Abstract
Hops were traditionally used in brewing for the addition of isomerized alpha acids that balance the sugary wort with bitterness, though modern breweries are dry-hopping to add complex and unique aromas to their beers. An unintended consequence of high amounts of dry-hopping is the phenomenon known as hop creep, causing an increase in alcohol, carbonation, and diacetyl concentrations. The amount of diacetyl in a beer can be directly correlated to yeast health and vitality, controlled by the amount of free amino nitrogen (FAN) available to the yeast. In this study, both diacetyl and amino acid concentrations were measured prior to and 24 h after dry-hopping, at terminal gravity, and at the start of fermentation. These values were compared across twelve yeasts that are commonly used in dry-hopped beer or offer unique characteristics to fermentation. Experimental yeasts BY881, WLP518, K-97, and CK S102 all exhibited diacetyl concentrations below the aroma threshold for beer. The addition of dry-hops added a significant (p < 0.05) increase in FAN content to the fermenting beers, with most of the increases in serine, arginine, glutamate/glutamine, alanine, and glycine. These increases did not parallel the amino acid content measured in the grist or hops, where asparagine/aspartic acid, proline, and glutamate/glutamine were most prominent. Correlations between total FAN content and diacetyl concentration were calculated, as diacetyl is a precursor in the biosynthesis pathway of the amino acids valine and leucine. However, in this experiment, no correlation of amino acid and diacetyl concentration was observed between dry-hopped and traditional fermentations.
Introduction
Traditionally, hops (Humulus lupulus) are used solely for brewing beer and are primarily added during brewhouse wort production in relatively small quantities. They contribute bitterness to balance the sugary wort, enhance flavor and aroma, enhance foam, provide microbiological stability in the finished beer, and can even increase clarity on the hot side.[Citation1–3] When hops are added to beer outside of the brewhouse, either during active fermentation or when the yeast has reached terminal gravity, it is termed dry-hopping. Dry-hopping with whole cones was performed historically to provide microbial stability in packaging and during transport,[Citation2,Citation4] but more recently, dry-hopping with pellets and advanced hop products is utilized to add intense hop aroma and flavor to finished beer without increasing the bitterness from additional isomerized alpha-acids.[Citation5,Citation6] In craft breweries, dry-hopping has become the standard operating procedure for many styles of beer, but most frequently it is employed in India Pale Ales (IPAs).[Citation6,Citation7] The largest trade group for craft brewers in the USA, the Brewers’ Association (BA), has reported IPAs as the most purchased beer style from their members for more than a decade. With more of these small BA-defined craft breweries operating than ever before, dry-hopping has become ubiquitous.[Citation8,Citation9] Dry-hopping has become so persistent in beer culture that even global brands such as Budweiser[Citation10] and Guinness[Citation11] are producing and advertising dry-hopped beers.
In an all-malt wort typically containing a saccharide content of 60% maltose, 15–20% maltotriose, 5–10% glucose, and less than 5% each of sucrose and fructose, Saccharomyces spp. brewing yeasts produce alcohol, carbon dioxide (CO2), and other desirable fermentation byproducts that contribute to the overall flavor of beer.[Citation12] In the presence of dry-hops, yeast can create unique flavors through a process called biotransformation, where terpenes in the hops are functionally changed to aromatic versions of those chemicals that are more enticing to modern brewers,[Citation13] with the liberation of these glycosylated aromatic compounds being effected by a choice in yeast.[Citation14] The remaining 5–10% of the wort is composed of longer chain oligosaccharides termed dextrins; these dextrins are typically deemed unfermentable by standard Saccharomyces yeasts.[Citation15–17] There is evidence that hops carry starch degrading α- and β-amylase enzymes that functionally change this saccharide composition in beer during the dry-hopping process.[Citation4,Citation18–20] These enzymes contribute to the hydrolysis of the aforementioned unfermentable dextrins into fermentable sugars.[Citation21–23] Other recent research suggests the enzymes responsible for this hydrolysis may not come from the plant material, but potentially from microbial growth on the hop cones,[Citation24] and a negative correlation was found between mildew fungicide application amount and enzymatic potential in some hop cultivars.[Citation25] In the presence of viable brewer’s yeast, the newly available fermentable sugars can lead to additional fermentation. This over-attenuation of the beer in presence of dry-hops is commonly referred to by brewers as the “freshening power of hops”[Citation4,Citation18,Citation21] or “hop creep.”[Citation19,Citation20,Citation26–29]
Hop creep can result in increased ethanol, higher CO2, and lower residual sugar contents if yeast is not removed with pasteurization or filtration before packaging. This can come with regulatory consequences from the Tax and Trade Bureau (TTB) in the United States, where a tolerance of only 0.3% above or below the alcohol content stated on the label exists.[Citation30] As is common in most craft breweries, unfiltered beer is packaged with residual yeast, creating additional CO2 from hop creep as well. When hop creep occurs and residual dextrins are hydrolyzed by hop enzymes, the remaining yeast causes over-attenuation, which in turn causes over-carbonation in the container. This can be a dangerous and frightening safety concern for the consumers of bottles or cans of this dry-hopped beer. Twelve-ounce glass, industry-standard bottles (ISBs), suggests no greater than three volumes of CO2 (6 g/L). Typical carbonation levels in beer fall around 2.5 volumes, or 5 g/L of CO2. An additional 0.1°P of fermentable sugar correlates to enough fermentation to approach the pressure limit of an ISB.[Citation15]
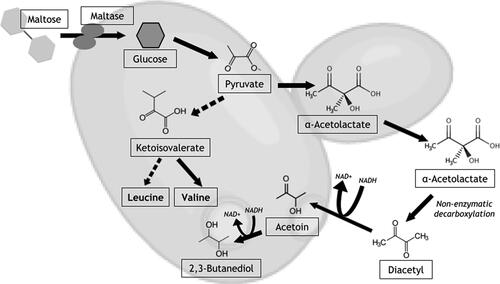
Additionally, when beers are dry-hopped after terminal gravity, hop creep can cause yeast to leave dormancy, as there are now more fermentable sugars available. Once fermentation is restarted, the yeast now have low vitality, yielding higher amounts of yeast-related off-flavors such as diacetyl and acetaldehyde.[Citation31–33] These off-flavors not only create a variation in consistency and quality for breweries and their consumers but can also extend the cellar schedule needed for beer production as diacetyl reduces over time. Diacetyl in particular, a flavor typically described as buttered popcorn or butterscotch, is formed as an intermediate in the anabolism of the amino acids valine and leucine during the anaerobic pathway of fermentation ().[Citation33] In Saccharomyces spp., pyruvate is converted to α-acetolactate before it is spontaneously decarboxylated into diacetyl. As the cell needs more energy, diacetyl is reduced to acetoin and finally 2,3-butanediol, with the anaerobic production of ATP by way of NADH.[Citation34] Yeasts of low vitality are no longer producing energy, and thus are no longer reducing diacetyl to 2,3-butanediol, a byproduct of fermentation that requires a much greater concentration than diacetyl before aroma threshold is reached.[Citation35]
Figure 1. Formation and subsequent reduction of diacetyl during the anaerobic pathway of fermentation. Pyruvate from glucose is also the substrate for the biosynthesis of the amino acids valine and leucine. Dotted arrows signify several intermediate steps in this pathway.
As seen in , the metabolism of nitrogen compounds plays an important role in fermentation. Brewers’ yeasts require nitrogen to synthesize new enzymes and structural proteins during cell growth, mainly from amino acids, ammonium, and shorter peptides.[Citation36,Citation37] The amino acids needed, however, are rarely used for the express purpose of the biosynthesis of proteins and other macromolecules in Saccharomyces spp. More often, they are catabolized from other compounds in wort, and any required amino acids for cell growth are synthesized from catabolic intermediates. Brewers measure available amino acid concentration in wort as free amino nitrogen (FAN), and 150 mg/L is often cited as required for a complete fermentation,[Citation1,Citation38] though successful fermentations have been performed with a wort FAN content as low as 51 mg/L.[Citation39] Insufficient wort FAN content may cause slow fermentations, low attenuation, and low yeast vitality, leading to previously mentioned off-flavors, such as diacetyl.[Citation34] Increased concentrations of FAN in the wort allow additional amino acids for cell protein synthesis, creating increased yeast growth rates, which in turn increases the rate of fermentation and ethanol production in the glycolytic pathway as no products are needed as carbon skeletons for amino acid synthesis. A byproduct of this increased fermentation rate can be the formation of higher fusel alcohols, as many are formed from intermediates in amino acid catabolic pathways.[Citation40]
The extended fermentation and resultant additional alcohol produced from hop creep can be mitigated through recipe changes in the brewhouse, or the arresting of fermentation and clarification to remove yeast for cessation of the production of alcohol. However, even with these procedures, many brewers still report an increase in diacetyl content, as the yeast has not been allowed the time needed to convert diacetyl to acetoin.[Citation12] Previous research from Bruner et. al[Citation26] has analyzed the fermentation characteristics of different Saccharomyces species in the presence and absence of dry-hops. While this research related yeast flocculation to hop creep and examined the effect of dry hopping on wort pH, no biochemical analyses were performed on the resultant beers.
In the present study, specific Saccharomyces species and strains were investigated that may have an ability to mitigate the diacetyl production associated with dry-hop creep, as this would be of great value to the commercial brewing industry. Amino acid contents were measured in the hops, grist bill, and resultant beers during fermentation to determine how FAN changes with the addition of dry-hops. Yeast health during fermentation has been directly related to FAN content, and the production of diacetyl from hop creep can be associated with yeast health. Therefore, this research aims to evaluate the correlation between diacetyl formation and the change in the concentrations of amino acids during the fermentation of beer with the addition of dry-hops. With a better understanding of the relationship between FAN content, diacetyl, and yeast selection, brewers can gain insight into dry-hop creep at an advanced level.
Experimental
Yeast selection and propagation
Twelve Saccharomyces yeasts () were chosen based on either their unique characteristics or ubiquitous use in dry-hopped beer. Four Saccharomyces cerevisiae and one S. cerevisiae var. diastaticus from Fermentis LeSaffre (Marcq-en-Baroeul, France, EU; fermentis.com/en/) were provided as active dry yeast in Mylar sachets with a sorbitan monostearate emulsifier (E491) and stored at 4 °C until use. One S. cerevisiae yeast from Berkeley Yeast (Oakland, CA, USA; berkeleyyeast.com) was provided on a freshly streaked peptone dextrose agar plate and stored at room temperature until propagation. Four S. cerevisiae and one S. bayanus from White Labs (San Diego, CA, USA; www.whitelabs.com) were provided in 35 mL PurePitch™ packages and stored at 4 °C until propagation. One S. cerevisiae yeast from the UC Davis Phaff Yeast Culture Collection (Davis, CA, USA; phaffcollection.ucdavis.edu) was revived from cryogenic storage and streaked onto a potato dextrose agar plate and incubated for 2 days at 30 °C then moved to room temperature storage until propagation. SafAle™ US-05 was used to perform triplicate paired fermentations from three separate brews for quality control purposes.
Table 1. Twelve yeasts used in the fermentations of experimental beer, reported in alphabetical order.
The same procedure was utilized to propagate all yeasts, regardless of source, in order to ensure consistency throughout this study. Yeasts were grown in 5.2 pH wort consisting of 10% w/v (10.0°P, 1.040 specific gravity) dry pilsner malt extract (Briess CBW® Pilsen Light; Chilton, WI, USA) hydrated in deionized water with 20 mg/L calcium chloride brewing salts and 0.10% w/v yeast nutrients (Kerry Yeastex® 82; Beloit, WI, USA). Propagation wort was boiled for 10 min, sterilized via autoclave, and finally sterile filtered to remove precipitated protein and trub. Yeast colonies were transferred from plate or package via sterile inoculation loop to propagation wort in a laminar flow hood. Yeasts were then propagated stepwise over the course of 11 days following the methods outlined in and as described in previous research.[Citation26,Citation41] All propagations were performed at room temperature on a platform orbital shaker (Innova™ 2000, New Brunswick Scientific; Edison, NJ, USA) set to 150 rpm. Yeast cell counts with methylene blue viability staining on all propagations and fermentations were performed following standard methods.[Citation42]
Figure 2. Yeast propagation schematic following previous methods.[Citation26] Yeasts were propagated to a final approximate total of 40.0 × 1010 cells in each bottle with a total of 390 mL of propagation wort, equivalent to the standard ale pitch rate of 1.0 × 106 cells per mL per °P[Citation12] for each 40 L, 10°P pilot fermentation. Figure created on BioRender.com, not to scale.
![Figure 2. Yeast propagation schematic following previous methods.[Citation26] Yeasts were propagated to a final approximate total of 40.0 × 1010 cells in each bottle with a total of 390 mL of propagation wort, equivalent to the standard ale pitch rate of 1.0 × 106 cells per mL per °P[Citation12] for each 40 L, 10°P pilot fermentation. Figure created on BioRender.com, not to scale.](/cms/asset/3440e67c-a29c-4477-bd70-82ebf4a8c36f/ujbc_a_2078946_f0002_c.jpg)
The twelve yeasts selected for analysis () included six standard brewing strains that are ubiquitous in industry, including two suppliers’ versions of the “Chico” American ale (UCDFST 96-12 and US-05), two yeasts commonly used in hazy and juicy IPAs (WLP066 and WLP095), a very highly flocculent English ale yeast (WLP002), and a German ale yeast typically used in Kölsch-style beers (K-97). The study also measured several non-conventional strains for the fermentation of beer, including a strain typically used for bourbon fermentation (USW-6), a wine strain used in aromatic whites and rosés (CK S102), a S. bayanus strain used in German Weissbier (WLP351), a Norwegian Kveik yeast (WLP518), a Belgian S. cerevisiae var. diastaticus strain (BE-134), and a genetically modified yeast that has an alpha-acetolactate decarboxylase (ALDC) gene inserted capable of preventing the formation of diacetyl by breaking down the precursor, alpha acetolactate (BY881).
Pilot scale wort production
Seven identical pilot scale brews were performed using the 1.8 hL Anheuser-Busch Research Pilot Brewery at the University of California, Davis, with an all-malt recipe and conditions chosen to mimic those of a typical production environment in an American craft brewery. Methods and brewing parameters, such as the grist bill, hops, water chemistry, mashing regime, pH, boil duration, whirlpool duration, and knockout temperatures followed those of previous research.[Citation26] The experimental beer recipe attempted to emulate an American Pale Ale or Session IPA, with targets of 10°P original gravity and 20 IBU from Centennial (8.3% AA, Hopsteiner, New York, NY), to produce a targeted 4.0% (v/v) alcohol beer when fermented under standard ale parameters. To allow each 1.8 hL brew to be used for multiple distinct fermentations, all brews were split evenly by volume between four 56 L fermenters with 40 L of cooled wort.
Pilot scale fermentations
To each of the 40 L pilot scale fermenters, 1.0 × 106 yeast cells per mL per °P were pitched at a standard ale temperature of 20 °C, which was held for the duration of the fermentation. Each yeast () was added to fermenters in duplicate pairs, with the S. cerevisiae US-05 pair fermented in biological triplicate for quality assurance, totaling 28 distinct fermentations. One fermenter in each pair was dry-hopped with 10 g/L Centennial (8.3% AA; Hopsteiner; New York, NY, USA) T-90 hop pellets when extract decreased to below 4.0°P, or at 7 days into fermentation, whichever occurred first. The second of the paired fermenter was allowed to continue fermenting without an addition of dry-hops. Yeast was not removed from fermenters prior to dry-hop in order to allow for maximum cell count and contact time while the beer was fermenting. End of fermentation was determined with an observed change in gravity of less than 0.10°P for 2 consecutive days following dry-hop, and is referred to here as “terminal gravity.”[Citation15]
Wort and beer sample collection/preparation
Beer was sampled daily from the fermenters using aseptic techniques within a 2 h window of the time of knockout transfer of wort to fermenter, and analyzed for gravity and other fermentation parameters as reported previously.[Citation26] For analysis of diacetyl content, additional samples were taken at three sample points during the fermentation: directly prior to dry-hopping, 24 h post dry-hop, and at terminal gravity. For amino acid analysis, samples were taken at all of these points, with the addition one sample at the start fermentation prior to yeast pitch. For each of these samples, 50 mL of wort or beer was centrifuged at 20 °C and 3,000 × g for 5 min in a sterile conical tube. The supernatant was decanted and degassed for 5 min using the degas setting on a VWR B1500A-DTH 1.90 L ultrasonic cleaner (VWR International; Radnor, PA, USA). For diacetyl analysis, 3.5 mL of the degassed sample was decanted into a 5.0 mL centrifuge tube containing 1.5 mL of a 1 M potassium phosphate buffer at pH 7.5 and held at −20 °C until delivery to Berkeley Yeast (Oakland, CA, USA) for analysis. For amino acid analysis, 0.8 mL of the degassed sample was decanted into a 1.0 mL centrifuge tube and stored at −20 °C until delivery to the UC Davis Molecular Structure Facility (Davis, CA, USA) for analysis.
Diacetyl analysis
Diacetyl content was determined using an Agilent 6890 N Network Gas Chromatograph (GC) coupled to a 5973 N transmission (single quadrupole) Mass Selective (MS) detector with electron ionization (Agilent Scientific; Santa Clara, CA, USA). Samples were incubated at 80 °C for 10 min, then cooled to room temperature and derivatized using 0.5 mL 20 mM 4,5-dichloro-o-phenylenediamine (DOP) in 1 M HCL (3.54 mg/mL). The samples were then incubated again at 80 °C for 5 min, cooled to room temperature, and extracted in 1.5 mL toluene. Samples were then injected into the GC/MS, where diacetyl was quantified against known standards and reported in mg/L of the original sample.[Citation43,Citation44]
Amino acid analysis
Amino acid composition was determined using an L-8800a (Hitachi High-Tech America; Santa Clara, CA, USA) amino acid analyzer at the Molecular Structure Facility at UC Davis. The analyzer utilizes ion-exchange chromatography to separate amino acids followed by a post-column ninhydrin reaction detection system,[Citation45] according to standard procedure outlined by the European Brewing Convention.[Citation46] For the wort or beer samples, 200 µL was diluted with 50 µL of 10% w/v aqueous solution of salicylic acid, frozen overnight, thawed, vortexed, then centrifuged and diluted with a norleucine standard in sodium citrate buffer before injection into the analyzer. The resultant molar concentrations of the individual amino acids were then converted to FAN (free amino nitrogen) by multiplying each by the molar mass of nitrogen, and it is reported in mg/L of the original sample for ease of comparison to previous literature. However, this is with the exception of proline, where the value reported here represents direct amino acid concentration, as the alpha-amino nitrogen in the proline molecule is bound in a ring structure, and thus is not typically freely available to Saccharomyces spp. as “FAN.” Malt and hop samples were hydrolyzed in both base (4.5 N NaOH at 110 °C) then acid (6 N HCl at 110 °C) for 24 h each, then diluted in a lithium citrate buffer before injection. Molar concentrations of the individual amino acids were then used to calculate the proportional mass of each by dry weight in both the hops and malt. Lithium citrate buffer allowed for the quantification of the amino acids cysteine and tryptophan in the solid malt and hop samples. Sodium citrate buffer was used in the beer samples for cost and time saving considerations with the volume of samples, but it is not as sensitive, thus the beer samples do not have measured values for cysteine and tryptophan.
Statistical analysis
Standard deviation for amino acid and FAN values, correlation with linear regression (R2 and Pearson’s) values for diacetyl and amino acid concentration, as well as one-tailed statistical analysis (t-test) and corresponding p value were performed in Microsoft® Excel 2019, Version 2102 (Build 13801.20360).
Results and discussion
Diacetyl analysis by yeast
The BY881 strain performed as expected, keeping the concentration of diacetyl below the published aroma threshold[Citation34] for the entirety of fermentation in both dry-hopped and standard fermentations due to its inclusion of the ALDC gene (). The K-97 Kölsch ale strain, the CK S102 Sauvignon Blanc strain, and the WLP518 Kveik strain all produced very low relative levels of diacetyl yet were above aroma threshold at some point prior to terminal gravity. Using the fermentation parameters of this study, the German ale K-97 strain only had a diacetyl concentration above aroma threshold on the dry-hopped sample 24 h post dry-hop. The Opshaug Kveik yeast WLP518 only produced diacetyl above the aroma threshold in the sample prior to dry-hop in this study, and no increase in diacetyl was measured following the addition of dry-hops. The CK S102 wine strain fermentation was below aroma threshold at terminal gravity in both dry-hopped and traditional treatments, with no observed increase in diacetyl concentration from the addition of dry-hops. Additional research should be performed with this yeast from a beer brewing perspective, as there is no known use of it in beer outside of this study. A clear increase in diacetyl concentration was seen in 7 of the 12 yeasts following the addition of dry-hops.
Figure 3. Diacetyl content in fermenting beer of the dry-hopped (W) and non-hopped (N) treatments on the day of dry-hopping, 24 h after dry-hopping, and at terminal gravity, as measured via GC/MS and reported in ppm (mg/L). n = 1 for each yeast pair represented here. Red line indicates the aroma threshold for diacetyl in beer at 0.1 ppm as reported previously.[Citation1]
![Figure 3. Diacetyl content in fermenting beer of the dry-hopped (W) and non-hopped (N) treatments on the day of dry-hopping, 24 h after dry-hopping, and at terminal gravity, as measured via GC/MS and reported in ppm (mg/L). n = 1 for each yeast pair represented here. Red line indicates the aroma threshold for diacetyl in beer at 0.1 ppm as reported previously.[Citation1]](/cms/asset/61afe2f5-c171-4f20-9a53-c1d40dcd2a36/ujbc_a_2078946_f0003_c.jpg)
Diacetyl concentration was expected to be similar prior to the addition of dry-hops in both fermentations of each yeast pair, but significant variability was observed with the BE-134 and USW-6 strains. While dry-hop procedures were performed when each specific fermenter reached the pre-determined gravity or time, the fermenter without dry-hops was not at the same gravity due to variable fermentation kinetics between tanks. Beer was still sampled from the same day on both the pre- and post-dry-hop samples notwithstanding if the non-dry-hopped was at the same gravity, then analyzed for both fermentation treatments. Additional brewing trials in tandem with analysis should be performed on all yeasts to determine statistical significance of the results reported in this study as the sample size was only one brew per yeast here.
Amino acid analysis
Grist and hop analysis
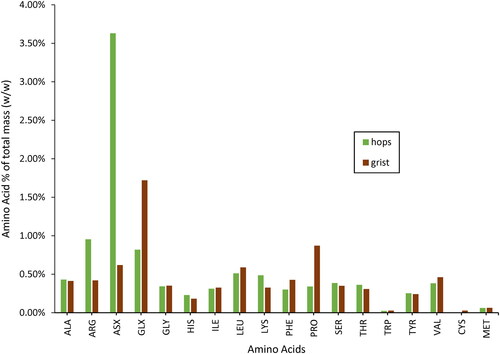
The amino acid content of the grist bill (malt blend) and hops used were also measured to estimate the potential contributions from each ingredient. The hops were measured to contain asparagine/aspartic acid in the highest relative concentration (), with arginine as the second highest measured amino acid. This composition does not appear to solubilize uniformly as free amino acids in the green beer, as the greatest increases from dry-hopping were seen in serine, arginine, and glutamate/glutamine (). All of these were greater than 0.5% of the total mass (%v/v, dry basis) of the Centennial hops measured here, well above previously reported (0.1%) total amino acid content of hops,[Citation47,Citation48] but in line with more recent research using similar quantification methods.[Citation49] Proline and glutamate/glutamine were the highest relative concentrations of amino acids in the grist (), which did translate to the wort, where proline was the amino acid with the highest concentration.
Figure 4. Amino acid content as percentage of total mass (% w/w) dry basis in the Centennial hops and malt blend (grist) used for this study. ASX and GLX are a combination measurement of asparagine/aspartic acid and glutamate/glutamine, respectively, and accounts for their much higher relative concentrations.
Total FAN
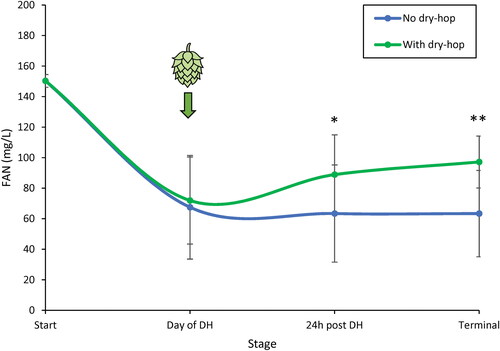
A clear downward trend of total FAN content was observed in all fermentations until the time at which time beers were deemed ready for dry-hopping based on pre-determined gravity conditions (). At 24 h following the addition of dry-hops, a marked increase in the total FAN content was observed for all yeast treatments, which was significantly different (p < 0.05) than the continued downward trend that was observed in the non-hopped treatments. The increased FAN content was maintained at higher concentration over the non-hopped treatments even at terminal gravity (p < 0.01). Whether this increase in FAN concentration is due to the direct contribution of amino acids from the dry-hop addition, yeast biosynthesis, protein hydrolysis, or some combination of these, has yet to be elucidated.
Figure 5. Mean FAN concentration in fermenting beer of all dry-hopped (green) and non-hopped (blue) treatments measured on the day of dry-hopping, 24 h after dry-hopping, and at terminal gravity. Mean shown as all twelve yeasts outlined above, including a biological triplicate of US-05, with total sample size of n = 14. Amino acid concentrations were measured via ion exchange chromatography, converted to FAN (excluding proline), and reported in mg/L. * designates p-value <0.05 and ** designates p-value <0.01.
Individual amino acids
This increase in FAN following dry-hopping was primarily due to increases in serine, arginine, glutamate/glutamine, and alanine (). Amino acid biosynthesis in S. cerevisiae has been noted for all branched chain amino acids,[Citation50] with genetic pathways for anabolism being well described in arginine, leucine, lysine, isoleucine, valine, glutamine, and histidine.[Citation51] Therefore, the increases in serine and glutamine may be linked to known biosynthesis pathways in S. cerevisiae, where serine is anabolized both in the phosphoglycerate pathway of glycolysis and the glyoxylate pathway of the tricarboxylic acid-cycle.[Citation52] Glutamine anabolism is also well-documented in its role to aid ammonia assimilation in the production of NAD+.[Citation53] The intermediates of these pathways should be measured during fermentation with dry-hops in future research to determine whether the observed increases in these amino acids are in fact a result of yeast biosynthesis.
Figure 6. Mean FAN concentration of each individual amino acid for all fermenting beers measured on the day of dry-hopping, 24 h after dry-hopping, and at terminal gravity, and separated by Saccharomyces spp. assimilation classes. Amino acid concentrations were measured via ion exchange chromatography, converted to FAN, and reported in mg/L. Note that proline is the only amino acid in Class D, and the scale for concentration is much higher than that of all other amino acids, with 50 mg/L as the maximum. This value does not represent FAN, though, as the alpha-amino nitrogen in the proline molecule is bound in a ring structure, and thus is not typically able to be assimilated by Saccharomyces spp. Left column is non-hopped and right column is dry-hopped.
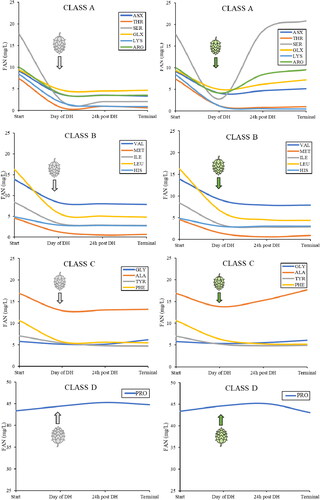
Biosynthesis may be the cause of the increase in alanine observed in this study, as previous research showed alanine catabolism during respiration in S. cerevisiae, but has yet to elucidate the alternate pathway for alanine synthesis during fermentation.[Citation54] A slight increase in glycine was also observed at terminal gravity in the non-hopped treatment of the yeast. This increase may be either due to glycine’s role as an intermediate product of an amino acid biosynthesis pathway in Saccharomyces,[Citation55,Citation56] or because of interference with glycerol in the reading of ion exchange chromatography. Slight increases in threonine and serine were also observed in the middle of non-hopped fermentation before trending downward, again likely due to biosynthesis during glycolysis, as all amino acids used in protein synthesis can be synthesized with the appropriate carbon and ammonium source.[Citation57,Citation58] Cystine and tryptophan were not measured in beer samples due to cost and time considerations, but their levels in the solid malt and hop samples were close to negligible ().
Analysis by yeast
It was readily apparent that some of the yeast strains used in this study decreased FAN more readily than others (). Some fermentations did not have amino acid analysis performed on the wort prior to yeast pitching, but the starting FAN concentrations of those measured were consistent at 152 +/− 3.9 mg/L. In this experiment, an increase in FAN was measured with the addition of dry-hops to all fermentations, except for the S. cerevisiae var. diastaticus strain BE-134. Previous research has shown that BE-134 ferments with such vigor that it has the capacity to sidestep the hop creep phenomenon,[Citation26] and its assimilation of FAN might offer further insight into this observation. The S. cerevisiae var. diastaticus BE-134 utilized nearly all the free amino nitrogen, to below 10 mg/L in the non-hopped treatment (). The proficiency of BE-134 is expected due to its ability to hydrolyze dextrins as a result of its STA-1 gene, an extracellular glucoamylase gene.[Citation59,Citation60]
Figure 7. Total FAN concentration in fermenting beer for each yeast treatment of the non-hopped (a) and dry-hopped (b) treatments, measured on the day of dry-hopping, 24 h after dry-hopping, and at terminal gravity. Amino acid concentrations were measured via ion exchange chromatography, converted to FAN (excluding proline), and reported in mg/L. n = 1 for each yeast pair represented here.
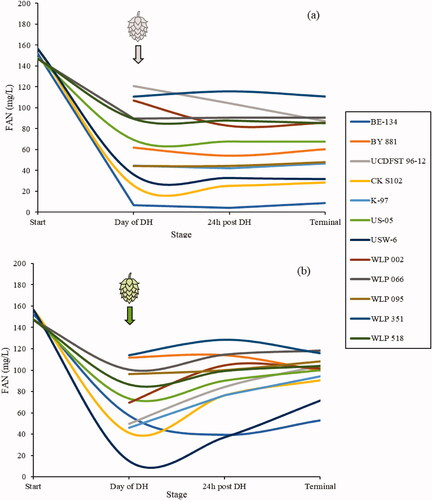
All dry-hopped fermentations finished with a higher FAN content when compared to its non-hoppped treatment pair. Additionally, fermentations with all but three yeasts—BY881, WLP002, and WLP351—showed a continuous increase in FAN to the end of fermentation, with higher values observed at terminal than at 24 h post-dry-hop (). Both of these observations may explain the reason hop creep causes continued fermentation for up to 30 days,[Citation19] even when beers would be deemed terminal, as FAN continues to rise while beer is exposed to the hops. Both the CK S102 Sauvignon Blanc and USW 06 American Whiskey strains assimilated FAN readily in both the non-hopped and dry-hopped fermentations, dropping the concentration more than 100 mg/L from the start of fermentation to the day of dry-hopping. Conversely, the S. bayanus strain WLP351 appeared to assimilate nitrogen poorly in both fermentations, with a pre-dry-hop measurement above 110 mg/L in both treatments.
The WLP066 London Fog strain, used by many brewers in the highly dry-hopped beers that show hop creep, did not efficiently assimilate amino acids, and FAN remained above 88 mg/L in the non-dry-hopped treatment and above 101 mg/L in the dry-hopped treatment at all points measured in this study. Burlington Ale WLP095 and German Ale K-97 both utilized FAN in an almost identical manner without the addition of dry-hops, though they diverged with dry-hopping, with a much greater increase in FAN in the K-97 fermentation (). The FAN requirements of yeast are known to vary by strain and species,[Citation38] and this study further elucidated this point. As stated previously, additional brewing trials in tandem with analysis should be performed on all yeasts to determine statistical significance of the results reported in this study as sample size was only one brew per yeast. Much research remains on how dry-hops affect the assimilation of nitrogen during fermentation and the connection of amino acid content to dry-hop creep.
Diacetyl and amino acid content correlation
For each of the twelve yeasts, the difference of total FAN content between the dry-hop treatments at 24 h post-dry-hop was compared to the difference in diacetyl concentration between dry-hop treatments at terminal gravity. Linear regression of these two values was performed and both the Pearson’s correlation coefficient (R = 0.402) and the measure of fit (R2 = 0.161) indicate very low correlation between the FAN after dry hopping and diacetyl concentration at terminal gravity for the beers in this study. The increase in concentration of each individual amino acid from prior to and 24 h following the addition of dry-hops was also correlated to the diacetyl concentration at terminal gravity. Pearson’s correlation showed only three amino acid concentrations to have a strong relationship with the final diacetyl quantity, with arginine (R = 0.76) exhibiting the highest correlation, followed by histidine (R = 0.65), and glutamate/glutamine (R = 0.54).
A provisional link between diacetyl and FAN concentration was observed in this study, when the WLP095 Burlington ale and K-97 German ale strains were more closely examined. In this study, both WLP095 and K-97 utilized FAN in a similar fashion without dry-hops (), but WLP095 continued to utilize FAN after the addition of dry-hops while K-97 did not, noted by an increase in FAN concentration with K-97 following dry-hop. The use of the WLP095 strain by brewers in highly dry-hopped beers that exhibit increased diacetyl from hop creep may be a result of its continued assimilation of FAN. Additionally, WLP095 produced more diacetyl in this study than K-97 (); from these data presented here, it may be inferred that the link between amino acid assimilation and diacetyl exists. Additional brewing trials with both of these yeasts coupled with amino acid and diacetyl analysis should be conducted to determine the extent of this relationship.
Previous research has found that the addition of exogenous lysine increased the final concetration of diacetyl in bench-top fermentations with S. pastorianus lager yeast,[Citation61] but the increase was also linked to an increase of cell biomass during fermentation. Diacetyl has also been shown to be intimately linked to the production of leucine and valine in the Saccharomyces yeast cell.[Citation33] None of these three amino acids—lysine, valine, or leucine—were observed to increase in wort with the addition of dry-hops in this study (), and thus here cannot be correlated to an increase in diacetyl. The amino acid arginine in relation to hop creep should be explored further due to its correlation to diacetyl production in this study, a link found in previous research,[Citation62] and its high content in the seeds of most plants.[Citation63] Preliminary discussions with other researchers may point to seed content being a determinant of diastatic potential in hops, with published results from 80 years past showing similar trends.[Citation21] The link between diacetyl production in beer and arginine concentration should be further explored, as well as the apparent lack of the link to diacetyl and currently known amino acid precursors, lysine, valine, and leucine, which was observed in this research.
Conclusions
The fermentation of beer in the presence of dry-hops has yet to be studied fully, with little data regarding the biochemical pathways of diacetyl formation[Citation23,Citation64] or the assimilation of amino acids[Citation49] in dry-hopped beer. Additionally, no previous data existed on the variability of Saccharomyces strains and species in their production of diacetyl or utilization of FAN in the presence of dry-hops until the publication of this research. Hop creep is not a new phenomenon, but the depth of research on why it happens and its implications for brewers are still being elucidated. In this study, a marked increase in diacetyl was observed with the addition of hops during fermentation in more than half of the yeasts used. BY881, a genetically engineered strain of S. cerevisiae that has the ALDC gene built in, effectively mitigated an increase in diacetyl, as expected. The use of genetically engineered yeasts with the alpha-acetolactate decarboxylase gene inserted, or exogenous ALDC enzymes, offers brewers effective means of mitigation if diacetyl is the primary concern resulting from hop creep. The WLP518 Kveik strain, CK S102 Sauvignon Blanc strain, and K-97 German ale strain all showed promise here for use in dry-hopped beer, as they all reached terminal gravity with diacetyl concentrations below the designated aroma threshold. Further brewing trials should be performed to analyze the extent to which these yeasts can help mitigate some of the deleterious effects of hop creep; some have begun at production levels with select commercial brewers, but more research scale analysis remains. This research provides an initial insight into the variability of diacetyl production of different yeasts in relation to hop creep, though research remains on these and other yeasts.
The addition of dry-hops added a significant increase in measured FAN content to the fermenting beers in this study, with the most prominent increases observed in serine, arginine, glutamate/glutamine, and alanine. This increase did not parallel the amino acid content measured in the grist or hops used here, where asparagine/aspartic acid, proline, and glutamate/glutamine were most prominent. Thus, it may be possible that many of these measured increases in the dry-hopped beer are byproducts of biosynthesis pathways of anaerobic fermentation in Saccharomyces, but the compositions of solely free amino acids in the malt and hops used here are unknown. This release of FAN into the beer needs to be further tracked and reviewed as it relates to dry-hopped beer, as this information may be crucial in the understanding of hop creep. At terminal gravity, all ferementatons with dry-hops added finished with a higher FAN content than those without. Additionally, in all dry-hopped ferementations, a continued increase in FAN content was observed until until the end of fermentation. At the end of fermentation, higher FAN was observed in all dry-hopped treatments than at 24 h after dry-hop, with the exception of BY881, WLP002, and WLP351. This may elucidate one reason hop creep causes fermentations to continue for over 30 days after the addition of dry-hops, as amino acid content continues to increase as they are released from the dry-hops, allowing viable yeast additional nutrients to complete fermentation. Linear regression of the difference between terminal gravity and measurements of diacetyl and FAN prior to dry-hop resulted in very low correlation for the fermentations in this study, so no concrete link between diacetyl concentration and amino acid presence was determined herein. Further analysis of amino acid content during fermentation in dry-hopped beers is required.
Authors’ contributions
J.B. conceived this study, performed the bulk of the research, gathered and transcribed data, and wrote the original manuscript. A.M. assisted in the brewing of beer and brew day sample collection, data curation with figure manipulation and statistics, and assisted with the final editing of the manuscript. G.F. supervised the work, offered insight, and assisted with final editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
Much gratitude to Anne Flesch and Kevin Lane of Fermentis, as well as Kyria Boundy-Mills and Irnayuli Sitepu from the UC Davis Phaff Collection for advice in yeast selection and revival of cryogenically stored yeast. Appreciation to Nick Harris of Berkeley Yeast for helping run diacetyl measurements on the resultant beers, as well as John Schulze at the Molecular Structure Facility of the University of California, Davis, for helping to run amino acid analysis.
Disclosure statement
The authors declare no conflict of interest as all research was performed and funded while the authors were students or professors at University of California, Davis in Davis, CA, USA.
Additional information
Funding
Literature cited
- Kunze, W. Technology Brewing & Malting; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei in Berlin (VLB): Berlin, Germany, 2019.
- Moritz, E. R.; Morris, G. H. A Text-Book of the Science of Brewing; Spon: London, 1891.
- Schönberger, C.; Kostelecky, T. 125th Anniversary Review: The Role of Hops in Brewing. J. Inst. Brew. 2011, 117, 259−267. DOI: 10.1002/j.2050-0416.2011.tb00471.x.
- Brown, H.; Morris, G. On Certain Functions of Hops Used in the Dry-Hopping of Beers. Trans. Inst. Brew. 1893, 6, 94–106.
- LaFontaine, S. R.; Shellhammer, T. H. How Hoppy Beer Production Has Redefined Hop Quality and a Discussion of Agricultural and Processing Strategies to Promote It. Tech. Q. Master Brew. Assoc. Am. 2019, 56, 1–12. DOI: 10.1094/TQ-56-1-0221-01.
- Lafontaine, S. R.; Shellhammer, T. H. Investigating the Factors Impacting Aroma, Flavor, and Stability in Dry-Hopped Beers. Tech. Q. Master Brew. Assoc. Am. 2019, 56, 13–23. DOI: 10.1094/TQ-56-1-0225-01.
- Hauser, D. G.; Simaeys, K. R. V.; Lafontaine, S. R.; Shellhammer, T. H. A Comparison of Single-Stage and Two-Stage Dry-Hopping Regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251−260. DOI: 10.1080/03610470.2019.1668230.
- Dykstra, J. 2020 Consumer Trends. The Beer Connoisseur, 2020, 1, 18–29.
- National Beer Sales & Production Data | Brewers Association. https://www.brewersassociation.org/statistics-and-data/national-beer-stats/.
- Bud Light Crisp. https://www.budlight.com/.
- Guinness® Nitro IPA | Guinness®. https://www.guinness.com/en/our-beers/guinness-nitro-ipa/.
- Bamforth, C. W. Scientific Principles of Malting and Brewing. St. Paul, MN: American Society of Brewing Chemists, 2006.
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of Hop-Derived Monoterpene Alcohols by Lager Yeast and Their Contribution to the Flavor of Hopped Beer. J. Agric. Food Chem. 2010, 58, 5050–5058. DOI: 10.1021/jf1000524.
- Sharp, D. C.; Steensels, J.; Shellhammer, T. H. The Effect of Hopping Regime, Cultivar and β-Glucosidase Activity on Monoterpene Alcohol Concentrations in Wort and Beer. J. Inst. Brew. 2017, 123, 185–191. DOI: 10.1002/jib.418.
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation. Wiley-Blackwell: Hoboken, NJ, 2001.
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L.; et al. Wort Composition and Its Impact on the Flavour-Active Higher Alcohol and Ester Formation of Beer – A Review. J. Inst. Brew. 2014, 120, 157–163. DOI: 10.1002/jib.145.
- Otter, G. E.; Taylor, L. Determination of the Sugar Composition of Wort and Beer by Gas Liquid Chromatography. J. Inst. Brew. 1967, 73, 570–576. DOI: 10.1002/j.2050-0416.1967.tb03086.x.
- Kirkendall, J. A.; Mitchell, C. A.; Chadwick, L. R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76, 1–7. DOI: 10.1080/03610470.2018.1469081.
- Kirkpatrick, K. R.; Shellhammer, T. H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. DOI: 10.1021/acs.jafc.8b03563.
- Olodokun, O.; Cowley, T.; James, S.; Smart, K. A. Dry-Hopping: The Effects of Temperature and Hop Variety on the Bittering Profiles and Properties of Resultant Beers. Brew. Sci. 2017, 70, 187–196.
- Janicki, J.; Kotasthane, W. V.; Parker, A.; Walker, T. K. The Diastatic Activity of Hops, Together with a Note on Maltase in Hops. J. Inst. Brew. 1941, 47, 24–36. DOI: 10.1002/j.2050-0416.1941.tb06070.x.
- Rubottom, L. N.; Lafontaine, S. R.; Hauser, D. G.; Pereira, C.; Shellhammer, T. H. Hop Kilning Temperature Sensitivity of Dextrin-Reducing Enzymes in Hops. J. Am. Soc. Brew. Chem. 2021, 80, 1–13. DOI: 10.1080/03610470.2021.1903290.
- Werrie, P.-Y.; Deckers, S.; Fauconnier, M.-L. Brief Insight into the Underestimated Role of Hop Amylases on Beer Aroma Profiles. J. Am. Soc. Brew. Chem. 2022, 80, 66–74. DOI: 10.1080/03610470.2021.1937453.
- Teraoka, R.; Kanauchi, M.; Bamforth, C. W. Do Starch-Degrading Enzymes in Hop Samples Originate in Microorganisms? Tech. Q. Master Brew. Assoc. Am. 2021, 58, 143–147. https://www.mbaa.com/publications/tq/tqPastIssues/2021/Pages/Number3.aspx.
- Stokholm, A.; Van Simaeys, K.; Gallagher, A.; Weaver, G.; Shellhammer, T. H. Investigating the Effect of Farm Management, Soil, and Climate on Hop Diastatic Potential. J. Am. Soc. Brew. Chem. 2021, 79, 1–12. DOI: 10.1080/03610470.2021.1977902.
- Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation. 2021, 7, 66. DOI: 10.3390/fermentation7020066.
- Bruner, J. R.; Williams, J.; Fox, G. P. Further Exploration of Hop Creep Variability with Humulus lupulus Cultivars and Proposed Method for Determination of Secondary Fermentation. Tech. Q. Master Brew. Assoc. Am. 2020, 57, 169–176. DOI: 10.1094/TQ-57-3-1002-01.
- Kirkpatrick, K. R.; Shellhammer, T. H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. DOI: 10.1080/03610470.2018.1546091.
- Stokholm, A.; Lindsey, N. R.; Shellhammer, T. H. Evaluating a Benchtop Fermentation Method for Estimating Dextrin Degradation by Hop Diastatic Enzymes during Dry-Hopping. Brew. Sci. 2020, 73, 140–148.
- US Gov., Tax and Trade Bureau. Chapter 1. Basic Mandatory Labeling Information for MALT BEVERAGES. In: The Beverage Alcohol Manual (BAM): A Practical Guide. US Gov., Tax and Trade Bureau: Washington DC, 2007; Vol. 3, Section 5.
- Otter, G. E.; Taylor, L. Estimation and Occurrence of Acetaldehyde in Beer. J. Inst. Brew. 1971, 77, 467–472. DOI: 10.1002/j.2050-0416.1971.tb03405.x.
- Tian, J. Determination of Several Flavours in Beer with Headspace Sampling–Gas Chromatography. Food Chem. 2010, 123, 1318–1321. DOI: 10.1016/j.foodchem.2010.06.013.
- Wainwright, T. Diacetyl-A Review: Part I-Analytical and Biochemical Considerations: Part II-Brewing Experience. J. Inst. Brew. 1973, 79, 451−470. DOI: 10.1002/j.2050-0416.1973.tb03567.x.
- Krogerus, K.; Gibson, B. R. 125th Anniversary Review: Diacetyl and Its Control during Brewery Fermentation. J. Inst. Brew. 2013, 113, 86–97. DOI: 10.1002/jib.84.
- Ouyang, X.; Yuan, G.; Ren, J.; Wang, L.; Wang, M.; Li, Y.; Zhang, B.; Zhu, B. Aromatic Compounds and Organoleptic Features of Fermented Wolfberry Wine: Effects of Maceration Time. Int. J. Food Prop. 2017, 20, 2234–2248. DOI: 10.1080/10942912.2016.1233435.
- Jones, M.; Pierce, J. S. Absorption of Amino Acids from Wort by Yeasts. J. Inst. Brew. 1964, 70, 307–315. DOI: 10.1002/j.2050-0416.1964.tb01996.x.
- Pierce, J. S. Horace Brown Memorial Lecture the Role of Nitrogen in Brewing. J. Inst. Brew. 1987, 93, 378–381. DOI: 10.1002/j.2050-0416.1987.tb04520.x.
- O’Connor-Cox, E. S. C.; Ingledew, W. M. Wort Nitrogenous Sources—Their Use by Brewing Yeasts: A Review. J. Am. Soc. Brew. Chem. 1989, 47, 102–108. DOI: 10.1094/ASBCJ-47-0102.
- Bajomo, M. F.; Young, T. W. Fermentation of Worts Made from 100% Raw Sorghum and Enzymes. J. Inst. Brew. 1994, 100, 79–84. DOI: 10.1002/j.2050-0416.1994.tb00810.x.
- Piddocke, M. P.; Fazio, A.; Vongsangnak, W.; Wong, M. L.; Heldt-Hansen, H. P.; Workman, C.; Nielsen, J.; Olsson, L. Revealing the Beneficial Effect of Protease Supplementation to High Gravity Beer Fermentations Using "-Omics" Techniques”. Microb. Cell Fact. 2011, 10, 27. DOI: 10.1186/1475-2859-10-27.
- Bruner, J.; Marcus, A.; Fox, G. Brewing Efficacy of Non-Conventional Saccharomyces Non-cerevisiae Yeasts. Beverages. 2021, 7, 68. DOI: 10.3390/beverages7030068.
- Schisler, D. O. Comparison of Revised Yeast Counting Methods. J. Am. Soc. Brew. Chem. 1986, 44, 81–85. DOI: 10.1094/ASBCJ-44-0081.
- Landaud, S.; Lieben, P.; Picque, D. Quantitative Analysis of Diacetyl, Pentanedione and Their Precursors during Beer Fermentation by an Accurate GC/MS Method. J. Inst. Brew. 1998, 104, 93–99. DOI: 10.1002/j.2050-0416.1998.tb00981.x.
- Ochando, T.; Mouret, J.-R.; Humbert-Goffard, A.; Sablayrolles, J.-M.; Farines, V. Vicinal Diketones and Their Precursors in Wine Alcoholic Fermentation: Quantification and Dynamics of Production. Food Res. Int. 2018, 103, 192–199. DOI: 10.1016/j.foodres.2017.10.040.
- Le Boucher, J.; Charret, C.; Coudray-Lucas, C.; Giboudeau, J.; Cynober, L. Amino Acid Determination in Biological Fluids by Automated Ion-Exchange Chromatography: Performance of Hitachi L-8500A. Clin. Chem. 1997, 43, 1421–1428. DOI: 10.1093/clinchem/43.8.1421.
- Lie, S. The EBC-Ninhydrin Method for Determination of Free Alpha Amino Nitrogen. J. Inst. Brew. 1973, 79, 37–41. DOI: 10.1002/j.2050-0416.1973.tb03495.x.
- Palamand, S. R.; Aldenhoff, J. M. Bitter Tasting Compounds of Beer. Chemistry and Taste Properties of Some Hop Resin Compounds. J. Agric. Food Chem. 1973, 21, 535–543. DOI: 10.1021/jf60188a005.
- Stevens, R. The Chemistry of Hop Constituents. Chem. Rev. 1967, 67, 19–71. DOI: 10.1021/cr60245a002.
- De Clippeleer, J.; Van Opstaele, F.; Gahr, A.; Forster, A. Reproducibility Trials in a Research Brewery and Effects on the Evaluation of Hop Substances in Beer - Part 3: Transfer Rates of Aroma Compounds from Hops to Beer and Their Ageing Behaviour. Brew. Sci. 2019, 72, 217–227. DOI: 10.23763/BrSc19-27gahr.
- Niederberger, P.; Miozzari, G.; Hütter, R. Biological Role of the General Control of Amino Acid Biosynthesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 1981, 1, 584–593. DOI: 10.1128/mcb.1.7.584-593.1981.
- Hinnebusch, A. G. Mechanisms of Gene Regulation in the General Control of Amino Acid Biosynthesis in Saccharomyces cerevisiae. Microbiol. Rev. 1988, 52, 248–273. DOI: 10.1128/mr.52.2.248-273.1988.
- Takada, Y.; Noguchi, T. Characteristics of Alanine: Glyoxylate Aminotransferase from Saccharomyces cerevisiae, a Regulatory Enzyme in the Glyoxylate Pathway of Glycine and Serine Biosynthesis from Tricarboxylic Acid-Cycle Intermediates. Biochem. J. 1985, 231, 157–163. DOI: 10.1042/bj2310157.
- Kumada, Y.; Benson, D. R.; Hillemann, D.; Hosted, T. J.; Rochefort, D. A.; Thompson, C. J.; Wohlleben, W.; Tateno, Y. Evolution of the Glutamine Synthetase Gene, One of the Oldest Existing and Functioning Genes. Proc. Natl. Acad. Sci. USA. 1993, 90, 3009–3013. DOI: 10.1073/pnas.90.7.3009.
- García-Campusano, F.; Anaya, V.-H.; Robledo-Arratia, L.; Quezada, H.; Hernández, H.; Riego, L.; González, A. ALT1-Encoded Alanine Aminotransferase Plays a Central Role in the Metabolism of Alanine in Saccharomyces cerevisiae. Can. J. Microbiol. 2009, 55, 368–374. DOI: 10.1139/W08-150.
- Björkeroth, J.; Campbell, K.; Malina, C.; Yu, R.; Di Bartolomeo, F.; Nielsen, J. Proteome Reallocation from Amino Acid Biosynthesis to Ribosomes Enables Yeast to Grow Faster in Rich Media. Proc. Natl. Acad. Sci. USA. 2020, 117, 21804–21812. DOI: 10.1073/pnas.1921890117.
- Ulane, R.; Ogur, M. Genetic and Physiological Control of Serine and Glycine Biosynthesis in Saccharomyces. J. Bacteriol. 1972, 109, 34–43. DOI: 10.1128/JB.109.1.34-43.1972.
- Jones, E. W.; Fink, G. R. Regulation of Amino Acid and Nucleotide Biosynthesis in Yeast. In: Cold Spring Harbor Monograph Archive, The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance. J.N. Strathern, E.W. Jones, and J.R. Broach, eds. Cold Spring Harbor Laboratory Press: Long Island, NY, 1982, pp 181–299.
- Ljungdahl, P. O.; Daignan-Fornier, B. Regulation of Amino Acid, Nucleotide, and Phosphate Metabolism in Saccharomyces cerevisiae. Genetics. 2012, 190, 885–929. DOI: 10.1534/genetics.111.133306.
- Sakai, K.; Fukui, S.; Yabuuchi, S.; Aoyagi, S.; Tsumura, Y. Expression of the Saccharomyces diastaticus STA1 Gene in Brewing Yeasts. J. Am. Soc. Brew. Chem. 1989, 47, 87–91. DOI: 10.1094/ASBCJ-47-0087.
- Yamashita, I.; Suzuki, K.; Fukui, S. Nucleotide Sequence of the Extracellular Glucoamylase Gene STA1 in the Yeast Saccharomyces diastaticus. J. Bacteriol. 1985, 161, 567–573. DOI: 10.1128/JB.161.2.567-573.1985.
- Lekkas, C.; Stewart, G. G.; Hill, A.; Taidi, B.; Hodgson, J. The Importance of Free Amino Nitrogen in Wort and Beer. Tech. Q. Master Brew. Assoc. 2005, 42, 113–116.
- Mathews, J. M.; Watson, S. L.; Snyder, R. W.; Burgess, J. P.; Morgan, D. L. Reaction of the Butter Flavorant Diacetyl (2,3-Butanedione) with N-α-Acetylarginine: A Model for Epitope Formation with Pulmonary Proteins in the Etiology of Obliterative Bronchiolitis. J. Agric. Food Chem. 2010, 58, 12761–12768. DOI: 10.1021/jf103251w.
- Winter, G.; Todd, C. D.; Trovato, M.; Forlani, G.; Funck, D. Physiological Implications of Arginine Metabolism in Plants. Front. Plant Sci. 2015, 6, 1–14. DOI: 10.3389/fpls.2015.00534.
- Baillo, A.; Ley, A.; Brigham, M. Dry Hopping and Stirring Pellets Increases Vicinal Diketones and Lowers Apparent Extract. 2017. https://www.asbcnet.org/events/archives/2017ASBCMeeting/proceedings/2017Proceedings/30_Baillo.pdf.
