence, a formal mechanism to resolve this level of uncertainty through RCT is not called for. As mentioned, not infrequently this level of uncertainty can shift in such a way that the value of a new therapy (which may have been tested in phase I/II trials) may become increasingly uncertain when compared with other existing alternatives eventually reaching equipoise.
One typical example was the use of stem cell transplant for breast cancer which resulted in tens of thousands of women getting aggressive toxic and very expensive treatment based on strong beliefs obtained in non-randomized trials. Slowly, however, doubts and uncertainties about true effects of this treatment have been propagated through the literature. Equipoise was eventually reached, which culminated in RCTs demonstrating no benefits but increased harms of stem cell transplants compared with conventional chemotherapy (CitationWelch & Mogielnicki, 2002).
III. ETHICISTS' AND TRIALISTS' ARTICULATION OF UNCERTAINTIES IN CLINICAL MEDICINE: “EQUIPOISE” VERSUS “UNCERTAINTY PRINCIPLE” VERSUS “INDIFFERENCE”
Perhaps ever since clinical trials have become an accepted method to test relative merits of competing treatments, the scientific and ethical justifications for enrollment of patients into trials, particularly RCTs, have revolved around the requirement of the substantial uncertainty concerning which of the treatments is more likely to benefit patients (CitationHill, 1952). Although it has taken several decades to articulate these questions in a more explicit manner, we should note that Bradford Hill wrote in 1963 that we should accept randomization “only in our state of ignorance, the treatment given [being] a matter of indifference” (CitationHill, 1963). Of interest, recently Veatch advocated “indifference of subjects” as the ethical and scientific alternative to equipoise in randomized clinical trials (CitationVeatch, 2002).
The still ongoing debate had started with the issues that ultimately relate to a question, “Whose uncertainty is morally relevant?” In 1974, Fried stipulated that a physician may offer enrollment into a trial to his/her patient only when he/she is genuinely uncertain as to the preferred treatment. He introduced the term “equipoise” which requires uncertainty at the level of individual physicians. A couple of the decades later, this view has been promulgated by Peto who coined the term “uncertainty principle” according to which RCTs are only ethically and scientifically permissible if both the treating physician and his/her patient are substantially uncertain regarding the merits of treatments being offered (CitationPeto & Baigent, 1998).
Lilford espoused similar views but in terms of theoretical patient's equipoise which he equated with expected utility threshold in decision-analytical parlance (CitationLilford & Jackson, 1995). In 1987, Friedman shifted the focus from the individual physician to the community (of expert practitioners) proposing that patients can be ethically and scientifically enrolled into a clinical trial only if there exists a state of honest, disagreement (i.e., uncertainty) in the community of expert practitioners regarding the preferred treatment.
In 2001, after intensive exchange about the usage of equipoise vs. uncertainty principle as an entry criterion for a RCT (CitationWeijer, Shapiro, Cranley, 2000), we pointed out that these are not mutually exclusive concepts and that the question would be better phrased as “How much uncertainty can we accept before entering a patient into a trial and by whom (patients, physicians, and community)?” (CitationLilford & Djulbegovic, 2001). Despite the strong opinions and forceful arguments put forward in favor of one view versus another, the general requirement for uncertainty before the trial is undertaken, although inadequately articulated, has actually never been challenged by ethicists.
One ingredient, however, which has been missing from this debate relates to the values and interests of each party whose uncertainties may drive a completely different research agenda. The research agenda issue is intimately linked to the question of “whose uncertainty matters” (public, researchers, sponsors, regulators)? As discussed in the next section, ultimately these uncertainties relate to the choice of comparator intervention(s). This is a very complex decision which is affected by the interests of many stakeholders. For example, the patient may be interested in participation in RCTs testing completely different treatments than the ones offered by the researchers.
Similarly, policy-makers, sponsors, or regulatory agencies may also want to see different types of testing, each of which addresses different categories of uncertainties. This is the major reason for possible discrepant views between a community of experts (who are bound by the equipoise precept) and individual physicians (who may hold their views according to the uncertainty principle).
Kenneth Arrow, Nobel laureate in economics, showed that when there are multiple choices and interests, it is impossible to provide consistent and unambiguous decisions that will be equally acceptable to all parties (CitationArrow, 1963). The choice of research question is determined by various judgments and potentially conflicting interests that are not addressable by precise mathematical solutions. I advocate the extension of the Rawlsian principle of reflective equilibrium/considered judgment (CitationRawls, 1999) in the clinical trial setting.
The equilibrium relates to the situation when principles and judgments coincide (CitationRawls, 1999). It is reflective since it can be derived from the key precepts of moral philosophy ultimately linking the theory of human experimentation with theory of rational choice. Judgments are deliberative and considered because they are derived systematically with the least likelihood of distortion. In the clinical trial setting, it is characterized by the need to acknowledge uncertainties, provide transparency in decision-making and clarity regarding the critical criteria that informed our choices, undertake a shared deliberation and provide an appeal process (via publicly available research protocols). This is known as “accountability for reasonableness” which may not completely resolve ethical and scientific dilemmas we face in clinical research, but may help legitimize specific choices that may favor one set of stakeholders over others ( e.g., the trial's patient interest over the future patients' interest) (CitationDaniel & Sabin, 1997).
Therefore, the approach to clinical uncertainty (equipoise) advocated here distinguishes the locus of equipoise (patient, physician, investigator, community, sponsor, regulator) from the evidentiary standards related to the merits of competing treatment alternatives.
IV. IMPLICATIONS FOR DESIGN OF CLINICAL TRIALS: CHOICE OF COMPARATOR AND ISSUE OF GENERALIZIBILITY
I believe that some of ethical issues related to the “uncertainty principle” (allowing individual researchers to exercise his/her hunches) versus clinical equipoise (allowing the trial to go forward only if there is split in the community of experts) is best addressed by considering their impact on the trial design.
Choice of Comparator and Effect Size
Therapeutic clinical trials are exercises in comparison and always involve some type of comparator. In observational and phase II studies, comparison is usually done indirectly, often against historical control. In RCT, two or more treatments are directly compared. The first step in undertaking a RCT relates to the choice of comparator interventions. This is a qualitative but arguably the most important step in the design of a clinical trial. For example, we showed that the study can adhere to all contemporary recommendations about a good trial design (i.e., CONSORT statement (CitationMoher, Schultz, & Altman, 2001)) and still produce predictably (biased) results (CitationDjulbegovic, Cantor, & Clarke 2003).
Is there a formal mechanism to select appropriate comparator, akin to the use of randomization to control for selection biases? Howard Mann and I have argued that acknowledgment of uncertainties around competing treatment alternatives (which may be expressed in terms of equipoise or the uncertainty principle) translates to the choice of comparator intervention (CitationMann & Djulbegovic, 2003; CitationMann & Djulbegovic, 2004). As discussed above, this is ultimately an epistemological question (CitationAshcroft, 1999) and whether there is uncertainty regarding the effects of comparator interventions will depend on the state of accumulated knowledge (CitationDjulbegovic, 2003). If our pre-existing knowledge makes clear that one of the treatments to be assessed is preferable, then research would not be justified. Scientifically, no substantial new knowledge would be learned and ethically, our patients would receive an inferior treatment. This means that the assessment of pre-existing knowledge is of profound moral value, and it behooves us to employ the best-known methods to address the state of existing uncertainties concerning the effects of competing interventions.
Arguably, the best current methods to assess the state of existing information are:
-
to employ systematic review of the totality of relevant research evidence (CitationGood, 1967) (with or without meta-analysis of all completed trials),
-
perform a formal survey of expert clinical practitioners and
-
request publication of the trial's protocol to solicit critical appraisal.
Systematic reviews are considered the scientific foundation of practice guidelines development (CitationGRADE Working Group, 2004). Practice guidelines represent the final link between basic and clinical research and actions to improve the health of patients. Only successful, effective interventions with benefits outweighing harms are expected to be used in clinical guidelines designed to improve patients' health outcomes (CitationChalmers, Grant, Cottrell, Clutcau, & Fawcet 2000).
Therefore, evidence-based practice guidelines can probably be used as the one of the best means to define the standard of care—i.e., appropriate comparators for a future study.
If these techniques show that there is a clear choice regarding comparator, or on the other hand, a substantial uncertainty or disagreement among experts concerning the merits of a proposed intervention, then the proper choice of a control intervention is affirmed (CitationMann & Djulbegovic, 2003; CitationMann & Djulbegovic, 2004). Under these circumstances it is less likely that the selected comparator would be (intentionally) inferior. The closest formal mechanism we may have when it comes to the choice of a comparator intervention is to ask the question: “Were the investigators uncertain (about treatment alternatives) when they designed the trial?” If the answer to this question is not affirmative comparator bias may be introduced into a design of RCT (CitationMann & Djulbegovic, 2004). In fact, comparator bias directly results from violating the principle that clinical trials should be done only when there is uncertainty about the relative merits of competing treatment alternatives.
In addition, many studies result in inconclusive findings, thus possibly violating the fundamental ethical precept that medical research is justified only if it aims to provide clear answers to research questions (CitationWorld Medical Association, 2000). When I analyzed the reasons behind these inconclusive findings I found that it is ill-informed, optimism or expectation bias—an unwarranted belief in the efficacy of new therapies-that was responsible for the high proportion of inconclusive findings (CitationKumar, Soares, Djulbegovic 2005a; CitationChalmers & Matthews, 2006). The chosen effect size was almost exclusively based on overoptimistic estimates of the effects obtained in Phase II studies, and not on systematic review of the totality of research evidence including cancer registry data, which might have been more helpful in determining the realistic effect size. Therefore, systematic review of the existing evidence can inform both qualitative choice (what treatments to study?) and quantitative aspects of clinical trial design (how much a difference between treatments we may hope to detect?). The concern appears to have been justified that no clinical trial should be done until it is preceded by systematic review of the totality of the existing research evidence (CitationChalmers, 2001).
Effect of Equipoise versus Uncertainty Principle on Generalizibility of Results
Although uncertainty considerations expressed in terms of equipoise versus uncertainty principle relates to the choice of comparator intervention, patients' characteristics do play a role in execution of the trials. Strictly speaking those physicians who accept equipoise as entry criterion are expected to adhere to agreed-upon eligibility criteria. However, the physicians who believe in “uncertainty principle” may be uncomfortable with some of the eligibility criteria, and therefore may be tempted to exclude some of these patients, or to offer treatment to those patients who did not meet eligibility criteria under the “equipoise agreement”. Theoretically at least, the results obtained using these principles as the entry criteria to RCT may have different applicability to non-trial patients. Empirical data shows that when the “uncertainty principle” (i.e., enrolling only those patients for whom there was individual uncertainty), was used as a sole eligibility criterion, an almost perfect balance in the wide range of important patients characteristics was achieved (CitationWarlow, 2002).
A theoretical argument against using “uncertainty principle” as an entry criterion to a clinical trial is that in a situation in which the community of experts are equally divided in favor of one treatment versus the other (i.e., as a group the experts may disagree, but personally they are not uncertain about the choice of treatment), the trial would not be possible under the “uncertainty principle” requirement while such a trial can be conducted under clinical equipoise prerequisite (Shapiro, 2006, personal Communication). I am not aware of any study where equipoise was explicitly used as an entry criterion with participation of many physicians that may have different views, but who nevertheless followed the trial's protocol as agreed upon by the group consensus. Such a trial would be of interest.
We would expect that internal validity remains intact since both equipoise and uncertainty principle relates to a pre-randomization phase of trial, and do not affect the post-randomization phase of the trial.
V. WHEN IS RATIONAL TO RANDOMIZE? A GAME THEORY APPROACH
As outlined above, a fundamental ethical (and scientific) dilemma revolves around seemingly never subsiding tension between societal value of clinical research and individuals volunteering for a research trial from which they may or may not benefit. This tension has never been more apparent than in a case of RCTs. In general, due to its reliability and credibility, society values evidence obtained from RCTs more than evidence generated using other types of clinical research design (CitationCollins & McMahon, 2001). However, the critique has charged that societal benefits is obtained at a price of asking patients to sacrifice for the benefit of future patients since many patients—arguably 50% of them—may not benefit from treatments being tested.
Over the years vociferous arguments have been made supporting one view or another. The debate has, however, crystallized one issue on which all parties agree: in clinical research, and particular one in which employs a RCT design, there is interplay between common and conflicting interest of two “players”—researcher (broadly considered as a representative of society) and patient. If we accept this premise, then viewed from this perspective, the problem of patients' enrollment into RCTs (and advances in therapeutics) can then be formulated in terms of game theory with two “players” (CitationDixit & Skeath, 2004): a patient and a researcher. The central solution of a game theory scenario is finding Nash equilibrium under which each player's strategy is optimal against the others' players' strategies (CitationDixit & Skeath, 2004). In the context of a RCT, the Nash equilibrium represent finding the probability of random allocation at which both researcher and patients are most likely to achieve their strategic goals.
A more complicated and calculation-intensive version of the game theory model is presented elsewhere. Here I will only state the obvious: if one treatment (experimental or standard) is known to be superior, neither patient nor researcher has interest in randomization. Naturally, patients would be expected to request the treatments that are believed to be superior—and researchers would be inclined to offer these treatments—making the use of RCT design both undesirable and impossible. It is only if the results cannot be predicted in advance, and the overall distribution of successes of experimental therapies is about equal to success of standard therapies, that both patient's and researcher's most rational course is to randomize.
Therefore, a key to answer the question regarding rationality of RCTs is to obtain empirical data about treatment success of experimental therapies. We (CitationKumar, Soares, Wells, Clarke, Hozo, Bleyer, Reaman, Chalmers, Djulbegovic, CitationSoares, 2005b) and others (CitationJoffe et al., 2004) have recently evaluated a distribution of treatment successes of innovative, experimental versus standard, conventional therapies in a large series of clinical trials.
shows our summary findings. As can be seen, a distribution of treatment successes follows an almost perfect Bell curve: new treatments tested in randomized controlled trials are, on average, as likely to be inferior as they are to be superior to standard treatments (or, they truly may not differ one from another). The figure illustrates the unpredictability of the results, i.e., the fact that researchers cannot predict in advance what they are going to discover. Data also show no evidence of any autocorrelation over time between success in one trial and success in another, (CitationKumar et al., 2005b), suggesting that each trial represents an independent experiment in a given time with the aim of addressing the uncertainty that existed when the trial was designed.
FIGURE 2 A) The distribution of treatment successes between standard and the experimental treatments in a large series of cancer trials. Data on primary outcomes (survival) shown. Log hazard <0 indicates superiority of experimental treatments and >0 survival advantage for standard treatments. B, Time series analysis of treatment effect (log hazard ratio) of the studies performed in large series of cancer trials. The results confirm to “white noise” pattern with no significant autocorrelation between studies performed at various time intervals indicating that each trial was undertaken to address uncertainty at the time it was designed. Log hazard <0 indicates superiority of experimental treatments and >0 survival advantage for standard treatments.
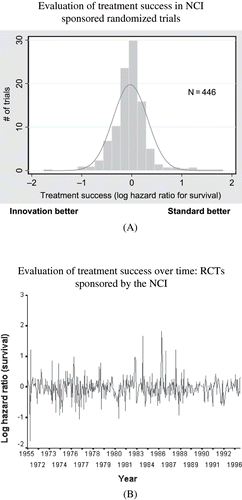
Therefore, if the probability of success of the experimental therapy is equal to standard treatment as shown in , then the most rational course for the patient is to accept, and for the researcher to employ the randomization scheme of allocating experimental and standard treatment at the probability that matches this treatment success distribution (which in our case is to randomize at 50:50% of allocation between new and standard treatments). The patient has the best odds that he/she will receive the beneficial treatment; similarly, the researcher has optimized his/her chances to learn if the treatment he/she proposed to test will turn out to be successful or not. It is important to realize that these odds apply prior to undertaking the trial. Once the trial is completed some patients/researchers will be “winners” and other “losers.” However, this simply cannot be known in advance of the trial- the best we can do is to optimize our chances of “winning,” which as argued here is to randomize.
This is, of course, a normative view and expresses what a rational decision-maker should do faced with the dilemma outlined above. However, what is a descriptive view-at what level of uncertainty would people actually enroll in a RCT? Johnson, Lilford, and Brazier showed that 50% of lay people would approve hypothetical RCT if 70% of experts favor one treatment and 30% favor another (CitationJohnson, Lilford & Brazier, 1991). We found that IRB members would, on average, approve a RCT if 60% favor one treatment over another (CitationDjulbegovic & Bercu, 2002). Interestingly, acceptance of clinical equipoise was crucial to actual patients' consent to randomization in one small study (CitationMills et al., 2003).
In the other study, however, cancer patients preferred the experimental arm despite their consent to enroll in a RCT and their apparent understanding of the purpose of randomization (CitationJoffe et al., 2006). Finally, our examination of research protocols of large cohorts of cancer trials supported by the US National Cancer Institute indicated that investigators are never in theoretical 50:50 equipoise as they almost always hope that new treatments are better.
VI. ACKNOWLEDGEMENT OF UNCERTAINTY DETERMINES A PATTERN OF SUCCESSES IN MEDICINE AND DRIVES CLINICAL DISCOVERIES
It can be further argued that the overall distribution of outcomes comparing new treatments with standard treatments shown in is the key to preserving the clinical trial system and making clinical advances. Without patients' willingness to participate in clinical trials, there would be no way to make new clinical discoveries.
Provided the uncertainty about treatment effects is clearly acknowledged, there is no a priori reason to be cautious about enrolling clinical trials, since new treatments tend to be, on average, neither better nor worse than standard therapies once they have reached the stage of being tested in a RCT. As outlined above, if treatment success could consistently be predicted, patients would be expected to request those successful treatments, making enrollment into clinical trials and randomization impossible. If this were to happen, the entire clinical trial system, as we know it, would come to a halt! Particular treatments may prove to be better or worse than standard treatments but this will only be known after completion of the trial.
This pattern of successes is not an accident. I have previously hypothesized that there a predictable relation between the acknowledgement of uncertainty (the moral principle) on which trials are based and the ultimate outcomes of clinical trials (CitationDjulbegovic, 2001). This initially dubbed “equipoise hypothesis” postulated that a pattern of therapeutic advances is bounded by the uncertainty constraint—that is, if equipoise or uncertainty principle is observed, we would expect, over time, to find an insignificant difference between the proportion of trials that favor experimental treatments and those that favor standard treatments (CitationDjulbegovic, 2001). Indeed, empirical data collected from RCTs so far appear to have corroborated this hypothesis (CitationKumar et al., 2005b; CitationSoares et al., 2005). Ultimately, it is this ethical principle which determines advances in medicine.
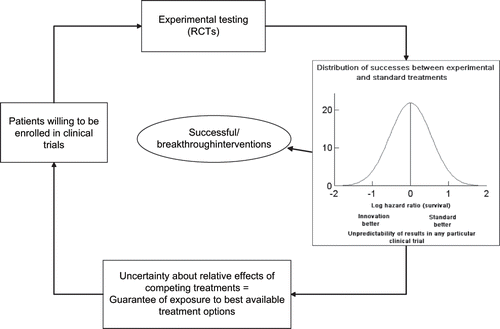
shows a proposed model of clinical discoveries—how the ethical principle converges to become a scientific principle driving treatment progress. To summarize this “law of clinical discoveries,” let me repeat that progress in clinical medicine can only occur because experimental testing has been feasible due to the willingness of patients to take part in experimental clinical trials (CitationWorld Medical Association, 2000). To be enrolled in clinical trials, patients require guarantees that they will not knowingly be harmed and will have an optimal chance of receiving the best available treatments. This guarantee is provided by the acknowledgment of uncertainty, which can be defined in the various ways—notably, as equipoise or “uncertainty principle.” This foundational requirement then serves as a basis for enrollment of patients into experimental trials, resulting in discovery of new successful and breakthrough interventions (CitationKumar et al., 2005b). Unpredictability of the individual results serves as both a driver of advances in medicine and help preserves the clinical trial system.
FIGURE 3 A proposed model of clinical discoveries-how the ethical principle converges to become a scientific principle driving treatment progress. Progress in clinical medicine can only occur because experimental testing has been feasible, due to willingness of patients to take part in experimental clinical trials. To be enrolled in clinical trials, patients require guarantees that they will not knowingly be harmed and will have an optimal chance of receiving the best available treatments. This guarantee is provided by the equipoise or “uncertainty principle,” which serves as a basis for enrolment of patients into experimental trials, resulting in discovery of new successful and breakthrough interventions. Graph insert shows actual distribution of treatment successes in trials conducted by the Children's Oncology Group. §RCT = randomized controlled trial.
One should, however, note that investigators rarely if ever postulate theoretical 50:50 equipoise in advance. While the null-hypothesis contains precise quantitative elements, the investigators' articulation of uncertainties is typically expressed in the qualitative terms. For example, when we examined the backgrounds of research protocols in a large series of cancer trials, it was clear that the researchers were not certain about the effect of the treatment in the trials but were almost always hoping that new treatments would be better than standard treatments (CitationKumar et al., 2005b). It appears, therefore, that
-
empirical data do not support theoretical (a priori), 50:50 equipoise,
-
despite the investigators' prior beliefs in new treatments, they were evidently substantially uncertain that outcomes of trials did in fact reflect perfect (a posteriori) equipoise!
VII. PARADOX OF UNCERTAINTY
The main argument proposed in this article is that ethical and scientific dilemmas relevant to clinical research revolves around the issue of unpredictability of hope-for-benefits and unknown risks of competing treatment alternatives and how we construe our responses to these epistemic uncertainties. I believe that once we have explicitly and transparently articulated the existing uncertainties, we will then be able to mount a proper response to their resolution (CitationDjulbegovic, 2001). The choice of scientific method should match the underlying level of uncertainty and, given the confusion in the field and poor understanding of the concepts among practitioners, my own view is that we should be insistent on articulation of the relevant uncertainties and avoid jargon. A notion of equipoise may be retained in a very narrow and specific meaning of the term when alternatives to be tested are clearly defined and there is belief about “equal” prospect of superiority of one intervention over another which warrants RCTs to settle a dispute.
However, there is a paradox in handling uncertainties, which I want to highlight in my concluding remarks:
As inevitable and unpleasant as many uncertainties are, one can argue that patients (and their doctors) should not even strive to completely eliminate uncertainties. Although the role of scientific method is to reduce uncertainties, a total elimination of uncertainty would be undesirable, since, it has been argued, it would lead to deterministic life—meaning that all events would be known in advance, in turn implying no hope, no ethics, no freedom of choice.
Hence, there is a paradox in dealing with uncertainty—we want to reduce uncertainty, but we do not want to eliminate it totally. Only because we do not know what the future holds can we have our hope and choices. In the context of informing patients about the effects of treatments, this means that the patients' basic right is whether to accept that uncertainty exists (which in practice often means disagreement among their doctors), and the proposed method for resolution of the existing uncertainties (which can include enrollment into a clinical trial as one of the means to resolve uncertainties) (CitationDjulbegovic, 2004).
ACKNOWLEDGMENTS
I wish to thank Howard Mann, Iztok Hozo, Mike Clarke, Stan Shapiro, Alex London, and Sir Iain Chalmers for their important feedback to the ideas expressed in this article. I also want to thank Robert Veatch, Frank Miller, Charles Weijer, Paul Miller, Howard Brody, and Fred Gifford for the stimulating exchange of ideas and the opportunity to read their articles which in turn influenced the final draft of this article. Although they may not necessarily agree with the final content of this text, many discussions I have had with most of them over the years had been invaluable in my own advancement of the ideas expressed herein.
This work was supported by the Research Program on Research Integrity, Office of Research Integrity/National Institute of Health collaboration, grants No: 1 R01 NS044417-01 and 1 R01 NS052956-01.
REFERENCES
- Arrow , K. 1963 . Social Choice and Individual Values , 2nd , New York : Wiley and Yale University Press .
- Ashcroft , R. 1999 . Equipoise, knowledge and ethics in clinical research and practice . Bioethics , 13 : 314 – 326 .
- Chalmers , I. 2001 . “ Using systematic reviews and registers of ongoing trials for scientific and ethical trial design, monitoring, and reporting ” . In Systematic Reviews in Health Care: Meta-Analysis in Context Edited by: Egger , M. , Smith , G. D. and Altman , D. G. 429 – 443 . London British Medical Journal
- Chalmers , I. 2004 . Well informed uncertainties about the effects of treatments . British Medical Journal , 328 ( 7438 ) : 475 – 476 .
- Chalmers , I. , Grant , J. , Cottrell , R. , Cluzeau , F. and Fawcett , G. 2000 . Evaluating “payback” on biomedical research: Biomedical funding decisions should be audited . British Medical Journal , 321 : 566
- Chalmers , I. and Matthews , R. 2006 . What are implications of optimism bias in clinical research? . Lancet , 367 : 449 – 450 .
- Collins , R. and McMahon , S. 2001 . Reliable assessment of the effects of treatment on mortality and major morbidity, I: clinical trials . Lancet , 357 : 373 – 380 .
- Courtney , H. , Kirkland , J. and Viguerie , P. 1999 . Strategy under uncertainty . Harvard Business Review , 75 November : 66 – 79 . 1999
- Daniel , N. and Sabin , J. 1997 . Limits to health care: Fair procedures, democratic deliberations, and the legitimacy problem for insurers . Philosophy and Public Affairs , 26 : 303 – 350 .
- Dixit , A. and Skeath , S. 2004 . Games of Strategy , 2nd , New York : W.W. Norton .
- Djulbegovic, B. (2000, June 12). Non-randomized trials that changed medical practice [On-line] http://www.hsc.usf.edu/∼bdjulbeg/oncology/NON-RCT-practice-change.htm (www.hsc.usf.edu/~bdjulbeg/oncology/NON-RCT-practice-change.htm) (Accessed: 6 March 2007 ).
- Djulbegovic , B. 2001 . Acknowledgment of uncertainty: A fundamental means to ensure scientific and ethical validity in clinical research . Current Oncology Reports , 3 : 389 – 395 .
- Djulbegovic , B. . Principles of research synthesis . ASCO Annual Meeting, (Educational Session) . Edited by: Perry , M. pp. 737 – 750 . Washington, DC : ASCO .
- Djulbegovic , B. 2004 . Well informed uncertainties about the effects of treatment: Paradox exists in dealing with uncertainty . British Medical Journal , 328 ( 7446 ) : 1018
- Djulbegovic , B. and Bercu , B. March 8–10 2002 . “ At what level of collective equipoise does a clinical trial become ethical for the IRB members? ” . In USF Third National Symposium—Bioethical Considerations in Human Subject Research March 8–10 , Clearwater, Florida
- Djulbegovic , B. , Cantor , A. and Clarke , M. 2003 . The importance of the preservation of the ethical principle of equipoise in the design of clinical trials: Relative impact of the methodological quality domains on the treatment effect in randomized controlled trials . Accountability in Research , 10 : 301 – 315 .
- Djulbegovic , B. and Hozo , I. 2002 . At what degree of belief in a research hypothesis is a trial in humans justified? . Journal of Evaluation in Clinical Practice , 8 : 269 – 276 .
- Foster , C. 2001 . The Ethics of Medical Research on Humans , Cambridge : Cambridge University Press .
- Freedman , B. 1987 . Equipoise and the ethics of clinical research . New England Journal of Medicine , 317 : 141 – 145 .
- Fried , C. , ed. 1974 . Medical Experimentation: Personal Integrity and Social Policy , Amsterdam : North Holland .
- Good , I. J. 1967 . On the principle of total evidence . British Journal for the Philosophy of Science , 17 : 319 – 321 .
- GRADE Working Group . 2004 . Grading quality of evidence and strength of recommendations . British Medical Journal , 328 ( 7454 ) : 1490
- Hastie , R. and Dawes , R. M. 2001 . Rational Choice in an Uncertain World , London : Sage Publications, Inc .
- Hill , A. B. 1952 . The clinical trial . New England Journal of Medicine , 247 : 113 – 119 .
- Hill , A. B. 1963 . Medical ethics and controlled trials . British Medical Journal , 2 : 1043 – 1049 .
- Hill , A. B. 1987 . Clinical trials and the acceptance of uncertainty . British Medical Journal , 294 : 1419
- Joffe , S. , Harrington , D. P. , George , S. , Emanuel , E. , Budzinski , L. and Weeks , J. 2004 . Satisfaction of the uncertainty principle in cancer clinical trials: Retrospective cohort analysis . British Medical Journal , 328 ( 7454 ) : 1463 – 1467 .
- Joffe , S. , Stumacher , M. , Clark , J. and Weeks , J. 2006 . Preferences for and expectations about experimental therapy among participants in randomized trials . Journal of Clinical Oncology , 24 ( 18s ) : 304s
- Johnson , N. , Lilford , J. R. and Brazier , W. 1991 . At what level of collective equipoise does a clinical trial become ethical? . Journal of Medical Ethics , 17 : 30 – 34 .
- Kant , I. 1964 . Groundwork of the Metaphysics of Morals , New York : Harper Torchbook . H.J. Paton (Trans.)
- Kumar , A. , Soares , H. P. and Djulbegovie , B. October 22–26 2005a . “ High proportion of high quality trials conducted by the NCI are negative or inconclusive ” . In XIII Cochrane Colloquium October 22–26 , Melbourne, , Australia
- Kumar , A. , Soares , H. P. , Wells , R. , Clarke , M. , Hozo , I. , Bleyer , A. , Reaman , G. , Chalmers , I. and Djulbegovic , B. 2005b . What is the probability that a new treatment for cancer in children will be superior to an established treatment? An observational study of randomised controlled trials conducted by the Children's Oncology Group . British Medical Journal , 331 : 1295 – 1301 .
- Lilford , R. J. and Djulbegovic , B. 2001 . Equipoise and “the uncertainty principle” are not mutually exclusive . British Medical Journal , 322 : 795
- Lilford , R. J. and Jackson , J. 1995 . Equipoise and the ethics of randomization . Journal of the Royal Society of Medicine , 88 : 552 – 559 .
- Mann , H. and Djulbegovic , B. 2003 . Choosing a control intervention for a randomized clinical trial . BMC Medical Research Methodology , 3 : 7
- Mann, H. & Djulbegovic, B. (2004, August 20). Why comparisons must address genuine uncertainties [On-line] www.jameslindlibrary.org/essays/bias/comparator-bias.html (Accessed: 7 March 2007 ).
- Miller , F. G. and Rosenstein , D. L. 2003 . The therapeutic orientation to clinical trials . New England Journal of Medicine , 348 ( 14 ) : 1383 – 1386 .
- Mills , N. , Donovan , J. L. , Smith , M. , Jacoby , A. , Neal , D. E. and Hamdy , F. C. 2003 . Perceptions of equipoise are crucial to trial participation: A qualitative study of men in the ProtecT study . Controlled Clinical Trials , 24 : 272 – 282 .
- Moher , D. , Schultz , K. F. and Altman , D. 2001 . For the CONSORT group. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials . Journal of the American Medical Association , 285 : 1987 – 1991 .
- Peto , R. and Baigent , C. 1998 . Trials: The next 50 years . British Medical Journal , 317 : 1170 – 1171 .
- Rawls , J. 1999 . A Theory of Justice , Revised edition). Cambridge, MA : Harvard University Press .
- Shannon , C. E. and Waever , W. 1962 . The Mathematical Theory of Communication , Urbana : The University of Illinois Press .
- Silverman , W. A. and Chalmers , I. 2001 . Casting and drawing lots: A time-honoured way of dealing with uncertainty and for ensuring fairness . British Medical Journal , 323 : 1467 – 1468 .
- Soares , H. P. , Kumar , A. , Daniels , S. , Swann , S. , Cantor , A. , Hozo , I. , Clark , M. , Serdarevic , F. , Gwede , C. , Trotti , A. and Djulbegovic , B. 2005 . Evaluation of new treatments in radiation oncology: Are they better than standard treatments? . Journal of the American Medical Association , 293 ( 8 ) : 970 – 978 .
- Veatch , R. M. 2002 . Indifference of subjects: An alternative to equipoise in randomized clinical trials . Social Philosophy & Policy , 19 : 295 – 323 .
- Warlow , C. 2002 . Advanced issues in the design and conduct of randomized clinical trials: The bigger the better? . Stat Med , 21 : 2797 – 805 .
- Weijer , C. , Shapiro , S. H. and Cranley , K. 2000 . Clinical equipoise and not the uncertainty principle is the moral underpinning of the randomized trial. For and against . British Medical Journal , 321 : 756 – 758 .
- Welch , H. G. and Mogielnicki , J. 2002 . Presumed benefit: Lessons from the American experience with marrow transplantation for breast cancer . British Medical Journal , 324 ( 7345 ) : 1088 – 1092 .
- Wittgenstein , L. 1969 . On Certainty , New York : Harper Torchbooks .
- World Medical Association . 2000 . World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects . Journal of the American Medical Association , 284 : 3043 – 3045 .