Abstract
A search was conducted for the sources of diastatic enzymes driving the over attenuation and continued fermentation of dry hopped beer known as “hop creep”. Microbial cultivation using starch containing media and assays of amylase enzyme activity were used to isolate and identify microbes from pellet hops that are potential sources of amylases associated with but exogenous to hops (Humulus lupulus). Bacteria and fungi associated with pellet hops produced amylases but did not produce hop creep in assays using finished beer and fermenting wort with added microbes. Cannabis sativa flower produced over attenuation of fermenting wort equivalent to that seen with hops. Comparative bioinformatic analysis of the Cannabis sativa proteome and H. lupulus genome revealed the genetic potential of H. lupulus to produce endogenous amylase enzymes. Sequence similarity of amylases annotated in the C. sativa proteome to previously unidentified genes in H. lupulus revealed 13 genes likely encoding amylases. PCR and sequencing confirmed the occurrence of genes that appear to encode α-amylase and β-amylase in the Citra® hop cultivar. Identifying a genetic basis for hop creep contributes knowledge that may lead to new approaches for controlling hop creep produced by endogenous amylases of H. lupulus.
Introduction
The step in the brewing process where hops are added to fermenting or finished beer is known as dry hopping. Hops utilized in this way contribute flavor and aroma to beer without imparting bitterness. Brewers have likely been dry hopping beer since the introduction of hops to brewing, which appears to have occurred in European brewing sometime between the 12th and 9th Centuries.[Citation1,Citation2] Contemporary brewers adjust the timing and quantity of dry hop additions to highlight a wide variety of hop flavor attributes found in different hop cultivars. Dry hopping gives the beer styles known as India Pale Ale (IPA) and New England IPA their distinctive qualities. The unique flavor, aroma, and hazy character of New England IPA is largely achieved through the application of hops in quantities up to 4-fold higher than has been used in other traditional dry hopped beer styles.[Citation3]
Large dry hop additions in the presence of metabolically active yeast can lead to over attenuation of fermenting beer or continued fermentation of finished beer. This phenomenon known as “hop creep” is characterized by unanticipated yeast fermentation that is fueled by fermentable substrates produced by enzymatic degradation of unfermentable dextrins.[Citation4] Dextrin oligosaccharides are converted by diastatic enzymes into fermentable sugars that are consumed by yeast, producing CO2 that can increase the carbonation of beer to undesirable levels from sensory and safety perspectives. The continued fermentation can generate off flavors in the form of diacetyl and produce alcohol concentrations higher than intended by the brewer. In a worst-case scenario, continued fermentation of finished, dry hopped beer can generate dangerously high pressure in cans and bottles leading to package failure. Various approaches based on an empirical understanding of the phenomenon have been proposed to minimize the impacts of hop creep.[Citation5]
Much work has been done to explain the variability seen in hop creep to help minimize the associated risks. One study focusing on Cascade hops demonstrated that the impact on beer of enzymes associated with hops, including amyloglucosidase, α-amylase, β-amylase, and limit dextrinase was time, temperature, and hops dose-dependent.[Citation4] Another effort identified hop cultivar as a source of the variation seen in hop creep by assessing amylolytic enzyme activity in 30 hop cultivars.[Citation6] Variation among hop cultivars showed patterns that were not related to pedigree, genetic makeup, or specific enzyme activities. The picture of factors contributing to the variability in hop creep was further complicated by one study showing that hops added to the kettle appear to contribute inhibitors of hop creep, including tannins released during wort boiling.[Citation7] Other factors that may contribute to the variability of hop creep include hop agricultural practices, processing, and storage.
The hop creep phenomenon is widely recognized, and intrinsic properties of hops are largely believed to be responsible for its manifestation. However, current understanding of the biology and genetics of H. lupulus responsible for the inferred dextrin degrading activities of hops remains incomplete. Starch metabolism is not a prominent feature of hop plant biology, so diastatic activity of hops is difficult to explain.[Citation8] The large, repetitive, and heterozygous genome of H. lupulus has presented obstacles to genetic approaches for exploring the existence of amylases endogenous to H. lupulus. However, new genomic resources, including a genome assembly of the Cascade hop cultivar,[Citation9,Citation10] the draft genome of H. lupulus generated using two cultivars, Saazer and Shinshu Wase, and a Japanese wild hop H. lupulus var. cordifolius[Citation11] provide new tools for exploring these questions.
Microbes associated with hops are another possible source of the amylolytic enzymes driving hop creep. It is noteworthy that fungi and bacteria are the main sources of exogenous enzymes, including α-amylase and β-amylase used in commercial brewing.[Citation12] H. lupulus is host to a diverse assemblages of bacteria and fungi, including some that cause plant disease.[Citation13] Current thinking points to a benign relationship between dry hopping and the potential impact of microbes associated with hops. For example, Guinard et al.[Citation14] demonstrated that viable fungi and bacteria can be found on Cascade and Chinook hops. However, the microbes could not be recovered by cultivation from dry hopped beer, suggesting that these microbes cannot survive in beer and therefore do not represent a threat of contamination during production of dry hopped beer.
Despite the currently accepted view that dry hopping is not a concern with respect to microbiological beer spoilage, recent work has raised the possibility that microbes may be responsible for the amylolytic activity driving hop creep. Addition of the biocide, sodium azide, to fresh hops and pellet hops inhibited α-amylase and β-amylase activity and prevented decreases in glucose and reducing sugar concentrations in incubations lasting up to 30 days.[Citation15] However, the inhibitory effect of sodium azide has not always been seen in studies exploring the amylolytic activity driving hop creep. For example, Kirkpatrick and Shellhammer[Citation4,Citation6] reported amylolytic activity associated with hops in the presence of sodium azide, including degradation of dextrin substrate analogues and production of dextrin hydrolysis products.
In this study, we examined potential sources of the amylolytic enzymes associated with pellet hops driving hop creep. The search included microbes isolated from pellet hops and the H. lupulus genome. Microbe isolates, including bacteria and fungi, were screened for the ability to degrade starch. Forced attenuations of fermenting wort and incubations of finished beer with and without added microbes were used to examine the potential role of microbes in hop creep. Genomic resources of H. lupulus PCR and DNA sequencing were used to determine if H. lupulus might itself be capable of producing endogenous amylases. The results contribute to knowledge of the genetic basis of amylases produced by H. lupulus that may point towards novel approaches for controlling hop creep.
Experimental
Source of hops, Cannabis sativa and Origanum vulgare
T90 pellet hops were purchased from Maryland Homebrew, Columbia, MD and kept sealed and refrigerated until use. The six hop cultivars examined, including Azacca®, Cascade, Centennial, Citra® and Mosaic® (Yakima Chief Hops, yakimachief.com) and Galaxy® (Brewers Supply Group, bsgcraft.com) are typically used in the production of dry hopped beers. Cannabis sativa (Northern Lights) containing <0.3% tetrahydrocannabinol was purchased from 529 Vape, Baltimore, MD. Origanum vulgare (oregano herb) was produced by McCormick & Co., Hunt Valley, MD. The pellet hops and whole flower C. sativa were ground using a manual spice grinder and the oregano was used as supplied by the vendor without any additional processing.
Media preparation
Two types of media in liquid and solid form were used for microbe isolation, including Fast Orange Wild Yeast medium (FOWY) (PIKA Weihenstephan, Pfaffenhofen, Germany) and Potato Dextrose Agar (PDA).[Citation16] The solid form of FOWY medium was prepared by adding an equal volume of autoclaved, 3% agar (Alpha Biosciences, Baltimore, MD) before dispensing into Petri plates. Potato Dextrose Agar was prepared by boiling 200 g of diced potato in 1 L of water for 30 min. The potato extract was filtered through cheese cloth to remove solids before adding 200 g of dextrose. The solid PD medium included 15 g/L of agar. The volume was brought to 1 L and the medium was sterilized by autoclaving at 121 °C for 40 min.
A medium containing soluble starch was used to assess starch degradation by the fungal and bacterial isolates. Starch Agar (SA) medium was prepared with 10 g soluble starch (Himedia, Mumbai, India), 15 g agar, 2 g yeast extract (Sigma-Aldrich, St. Louis, MO), and 2 g tryptone (Sigma-Aldrich, St. Louis, MO) per L of deionized water. The medium was autoclaved at 121 °C for 40 min, cooled and dispensed into Petri plates.
Microbe isolation from pellet hops
Microbes were isolated from pellet hops using liquid and solid media. Isolation using liquid medium was performed by adding 2 g of pellet hops to 100 mL of liquid FOWY or PD medium in 250 mL Erlenmeyer flasks. Enrichment using solid FOWY and PDA medium was performed by spreading the hops on the surface of the solid medium in a Petri plate. Pure strains of bacteria and fungi were obtained by transfer of single colonies to solid SA medium with a flamed wire loop. Incubations were carried out aerobically at 30 °C with the goal of isolating microbes growing in the aerobic environment of hop cones, while recognizing the selective pressure that the microaerophilic environment of beer would impose on any microbes potentially contributing to hop creep.
Microbe identification
Microbes were classified as a bacterium or fungus based on cell size using a Leica DMLB microscope (Wetzlar, Germany) fitted with a 40X objective (800X total magnification). Wet mounts were prepared on glass slides using 2 µL of distilled water and a coverslip. Cells larger than and less than 2 µm in diameter were classified as fungus or bacterium, respectively.
Fungal isolates were classified using PCR and partial sequencing of the rRNA internal transcribed spacer (ITS), including ITS 1, 5.8S rRNA, and ITS 2.[Citation17] Similarly, bacterial isolates were classified using PCR and partial sequencing of the 16S rRNA gene.[Citation18] Sequencing was performed by Azenta Life Sciences (South Plainfield, NJ).[Citation19] Taxonomic assignments were made based on closest Basic Local Alignment (BLAST) matches to the National Center for Biotechnology nr database.[Citation20]
Starch degradation assay
Starch degradation by the isolated fungal and bacterial isolates was assessed using SA medium. Microbe isolates were grown on the solid medium for three days at 30 °C. The plate was then flooded with a 1% iodine solution (Topco Associates, Elk Grove, IL) for 3 min at room temperature. The iodine solution was removed, and the plate was rinsed once with distilled water producing a blue-black staining of starch in the medium. Starch degrading microbes were identified by the unstained boarder adjacent to microbe growth that is indicative of starch degradation. A bacterium that does not degrade starch served as a negative control.
Amylolytic enzyme activity
The α-amylase and β-amylase activities of microbes isolated from pellet hops were assessed using spectrophotometric assays with para-nitrophenyl blocked oligosaccharide substrates (Megazyme, Bray, Ireland). The α-amylase and β- amylase activities were measured using the CERALPH (cat. no. K-CERA) and BETAMYL-3 (cat no. K-BETA3) assay kits, respectively. The substrate mixes containing blocked p-nitrophenyl maltoheptaoside plus thermostable α-glucosidase and p-nitrophenyl-β-D-maltorioside plus β-glucosidase were dissolved in distilled water and stored at −20 °C as directed by the manufacturer. Samples (60 µL) of microbial cultures or filter sterilized spent media collected after microbe growth were combined with 60 µL of each substrate mix and incubated at 40 °C for 3 h. The reactions were terminated by adding 1 mL of 20% tri-sodium phosphate solution (pH 11) or 0.1 M Tris-HCl (pH 8) to the reactions containing the CERALPH and BETAMYL-3 kit reagents, respectively. The p-nitrophenol product was determined by measuring the absorbance at 400 nm (Thermo Scientific GENESYS 10 UV/Visible Spectrophotometer) relative to a blank prepared with SB medium and the assay reagents.
Limit of attenuation
Wort used in limit of attenuation assays was prepared using 107 g/L Pilsen Light dried malt extract (Briess Malt and Ingredients, Chilton, WI), 53 g/L maltodextrin (LD Carlson, Kent, OH), and 0.33 g/L Magnum pellet hops containing 18.9% alpha acids (LD Carlson, Kent, OH). The wort was boiled for 1 h, cooled in a water bath to 20 °C and 150 mL aliquots were dispensed into 250 mL Erlenmeyer flasks. Chico yeast (White Labs, San Diego, CA) slurry was concentrated by vacuum filtration using a Buchner funnel and qualitative filter paper. The yeast pitch rate was 66 g wet weight per L.
Limit of attenuation assays were conducted to assess the impact of different plant materials on extract available to yeast fermentation. The plant materials included H. lupulus (Citra® pellet hops), moldy pellet hops, dried C. sativa flower, and dried oregano herb. The moldy pellet hops were prepared using a one-month incubation at 30 °C of 1.5 g of Citra® pellet hops on a petri plate containing SA medium solidified with 15 g agar per L. The plant materials were added to limit of attenuation assays at a rate of 10 g/L.
Continued attenuation of packaged beer
Aliquots of an amylolytic bacterium (isolate H10.1) and an amylolytic fungus (isolate H6.3.1) were added to packaged beer to assess the ability of the microbes to produce continued fermentation when the cells are in direct contact with beer. The assay utilized a commercially available American Pale Ale (2.55 °P Ea, 5.7% ABV, 2.6 vol. CO2) packaged in 12 oz. bottles. After four days of growth in SB medium, the fungus and the bacterium were removed from the medium. The bacterium was centrifuged at 3,600 X g for 3 min and resuspended four times with distilled water to minimize carryover of nutrients into the beer. The fungus was collected by vacuum filtration onto a 0.45 µm pore size, 25 mm dia. Durapore filters (Millipore, Burlington, MA) and rinsed four times with distilled water. The bacterium was added to 12 oz. bottles of beer at a concentration of 1E6 cells/mL. The fungal biomass produced in 1 mL of SB medium in four days was added to each 12 oz. bottle of beer. Samples of the rinsed fungus and bacterium were inoculated into SB medium to confirm viability after the rinsing step.
Spent medium from the fungal and bacterial cultures was collected and assayed for amylolytic activity as described above using substrate analogues. The spent medium from the fungal and the bacterial cultures was filtered through 0.22 µm pore size, 25 mm dia. sterile PES syringe filters (www.membrane-solutions.com) to remove any remaining microbes. Treatments receiving the filter sterilized media were dosed at a concentration of 1 mL/12 oz. bottle. Samples of the filter sterilized spent media were added to SB medium to confirm the absence of a viable fungus or bacterium.
Chico yeast was grown for two days in Yeast Peptone Dextrose (YPD) medium (Sigma-Aldrich, St. Louis, MO). Yeast cells were collected by centrifugation, washed four times with distilled water and added at a concentration of 1E6 cells/mL to the bottled beer that received the fungus, bacterium, and pellet hops additions. Citra® pellet hops (10 g/L) served as a hop creep positive control and negative control incubations received only the yeast addition.
Treated bottles were sealed with crowns and incubated in the dark at 20 °C. Periodically, bottles representing each of the four treatments and the control were assayed in triplicate for apparent extract using a DMA 500 M instrument (Anton-Paar, Graz, Austria) and alcohol content using an Alcolyzer Beer ME instrument (Anton-Paar, Graz, Austria). Viability of the fungus and the bacterium added to beer was monitored by filtering an entire bottle onto a 0.45 µm pore size, 47 mm dia. filter that was then placed onto SA medium in a Petri plate that was then incubated at 30 °C for 7 days.
Bioinformatic search for amylase genes in C. sativa and H. lupulus
Amylases of C. sativa were identified by searching the UniProtKB database (www.uniprot.org) with the query “name:amylase AND organism:"Cannabis sativa (Hemp) (Marijuana) [3483]" AND proteome: UP000525078”. Cluster analysis of the C. sativa amylases was performed at 50% and 90% sequence similarity using the queries “uniprot:(name:amylase organism:"Cannabis sativa (Hemp) (Marijuana) [3483]" proteome:up000525078) AND identity:0.5” and “uniprot:(name:amylase organism:"Cannabis sativa (Hemp) (Marijuana) [3483]" proteome:up000525078) AND identity:0.9”, respectively. The C. sativa sequences were used to identify amylase genes in the genome assembly of H. lupulus by sequence similarity using a TBLASTN search of GenBank assembly GCA_000831365.1.
PCR amplification and sequencing of H. lupulus amylase genes
PCR primers Hlaa2F (5′-ATGTAACTGCACGCTCATCC-3′) and Hlaa2R (5′-CCACAGAACATTTACTCTGTCAATTC-3′) targeting the α-amylase 2 gene of H. lupulus (GenBank Accession LD152561.1:14054-14320) and PCR primers Hlba2F (5′-CTCGTGAGCGTAATCGTCATAG-3′) and Hlba2R (5′-GAGGTTGGAACGGTACTTATGG-3′) targeting the β-amylase 2 gene of H. lupulus (GenBank Accession LD162710.1:13476-14282) were designed using the Integrated DNA Technologies (IDT) PrimerQuest Tool (www.idtdna.com) using the default design parameters. PCR primers were synthesized by Gene Link (www.genelink.com).
H. lupulus DNA was extracted using the Quick-DNA Fungal/Bacterial Microprep Kit (Zymo Research, www.zymoresearch.com) following the manufacturer’s protocol without beta-mercaptoethanol added to the Genomic Lysis Buffer. A 0.1 g aliquot of Citra® T90 pellet hops was resuspended in 200 µL of distilled water, transferred to a lysis tube and processed using buffer additions and a vortex mixer (Weber Scientific, Hamilton Township, NJ). The procedure yielded 20 µL of a 1 ng/µL DNA extract as determined using a Qubit 2.0 fluorometer (Invitrogen, Waltham, Ma) with SYBR Green I stain (Invitrogen, Waltham, MA).
PCR reactions included 0.5 nmol each of forward and reverse primer, 5 µL of DNA extract, 2.5 µL of PrimeTime Gene Expression Master Mix (IDT) and water added to a final volume of 25 µL. Thermal cycling was accomplished using a Chai Open qPCR instrument (www.chaibio.com) following a program that included an initial step at 95 °C for 2 min and 35 cycles of 95 °C for 15 sec, 60 °C for 30 sec and 72 °C for 1 min. The final cycle was followed by a step at 72 °C for 10 min.
PCR products were prepared for sequencing using ExoSAP-IT PCR Product Cleanup Reagent (Applied Biosystems, www.thermofisher.com) following the manufacturer’s protocol. Sanger sequencing was performed by Azenta Life Sciences (www.genewiz.com).
Results
Microbes isolated from pellet hops
Microbes were successfully isolated from pellet hops of every cultivar examined. A total of 68 microbe isolates were purified, including 28 bacterial and 40 fungal isolates. Two to seven bacterial isolates and three to 10 fungal isolates were purified from pellet hops of the various hop cultivars. Microbe colonies that grew from the pellet hops of the various hop cultivars were morphologically diverse, as represented by the 18 colonies seen on the FOWY agar plate inoculated with pelletized Cascade hops (). The number of microbe isolates purified from Azacca®, Cascade, Centennial, Citra®, Galaxy®, and Mosaic® pellet hops ranged from 10 to 15 isolates ().
Figure 1. Photograph of an FOWY agar plate inoculated with 0.1 g of Cascade hops after seven days of incubation at 30 °C. The image shows approximately 18 fungal colonies. The change in color of the medium from pink to yellow in the upper right portion of the plate indicates a decreased pH of the medium. The Petri plate diameter is 60 mm.
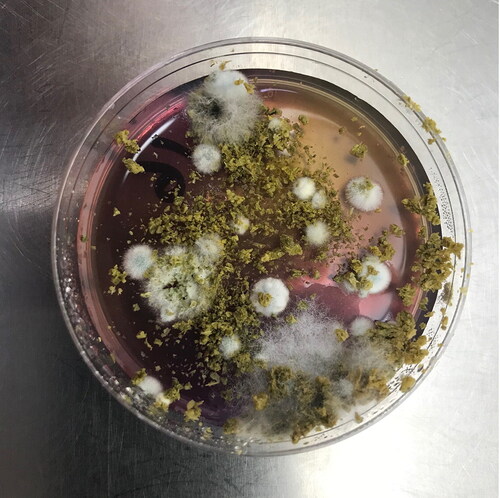
Table 1. Numbers of bacterial and fungal isolates obtained from pellet hops of six cultivars. Isolates degrading starch were identified using a starch containing medium and iodine staining.
Every medium used in the primary microbe isolation step yielded isolates. A total of 18, 16, 10, and 25 microbe isolates were purified from primary microbe isolations starting with FOWY liquid medium, PD liquid medium, FOWY agar, and PD agar, respectively (). The number of bacterial and fungal isolates varied among the different media used for primary isolation. Two bacterial isolates were purified from PD liquid and FOWY agar media cultures. The number of bacterial isolates purified from PD agar and FOWY liquid were 10 and 14, respectively ().
Table 2. Numbers of microbe isolates obtained from pellet hops of six cultivars using different primary isolation media.
Starch degradation by microbes associated with pellet hops
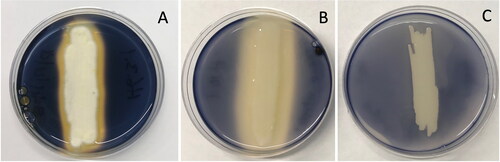
Starch degrading microbes were successfully cultivated from four of the six hop cultivars examined. Bacterial and fungal isolates were identified as starch degraders based on the clearing of iodine staining from solid medium containing starch (). Fungal isolate H6.3.1 and bacterial isolate H10.1 cleared iodine staining from the medium extending a few millimeters from where the microbes grew on the agar surface. Isolate H10.1 was the only starch degrading bacterium seen in this study and was isolated from Citra® pellet hops (). Isolate H6.3.1 was one of two starch degrading fungal isolates obtained from Mosaic® pellet hops. Three starch degrading fungal isolates were isolated from the pelletized Cascade and Centennial hops.
Figure 2. Photographs of SA plates inoculated with fungal and bacterial isolates to assess their ability to degrade starch. The blue-black color of the medium indicates starch stained with iodine. Absence of blue-black staining in the medium adjacent to microbial growth indicates starch degradation. Fungus isolate H6.3.1 (A) and bacterium isolate H10.1 (B) produced clearing zones adjacent to the microbial growth. The agar medium was stained uniformly blue-black on the negative control plate with isolate H2.1 (C) indicating no starch degradation.
Microbes isolated from the pellet hops were capable of starch degradation. Nine of the 68 microbe isolates (13.2%) were shown to be capable of degrading starch (). The prevalence of starch degradation seen in the bacteria isolates was one out of eight (12.5%). In the case of the fungal isolates, eight out of 40 isolates (20%) were able to degrade starch. No starch degrading microbes were seen among the five and six bacterial and fungal isolates purified from Azacca® pellet hops. Similarly, among the microbes purified from Galaxy® pellet hops, none of the seven fungi and three bacterial isolates were capable of degrading starch ().
The different primary isolation media were successful in isolating starch degrading microbes. Of the nine starch degrading microbes, the three starch degrading fungal isolates obtained from Cascade hops were isolated using PD liquid and PD solid media (). Similarly, the three starch degrading fungal isolates obtained from Centennial and Mosaic® pellet hops were obtained using PD liquid and PD solid media. In contrast, the starch degrading bacterium isolated from Citra® pellet hops was obtained using FOWY liquid medium. All the starch degrading microbes produced clearing zones similar to that seen with fungus isolate H6.3.1 () and bacterium isolate H10.1 (). FOWY agar was the only primary isolation medium from which a starch degrading microbe was not purified.
Table 3. Starch degrading microbes isolated from pelletized Cascade, Centennial, Citra®, and Mosaic® hops. Starch substrate analogues were used to assess α- and β-amylase activities. Partial 16S rRNA (bacteria) and rRNA ITS (fungi) gene sequences were used to determine the most similar sequence matches to previously identified microbes.
Amylase activity of microbes isolated from pellet hops
The eight microbes producing clearing zones on starch containing medium tested positive for α-amylase production with the CERALPH assay. Cultures grown for three days in SB medium on three occasions yielded levels of α-amylase activity averaging 0.8 A400 (n = 24), ranging from 0.1 to 2.5 A400 in a 3-h assay incubation (). The fungus isolate H6.3.1 generated the highest average activity (1.97 ± 0.22 A400). The amylolytic bacterium H10.1 generated α-amylase activity of 0.43 ± 0.08 A400, which was 66% of the average activity of the seven fungal isolates.
Figure 3. Amylolytic activity of microbes isolated from pelletized hops assessed using p-nitrophenol conjugated oligosaccharide substrates. Values represent the average absorbance at 400 nm after a 3-h incubation assay (n = 3 cultures). α-Amylase activity determined using the CERALPH assay (A). β-Amylase activity determined using the BETAMYL-3 assay (B). Error bars represent one s.d.
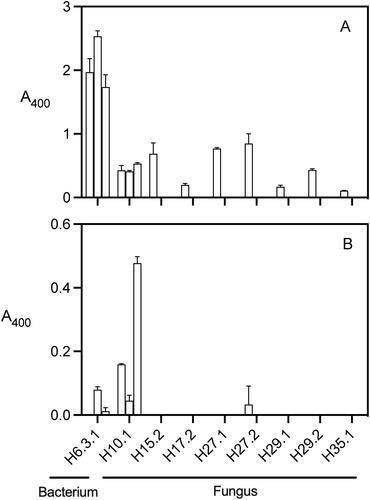
The picture generated by the BETAMYL-3 assay for β-amylase was different from that for α-amylase. Only three of the microbe isolates had activity with the BETAMYL-3 assay, including the fungal isolates H6.3.1 and H27.2 and the bacterial isolate H10.1. The assay with 3-day old cultures revealed an activity generating an A400 absorbance in a 3-h assay of 0.16 ± 0.003 A400 and 0.03 ± 0.06 A400 for the bacterium and the fungus, respectively. In contrast, in the assay with the 4-day old cultures, the fungus H6.3.1 and the bacterium H10.1 yielded activities of 0.08 ± 0.01 and 0.05 ± 0.02 A400 in 3-h assays.
Microbe identification by 16S rRNA gene and rRNA ITS sequencing
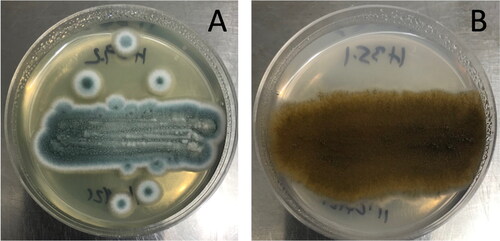
The microbes isolated from pellet hops include eight fungi, including isolates H6.3.1, H15.2, H17.2, H27.1, H27.2, H29.1, H29.2 and H35.1 and one bacterium, isolate H10.1. Partial sequence analysis of the fungal rRNA ITS identified the fungal isolates as Penicillium[Citation21] and Alternaria[Citation22] genera (). ITS sequence diversity of the fungal isolates was very low. ITS sequences were identical for all the Penicillium isolates. Similarly, the Alternaria fungal isolates all shared identical ITS sequences. The Penicillium isolates produced blue colonies with a white border while colonies of the Alternaria isolates were brown ().
Figure 4. Photographs showing colony morphology of the Penicillium strain H29.2 (A) and Alternaria strain H35.1 (B) grown on SA medium for seven days.
Partial sequencing of the 16S rRNA gene of the bacterium H10.1 revealed highest similarity to the genus Klebsiella. BLAST analysis demonstrated 100% similarity over the entire 493 bp sequence to various species of Klebsiella, including K. oxytoca (accession MT568561.1), K. michiganensis (accession CP048108.1), and K. spallanzanii (accession MN091365.3).
Continued attenuation of packaged beer
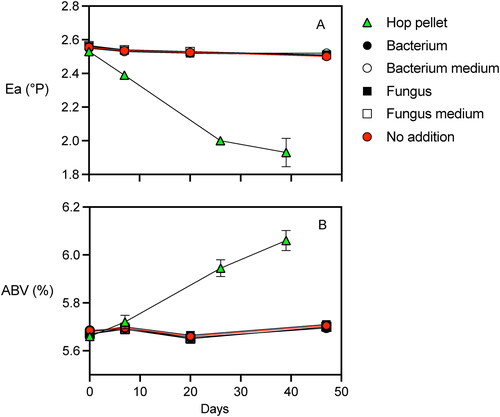
The impact of starch degrading microbes isolated from pellet hops on continued fermentation was examined using a packaged Pale Ale. Beer incubated for two months with microbes or amylases released by the microbes showed no changes in apparent extract or alcohol content compared to the no addition control (). Apparent extract of the Pale Ale was 2.55°P at the start of the incubation and remained steady for 48 days in samples with additions of bacterial strain H10.1, fungal strain H6.3.1, filter sterilized spent medium containing amylases released by the two microbes, and the control with no addition. In contrast, apparent extract decreased 25% from 2.55°P to 1.93°P in the beer with Citra® hops added (). Similarly, alcohol concentration of the beer remained steady at 5.67% ABV for 48 days in incubations with added starch degrading microbes, spent medium containing their amylases, and the control with no addition. In the beer with hops added, alcohol concentration increased 7% from 5.67% ABV to 6.06% ABV. Previous studies have seen increases in ABV % as large as 25% after hops were added to finished beer.[Citation4]
Figure 5. Packaged beer attenuation assay examining the impact of pellet hops, starch degrading bacterium, and starch degrading fungus additions on (A) apparent extract and (B) alcohol concentration. The microbial biomass and the growth media were separated and added to different incubations. The beer was an American Pale Ale. Three bottles were incubated for each treatment and error bars represent one s.d.
Fungus isolate H6.3.1 and bacterium isolate H10.1 remained viable after being rinsed with distilled water. Growth of the microbes was evident in SA medium with the accumulation of biomass after two days. The fungus H6.3.1 and the bacterium H10.1 remained viable in the beer for seven days as demonstrated by growth on SA medium. In contrast, no growth was seen after seven days of incubation with the one mL of filter sterilized spent medium added to SA medium, confirming the successful removal of microbes by filtration from the spent medium.
Limit of attenuation of a test wort
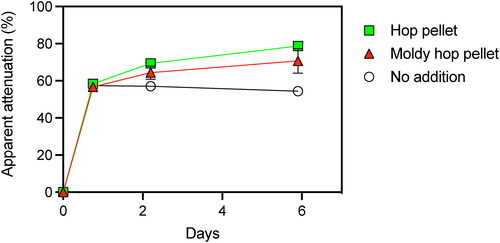
A limit of attenuation assay was conducted to test the idea that microbes associated with pellet hops play a role in the over attenuation of fermenting wort. The assay utilized pellet hops that were enriched with their microbiome, i.e., moldy hops produced by incubating hops on nutrient agar for a month. Pellet hops that had been stored refrigerated in the original packaging served as a control. The pellet hops, moldy pellet hops, and no hops addition control incubations achieved 58% attenuation after 24 h (). Apparent attenuation remained steady in the control without hops added for the remaining five days of incubation at 30 °C. In contrast, apparent attenuation increased to 78.8% ± 1.5% and 70.7% ± 6.7% in the pellet hops and moldy pellet hops incubations, respectively (). The enriched microbial biomass of moldy pellet hops had no impact on attenuation of the test wort. Apparent limits of attenuation after six days with the hop pellet and moldy hop pellet treatments were not significantly different (p = 0.11, student’s t-test).
Endogenous amylases of H. lupulus
The absence of evidence supporting the idea that microbes associated with pellet hops contribute to hop creep prompted a closer examination of the potential role of endogenous amylases of H. lupulus. We began by determining if the amylolytic activity associated with hops is specific to the hop plant by examining a plant material that is similar to but genetically different from hops. A limit of attenuation assay with pellet hops and female flowers of C. sativa, which is closely related to H. lupulus, demonstrated that the ability to produce over attenuation is not unique to hops (). H. lupulus and C. sativa each have sufficient amylolytic activity to generate a ∼50% increase in apparent attenuation of fermenting wort. The apparent limit of attenuation with additions of pellet hops or C. sativa flower was 69.8% ± 10% and 72.8% ± 1.6%, respectively. The apparent limit of attenuation of the test wort with no addition of plant material was 52.2% ± 0.12% ().
Figure 7. Limit of attenuation assay examining the impact of (A) C. sativa and (B) oregano on apparent attenuation of a test wort. Hop pellet and ‘No addition’ treatments served as positive and negative controls, respectively. Error bars represent one s.d.
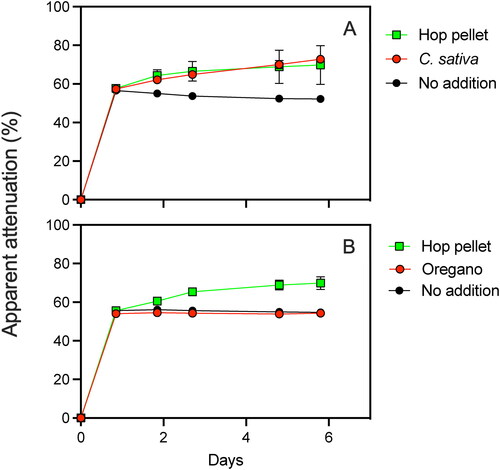
Additions of oregano herb to fermenting wort demonstrated that the over attenuation seen with H. lupulus and C. sativa is not a non-specific consequence of adding plant material to a fermentation. The limits of attenuation in a control assay with no plant material added and with oregano herb addition were not significantly different (p = 0.001, student’s t-test). The limit of attenuation in the control and with the oregano herb addition were 54.7% ± 0.3% and 54.3% ± 0.7%, respectively. As expected, the limit of attenuation of the test wort increased to 69.8% ± 3.3% with the addition of pellet hop material ().
Amylase genes of H. lupulus identified by sequence similarity to amylases in C. sativa
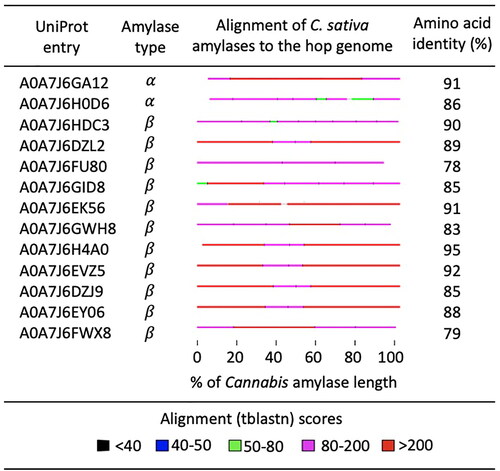
Amylases of C. sativa were identified using a keyword search of the C. sativa proteome in the UniProtKB database (Proteome ID UP000583929). The search returned amino acid sequences for 13 proteins, including two α-amylases and 11 β-amylases. The α-amylases corresponded to UniProt entries A0A7J6GA12 and A0A7J6H0D6. The β-amylases corresponded to UniProt entries A0A7J6HDC3, A0A7J6DZL2, A0A7J6FU80, A0A7J6GID8, A0A7J6EK56, A0A7J6GWH8, A0A7J6H4A0, A0A7J6EVZ5, A0A7J6DZJ9, A0A7J6EY06, and A0A7J6FWX8. Cluster analysis revealed that the 13 C. sativa amylases are diverse, belonging to 12 different clusters defined at 50% sequence similarity. C. sativa β-amylases A0A7J6EVZ5 and A0A7J6EY06 were >90% similar and belonged to the UniRef50_O23553, Beta-amylase 3, chloroplastic cluster. C. sativa β-amylases A0A7J6DZL2 and A0A7J6GWH8 belonged to different chloroplastic clusters UniRef50_Q9LIR6 and UniRef50_O6525, respectively. C. sativa β-amylases A0A7J6FU80 belonged to the UniRef50_A0A6J0PS46 cluster that includes a β-amylase of Hordeum vulgare subsp. vulgare, domesticated barley.
The UniProtKB database contains no entry for the H. lupulus proteome, so the amino acid sequences of C. sativa amylases were used to search the H. lupulus genome for genes encoding similar proteins. The TBLASTN search identified a match for each of the 13 C. sativa amylases in the H. lupulus genome. Amino acid sequence identity between the C. sativa and H. lupulus amylases ranged from 78% to 95% (average = 87%) (). The matches were distributed uniformly over the length of the amino acid alignments that typically included >90% of length of the C. sativa amino acid sequence. Similarity scores ranged from 80 to 200 bits.
Figure 8. Amino acid identity between C. sativa amylases and conceptual translations from the H. lupulus genome identified by a TBLASTN search. The horizontal lines represent amino acid alignments that are color coded according to sequence similarity. UniProt entry refers to amylases in the C. sativa proteome.
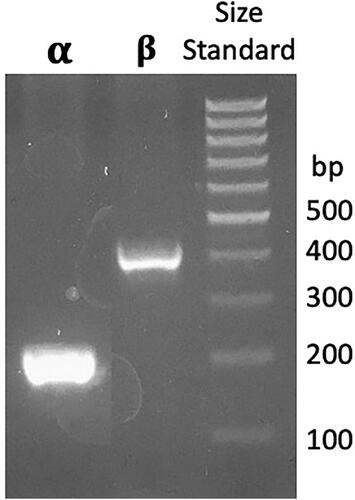
PCR primers were designed for C. sativa α-amylase (A0A7J6H0D6) and β-amylase (A0A7J6DZL2) that are 86% and 89% identical to genes in H. lupulus, respectively. PCR reactions with DNA extracted from the Citra® hop cultivar of H. lupulus produced successful amplification for the α-amylase and β-amylase genes. Agarose gel electrophoresis revealed approximately 200 bp α-amylase and 400 bp β-amylase amplicons (), which was consistent with the expected 202 bp and 422 bp amplicon size for the α-amylase and β-amylase, respectively. Sequencing of the H. lupulus α-amylase and β-amylase amplicons showed that the H. lupulus and C. sativa amylase genes would encode proteins that are 99% identical at the amino acid level with only one amino acid difference out of 67 and 133 amino acids for the α-amylase and β-amylase, respectively. The H. lupulus and C. sativa amylase sequences were 99% identical at the nucleotide level, sharing 201 of 202 and 395 of 400 bases in common between the H. lupulus and C. sativa α-amylase and β-amylase partial gene sequences, respectively.
Figure 9. Agarose gel of H. lupulus (Citra® cultivar) amylase gene PCR products obtained with primers Hlaa2F and Hlaa2R targeting an α-amylase (α) and Hlba2F and Hlba2R targeting a β-amylase (β). The third lane shows a size standard with bands ranging in size from 100 bp to 1,000 bp in 100 bp increments.
Sequence data availability
The sequences reported in this study are available through GenBank accessions ON553555, ON553556, ON553557, ON553558, ON553559, ON553560, ON553561, ON553956, ON585712, and ON585713.
Discussion
Identifying the mechanisms underlying hop creep is an important first step towards controlling the detrimental impacts on beer quality of over attenuation and continued fermentation associated with dry hopping. This study demonstrated the potential role in hop creep of exogenous amylases of microbes and endogenous amylases of hop. Bacteria and fungi were successfully isolated from hops using solid and liquid media containing starch. Pure cultures of the hop associated microbes produced starch degrading enzymes, including α-amylase and β-amylase. Survival in beer for as long as seven days indicated that the microbes isolated from hops remain metabolically competent after being introduced into beer with a dry hop addition. However, microbes alone do not appear to be the sole source of amylases introduced into beer with a dry hop addition. A search for H. lupulus genes encoding amylases revealed several that appear to encode α-amylase and β-amylase. Greater appreciation of the hop microbiome is needed to inform our understanding of amylase-producing microbes having the potential to drive hop creep. In addition, greater knowledge of the genetic determinants of amylase production by H. lupulus could inform genetic approaches for controlling the endogenous amylases of H. lupulus driving hop creep.
Our approach utilized starch containing media and cultivation of amylolytic microbes from hops to identify exogenous sources of amylases produced by microbes. The Klebsiella sp. bacterium isolated from pellet hops produced enzymatic activity consistent with that of α-amylase and β-amylase and degraded starch in solid medium. Klebsiella sp. are members of the Enterobacteriaceae, which represents a large family of Gram negative facultatively anaerobic bacteria within the Gammaproteobacteria. In the brewery, Klebsiella sp. are recognized wort spoilers producing phenolic off flavors such as 4-vinylguaiacol by the decarboxylation of ferulic acid present in wort.[Citation24] Klebsiella sp. are a type of coliform bacteria that are commonly regarded as indicators of poor sanitation in breweries. Typical introduction into wort is thought to be through contaminated water or wastewater systems.[Citation25] The results of this study suggest that hops in the brewery may represent another potential source of Klebsiella sp. spoilage bacteria that has not been previously recognized. Therefore, care should be taken when hops are handled in areas of the brewery where cooled wort is present. We suggest that knocking out wort directly onto hops should be avoided. This approach has been used to maximize biotransformation of hop compounds by yeast.[Citation26] Wort that contacts hops before brewer’s yeast can become established could risk becoming contaminated with bacteria, including Klebsiella sp. spoilage bacteria associated with the hops.
The fungi cultivated from hops were identified as Penicillium and Alternaria genera, which include species that are commonly associated with plants and that produce amylolytic enzymes. Alternaria fungi are widespread in nature and includes species that are associated with plants in saprophytic, endophytic, or pathogenic relationships.[Citation27] Many Penicillium spp. have been implicated in agricultural postharvest spoilage,[Citation28] and various Penicillium and Alternaria fungi produce α-amylase.[Citation29,Citation30] The fungi isolated from pellet hops likely do not represent the entire diversity of fungi associated with hops due to the selective pressure inherent in cultivation. Additional work is needed to assess the full potential for fungi associated with hops to produce hop creep.
In addition to the cultivation results, other information about fungi on hops points to a potential role in hop creep. H. lupulus is afflicted with two fungal diseases, including downy mildew and powdery mildew caused by Pseudoperonospora humuli and Podosphaera macularis, respectively.[Citation31] These fungi are potential sources of starch degrading enzymes because their genomes or the genomes of related fungi include genes encoding glycosyl hydrolases.[Citation32,Citation33] While the production of such enzymes by these hop pathogens has yet to be shown, Stokholm et al.[Citation34] reported that amylase activity associated with hops decreased with fungicide application on farms producing hops. This result suggests that the enzyme activity associated with the hops may have decreased by reducing the abundance of fungi on the hops. Improved control of fungi on hops might represent a step towards controlling hop creep.
Results from the microbe cultivation work are consistent with previous observations of amylolytic microbes associated with hops that potentially contribute to hop creep. A search of the National Center for Biotechnology Information Protein database[Citation23] (accessed January, 2022) for "Humulus lupulus” [All Fields] AND amylase [All Fields] returned 18 occurrences of amylases seen in whole genome sequences of the bacteria Pantoea agglomerans, Pseudomonas fluorescens, and Pseudomonas stutzeri that were cultivated from hop cones.[Citation35] The P. stutzeri (accession TGY14475.1), P. fluorescens (accession TGY19095.1), and P. agglomerans (accession TGX89808.1) sequences include an α-amylase catalytic domain. P. agglomerans is commonly associated with plant surfaces and has been shown to outcompete certain plant pathogens, such as Erwinia amylovora, which is commonly found associated with pears and apples and causes Fire Blight disease.[Citation36,Citation37] Some P. fluorescens strains have biocontrol properties, protecting plant species from parasitic fungi and phytophagous nematodes.[Citation38] Certain strains of P. stutzeri are capable of nitrogen fixation and therefore may provide a source of reduced N to plants when found in the plant rhizosphere.[Citation39] The presence of genes encoding α-amylase in these P. stutzeri isolated from hop cones suggest that dry hopping with hops likely introduces diastatic enzymes into dry hopped beer.
Dry hopping is generally regarded as safe from the perspective of microbiological contamination. A study by Guinard et al.[Citation14] lead to the conclusion that dry hopping presents little risk of microbial contamination of beer. Like the results presented in this study, bacteria (enterics) and wild yeast (Saccharomyces, Candida, and Cryptococcus species) were readily isolated from different hop cultivars, including Cascade and Chinook whole hops and Willamette pellet hops. After the Cascade and Chinook hops were added to fermenting beer, microbiological monitoring revealed that the microbes did not survive in the fermenting beer for longer than two days. In contrast, the results of this work suggest that there are bacteria and fungi found on hops that can survive in beer for as long as one week. The study by Guinard et al.[Citation14] did not include fungi. This work points to the need for a better understanding of how hop creep might be impacted by the fungi introduced into beer by dry hopping and the factors influencing the presence and survival of fungi on hops.
The quality of hops for brewing is highly dependent on the processing steps needed to maintain microbiological stability during storage. Kiln drying to remove moisture is the main tool used to achieve microbiological stability of stored hops. In the absence of drying to 10% moisture, fresh hops are subject to microbiological spoilage known as composting.[Citation40] In extreme cases, heat generated by the exothermic microbial activity of decomposing hops can be sufficient to cause ignition. In the past decade, hop processors have lowered kilning temperatures by 8 to 11 °C.[Citation41,Citation42] Lower kilning temperature increases the retention of essential oil, which contains the aromatic compounds that are made more prominent in beer by dry hopping. The goal of kilning is to remove moisture, which reduces microbial activity during storage of hops, so it seems reasonable to suggest that less aggressive kilning might result in higher residual microbial activity associated with stored hops. The results showing that fungi on hops produce amylases is consistent with the observation that higher kiln temperatures reduce the enzyme activity associated with hops and reduces hop creep seen during fermentation and in packaged beer.[Citation42]
The current understanding of hop creep has produced different approaches to minimize continued attenuation after dry hopping. The approaches fall into categories characterized by speeding up the process or slowing it down and by inactivation of enzymes by pasteurizing hops to eliminate diastatic activity.[Citation3,Citation5] These approaches can successfully control over attenuation, but do not address the root cause. The approach aimed at speeding up the process by including the dry hop step early in production when the fermenting beer is warm allows enough time for the hop creep effect to run its course before the beer is packaged. The alternative approach aiming to slow down the hop creep process includes a dry hop step with short duration, two days for example, at a cool temperature, i.e., 10 °C (50 °F). Brewers have had success with both approaches, but each represents a workaround to minimize impacts not a remedy.
A greater understanding of the hop microbiome could lead to the development of biological control tools. In the case of fungi, antagonistic bacteria have shown promise as effective control agents of Penicillium fungi post-harvest. For example, Pantoea agglomerans has proven to be an effective antagonist for controlling blue and grey mold on apples.[Citation43] Viruses that kill bacteria, i.e., a bacteriophage might be used to control bacteria contributing to hop creep. For example, bacteriophage cocktails have been shown to be effective at controlling pathogenic bacteria on fresh-cut leafy greens and other produce.[Citation44,Citation45] Efforts to control microbes contributing to hop creep would require a detailed understanding of the risk posed by different members of the microbial community associated with hops.
The diastatic microbes associated with hops examined in this study did not appear to produce hop creep under the conditions and with the beers that were examined. In beer assays with a commercially available Pale Ale, adding the diastatic fungus isolate H6.3.1 and bacterium isolate H10.1 did not cause continued fermentation as shown by no change in apparent extract or alcohol content in incubations that approached a two-month duration. Similarly, the spent medium used to grow the microbes and containing amylases had no impact on continued fermentation. These results suggest that while diastatic microbes are associated with hops they may not always represent a risk of over attenuation to finished beer. However, it is important to recognize that the beer assays that were conducted are not an exact simulation of dry hopping of beer in production. The physiological state of the yeast added to the beer experiments likely differed from that of the yeast in a fermenting beer that is dry hopped. In addition, interactions between the hops and microbes cannot be ruled out that would be absent from the beer assays with added microbes and yeast without added hops. Growth of the microbes associated with hops likely benefits from or is possibly even dependent on the hops and the beer assays would not have incorporated those interactions.
Forced attenuation assays with moldy hops provided no evidence that microbes associated with hops promote hop creep. The goal of these assays was to simulate dry hopping of actively fermenting beer and the results suggested that microbes associated with hops may not add risk of hop creep in addition to the risk linked to the hops. The moldy hops addition assays included the opportunity for interactions between microbes and hops that might be involved in hop creep. However, the possibility cannot be ruled out that hop creep failed to occur in the forced attenuation assays because of shorter duration together with the higher yeast abundance in forced attenuation assays compared to that used in beer production. Any approach to further examine the possible role of microbes in hop creep should address issues of timing, yeast abundance, and yeast physiological state.
The lack of evidence for a microbial role in hop creep redirected our attention to the potential role of endogenous amylases of H. lupulus. The search for endogenous amylases of H. lupulus began by determining if hop creep is specific to hops and to rule out the possibility that adding any type of plant material to beer or wort would produce hop creep. C. sativa was chosen as a control plant material because it is closely related to H. lupulus and the female flower material analogous to the female hop flower cones is commercially available. The expectation was that the C. sativa plant material would prove to be not diastatic because no diastatic activity of C. sativa has been previously reported. However, adding female C. sativa flower to a forced attenuation assay had the same effect on over attenuation as seen with hops. In contrast, additions of oregano herb to fermenting wort did not cause over attenuation, demonstrating that hop creep is not the result of adding any plant material. This observation inspired the next step to enlist knowledge of amylase genes in C. sativa to the search for endogenous amylase of H. lupulus.
The currently available genome sequences of H. lupulus do not include annotation that would enable finding genes by name such as “amylase”. To overcome this limitation, a comparative genomic approach was employed relying on the genetic similarity between H. lupulus and the only other member of the Cannabaceae family, C. sativa. A keyword search of the C. sativa proteome revealed 13 amino acid sequences annotated as “amylases”. Using the C. sativa amylase amino acid sequences to query the H. lupulus genome identified highly similar sequences in the H. lupulus genome that likely encode amylases. PCR and sequencing showed that the amylase gene sequences identified in the Saazer and Shinshu Wase hop genome sequences are found in DNA extracted from the Citra® hop cultivar that is widely used in contemporary craft beer brewing.
Greater knowledge of the amylase genes of H. lupulus identified in this study will contribute to the understanding of hop biology. Sequence similarity suggests that the amylases identified might play a role in starch metabolism in C. sativa and H. lupulus. Amylases have been shown to play a role in cold tolerance of plants,[Citation46] which offers a hypothesis to test for the amylases in C. sativa and H. lupulus. Four of the H. lupulus amylase genes identified are highly similar to β-amylase genes in C. sativa belonging to protein clusters identified as chloroplastic amylases. The β-amylases localized to the chloroplast appear to play a role in producing maltose that is protective during cold temperature shock.[Citation47] However, caution is warranted until amylase enzyme activity linked to these genes can be demonstrated. Examples where sequence expression analysis revealed an activity differing from that suggested by sequence similarity includes certain genes identified as bacterial cellulase genes that were later shown by expression analysis to have peptidase activity.[Citation48]
We propose that the amylase genes of H. lupulus identified in this study represent the genetic basis underlying hop creep driven by endogenous amylase activity of hops. Knowledge of the H. lupulus genes involved in hop creep opens the possibility of applying genome editing tools to intervene and eliminate hop creep from hop cultivars used to produce dry hopped beer. Clustered regularly interspaced short palindromic repeats (CRISPR) have proven useful for removing undesirable traits of plants by knocking out specific genes.[Citation49] Applying such an approach to H. lupulus seems possible, as CRISPR has been used successfully to edit the phytoene desaturase gene in H. lupulus, interfering with plant pigment synthesis.[Citation50] If endogenous amylases are not essential for the growth of H. lupulus, it may be possible to produce hop cultivars lacking the genes at the root cause of hop creep. Developing a better understanding of endogenous amylases of H. lupulus has implications for understanding hop biology and for the application of hops in industrial brewing of dry hopped beer styles.
Acknowledgements
This work was inspired by the TSC Project for Excellence in Microbial Ecology. Heavy Seas Beer provided laboratory space and analytical instrumentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Literature cited
- Hieronymus, S. 2012. A Plant with a Past. In For the Love of Hops: The Practical Guide to Aroma, Bitterness, and the Culture of Hops; Brewers Publications, A Division of the Brewers Association: Boulder, CO; pp. 45–63.
- Hornsey, I. S. 2003. The British Isles and Europe. In A History of Beer and Brewing; Royal Society of Chemistry: Cambridge, UK; pp. 165–276.
- Janish, S. Dry Hop Best Practices: Using Science as a Guide for Process and Recipe Development. Tech. Q. Master Brew. Assoc. Am. 2021, 58, 59–65.
- Kirkpatrick, K. R.; Shellhammer, T. H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus)). J Agric Food Chem. 2018, 66, 9121–9126. DOI: 10.1021/acs.jafc.8b03563.
- Stokholm, A.; Shellhammer, T. H. 2020. Hop Creep – Technical Brief. In Educational Publications. Published online at https://www.brewersassociation.org/educational-publications/hop-creep-technical-brief/. Brewers Association.
- Kirkpatrick, K. R.; Shellhammer, T. H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. DOI: 10.1080/03610470.2018.1546091.
- Kirkendall, J. A.; Mitchell, C. A.; Chadwick, L. R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. DOI: 10.1080/03610470.2018.1469081.
- Bocquet, L.; Sahpaz, S.; Hilbert, J. L.; Rambaud, C.; Rivière, C. Humulus lupulus L., a Very Popular Beer Ingredient and Medicinal Plant: Overview of Its Phytochemistry, Its Bioactivity, and Its Biotechnology. Phytochem. Rev. 2018, 17, 1047–1090. DOI: 10.1007/s11101-018-9584-y.
- Hill, S. T.; Sudarsanam, R.; Henning, J.; Hendrix, D. 2017. HopBase. In HopBase: A Unified Resource for Humulus Genomics. Published online at http://hopbase.cgrb.oregonstate.edu/index.html.
- Padgitt-Cobb, L. K.; Kingan, S. B.; Wells, J.; Elser, J.; Kronmiller, B.; Moore, D.; Concepcion, G.; Peluso, P.; Rank, D.; Jaiswal, P.; et al. A Draft Phased Assembly of the Diploid Cascade Hop (Humulus lupulus) Genome. Plant Genome 2021, 14, e20072. DOI: 10.1002/tpg2.20072.
- Natsume, S.; Takagi, H.; Shiraishi, A.; Murata, J.; Toyonaga, H.; Patzak, J.; Takagi, M.; Yaegashi, H.; Uemura, A.; Mitsuoka, C.; et al. The Draft Genome of Hop (Humulus Lupulus), an Essence for Brewing. Plant Cell Physiol. 2015, 56, 428–441. DOI: 10.1093/pcp/pcu169.
- Windhausen, A. B. 2020. Practical Enzymatic Brewing: An Intermediate Exploration of Brewing Enzymes. In Craft Brewers Conference & BrewExpo America. Published online at https://www.brewersassociation.org/wp-content/uploads/2020/05/CBC-Online-Seminar-Presentation-Practical-Enzymatic-Brewing.pdf. Brewers Association.
- Pethybridge, S.; Mahaffee, W. 2007. Diseases of Hop (Humulus lupulus L.); The American Phytopathological Society. Published online at https://www.apsnet.org/edcenter/resources/commonnames/Pages/DiseasesofHop.aspx.
- Guinard, J. X.; Woodmansee, R. D.; Billovits, M. J.; Hanson, L. G.; Gutierrez, M. J.; Snider, M. L.; Miranda, M. G.; Lewis, M. J. The Microbiology of Dry-Hopping. Tech. Q. Master Brew. Assoc. Am. 1990, 27, 83–89.
- Teraoka, R.; Kanauchi, M.; Bamforth, C. W. Do Starch Degrading Enzymes in Hop Samples Originate in Microorganisms. Tech. Q. Master Brew. Assoc. Am. 2021, 58, 143–147. DOI: 10.1094/TQ-58-3-0705-01.
- Aryal, S. 2019. Potato Dextrose Agar (PDA) - Principle, Uses, Procedure & Characteristics. Published online at https://microbiologyinfo.com/potato-dextrose-agar-pda-principle-uses-composition-procedure-and-colony-characteristics/MicrobiologyInfo.com.
- Ryberg, M.; Kristiansson, E.; Sjökvist, E.; Nilsson, R. H. An Outlook on the Fungal Internal Transcribed Spacer Sequences in GenBank and the Introduction of a Web-Based Tool for the Exploration of Fungal Diversity. New Phytol. 2009, 181, 471–477. DOI: 10.1111/j.1469-8137.2008.02667.x.
- Tringe, S. G.; Hugenholtz, P. A Renaissance for the Pioneering 16S rRNA Gene. Curr. Opin. Microbiol. 2008, 11, 442–446. DOI: 10.1016/j.mib.2008.09.011.
- GENEWIZ from Azenta | Bacterial and Fungal Identification. Published online at https://www.genewiz.com/en/Public/Services/Molecular-Genetics/Bacterial-and-Fungal-Identification.
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A Greedy Algorithm for Aligning DNA Sequences. J. Comput. Biol. 2000, 7, 203–214. DOI: 10.1089/10665270050081478.
- Visagie, C. M.; Houbraken, J.; Frisvad, J. C.; Hong, S.-B.; Klaassen, C. H. W.; Perrone, G.; Seifert, K. A.; Varga, J.; Yaguchi, T.; Samson, R. A. Identification and Nomenclature of the Genus Penicillium. Stud. Mycol. 2014, 78, 343–371. DOI: 10.1016/j.simyco.2014.09.001.
- Lawrence, D. P.; Rotondo, F.; Gannibal, P. B. Biodiversity and Taxonomy of the Pleomorphic Genus Alternaria. Mycol. Prog. 2016, 15, 1–22. DOI: 10.1007/s11557-015-1144-x.
- National Center for Biotechnology Information Protein database. In: National Center for Biotechnology Information Protein database. Published online at https://www.ncbi.nlm.nih.gov/protein/. NCBI Protein Database.
- Ashtavinayak, P.; Elizabeth, H. A. Review: Gram Negative Bacteria in Brewing. Adv. Microbiol. 2016, 06, 195–209. DOI: 10.4236/aim.2016.63020.
- Vaughan, A.; O’Sullivan, T.; Sinderen, D. Enhancing the Microbiological Stability of Malt and beer - A Review. J. Inst. Brew. 2005, 111, 355–371. DOI: 10.1002/j.2050-0416.2005.tb00221.x.
- Meiners, L.; Cavanna, M. Piecing Together What we Know and What We Don’t on Biotransformation and Its Organoleptic Impact. Tech. Q. Master Brew. Assoc. Am. 2021, 58, 148–153. DOI: 10.1094/TQ-58-3-0812-01.
- Thomma, B. P. H. J. Alternaria Spp.: From General Saprophyte to Specific Parasite: Alternaria. Mol. Plant Pathol. 2003, 4, 225–236. DOI: 10.1046/j.1364-3703.2003.00173.x.
- Louw, J. P.; Korsten, L. Pathogenic Penicillium Spp. on Apple and Pear. Plant Dis. 2014, 98, 590–598. DOI: 10.1094/PDIS-07-13-0710-RE.
- El Aty, A. A. A.; Aty, E.; Mostafa, F. Production and Characterization of Fungal α-Amylase from Marine Alternata Utilizing Lignocellulosic Wastes and Its Application. Res. J. Pharm., Biol. Chem. Sci. 2015, 6, 975–8585.
- Balkan, B.; Ertan, F. Production and Properties of Alpha-Amylase from Penicillium chrysogenum and Its Application in Starch Hydrolysis. Prep. Biochem. Biotechnol. 2005, 35, 169–178. DOI: 10.1081/PB-200054740.
- Gent, D. H.; Barbour, J. D.; Dreves, A. J.; James, D. G.; Parker, R.; Walsh, D. B. Field Guide for Integrated Pest Management in Hops, 2nd ed.; Oregon State University, University of Idaho, USDA Agricultural Research Service, Washington State University: Pullman, WA, 2010.
- Fonseca, N. R.; Ibarra Caballero, J.; Kim, M.-S.; Stewart, J. E.; Guimarães, L. M. S.; Alfenas, A. C.; Klopfenstein, N. B. Transcriptome Analysis of a Powdery Mildew Pathogen (Podosphaera pannosa) Infecting Eucalyptus urophylla: De Novo Assembly, Expression Profiling and Secretome Prediction. For. Path. 2019, 49, e12508. DOI: 10.1111/efp.12508.
- Purayannur, S.; Cano, L. M.; Bowman, M. J.; Childs, K. L.; Gent, D. H.; Quesada-Ocampo, L. M. The Effector Repertoire of the Hop Downy Mildew Pathogen Pseudoperonospora humuli. Front. Genet. 2020, 11, 910. DOI: 10.3389/fgene.2020.00910.
- Stokholm, A.; Van Simaeys, K.; Gallagher, A.; Weaver, G.; Shellhammer, T. H. Investigating the Effect of Farm Management, Soil, and Climate on Hop Diastatic Potential. J. Am. Soc. Brew. Chem. 2021, 1–12. DOI: 10.1080/03610470.2021.1977902.
- Sevigny, J. L.; Lloyd, B.; McComish, C.; Ramsey, A.; Koziol, L. Whole-Genome Sequences of Pantoea agglomerans BL3, Pseudomonas fluorescens BL, and Pseudomonas stutzeri CM14, Isolated from Hops (Humulus lupulus). Microbiol. Resour. Announce. 2019, 8, 1–2. DOI: 10.1128/MRA.00545-19.
- Anderson, L. M.; Stockwell, V. O.; Loper, J. E. An Extracellular Protease of Pseudomonas fluorescens Inactivates Antibiotics of Pantoea agglomerans. Phytopathology 2004, 94, 1228–1234. DOI: 10.1094/PHYTO.2004.94.11.1228.
- Lim, J.-A.; Lee, D. H.; Kim, B.-Y.; Heu, S. Draft Genome Sequence of Pantoea agglomerans R190, a Producer of Antibiotics against Phytopathogens and Foodborne Pathogens. J. Biotechnol. 2014, 188, 7–8. DOI: 10.1016/j.jbiotec.2014.07.440.
- Haas, D.; Keel, C. Regulation of Antibiotic Production in root-colonizing Pseudomonas spp. and Relevance for Biological Control of Plant Disease. Annu. Rev. Phytopathol. 2003, 41, 117–153. DOI: 10.1146/annurev.phyto.41.052002.095656.
- Lami, M. J.; Adler, C.; Caram, Di Santo, M. C.; Zenoff, A. M.; Cristóbal, R. E.; Espinosa, Urgel, M.; Vincent, P. A. Pseudomonas stutzeri MJL19, A Rhizosphere-Colonizing Bacterium that Promotes Plant Growth Under Saline Stress. J. Appl. Microbiol. 2020, 129, 1321–1336. DOI: 10.1111/jam.14692.
- Peacock, V.; Arendt, B.; Thiel, R.; Gura, M.; Chadwick, L. A Comparison of Hop Drying with Unheated, Dehumidified Air versus Traditional Drying with Heated Air. Tech. Q. Master Brew. Assoc. Am. 2018, 55, 63–66. DOI: 10.1094/TQ-55-3-1108-01.
- Adolf, R.; Karel, K.; Petr, H.; Ivo, H.; Jaroslav, P. Effect of Drying Temperature on the Content and Composition of Hop Oils. Plant. Soil Environ. 2018, 64, 512–516. DOI: 10.17221/482/2018-PSE.
- Rubottom, L. N.; Lafontaine, S. R.; Hauser, D. G.; Pereira, C.; Shellhammer, T. H. Hop Kilning Temperature Sensitivity of Dextrin-Reducing Enzymes in Hops. J. Am. Soc. Brew. Chem. 2022, 80, 75–83. DOI: 10.1080/03610470.2021.1903290.
- Nunes, C.; Usall, J.; Teixidó, N.; Fons, E.; Viñas, I. Post-Harvest Biological Control by Pantoea agglomerans (CPA-2) on Golden Delicious Apples. J. Appl. Microbiol. 2002, 92, 247–255. DOI: 10.1046/j.1365-2672.2002.01524.x.
- Boyacioglu, O.; Sharma, M.; Sulakvelidze, A.; Goktepe, I. Biocontrol of Escherichia coli O157: H7 on Fresh-Cut Leafy Greens. Bacteriophage 2013, 3, e24620. DOI: 10.4161/bact.24620.
- Moye, Z. D.; Das, C. R.; Tokman, J. I.; Fanelli, B.; Karathia, H.; Hasan, N. A.; Marek, P. J.; Senecal, A. G.; Sulakvelidze, A. Treatment of Fresh Produce with a Salmonella-Targeted Bacteriophage Cocktail is Compatible with Chlorine or Peracetic Acid and More Consistently Preserves the Microbial Community on Produce. J. Food Saf. 2020, 40, 1–15. DOI: 10.1111/jfs.12763.
- Sun, S.; Fang, J.; Lin, M.; Qi, X.; Chen, J.; Wang, R.; Li, Z.; Li, Y.; Muhammad, A. Freezing Tolerance and Expression of β-Amylase Gene in Two Actinidia arguta Cultivars with Seasonal Changes. Plants (Basel) 2020, 9, 515. DOI: 10.3390/plants9040515.
- Kaplan, F.; Guy, C. L. Beta-Amylase Induction and the Protective Role of Maltose during Temperature Shock. Plant Physiol. 2004, 135, 1674–1684. DOI: 10.1104/pp.104.040808.
- Cottrell, M. T.; Yu, L.; Kirchman, D. L. Sequence and Expression Analyses of Cytophaga-Like Hydrolases in a Western Arctic Metagenomic Library and the Sargasso Sea. Appl. Environ. Microbiol. 2005, 71, 8506–8513. DOI: 10.1128/AEM.71.12.8506-8513.2005.
- Zhang, Y.; Malzahn, A. A.; Sretenovic, S.; Qi, Y. The Emerging and Uncultivated Potential of CRISPR Technology in Plant Science. Nat. Plants 2019, 5, 778–794. DOI: 10.1038/s41477-019-0461-5.
- Awasthi, P.; Kocábek, T.; Mishra, A. K.; Nath, V. S.; Shrestha, A.; Matoušek, J. Establishment of CRISPR/Cas9 Mediated Targeted Mutagenesis in Hop (Humulus lupulus). Plant Physiol. Biochem. 2021, 160, 1–7. DOI: 10.1016/j.plaphy.2021.01.006.