Abstract
There is an increasing drive across the global distilling industry for a greater understanding of the development of sensory properties in whisky. Origins of the fruity aroma descriptor have been reasonably well-explored in the current literature, but this umbrella term captures a wide range of quite varied sub-descriptors. One such fruit-type lexicon term that has received limited attention is that of mango. The present trial assessed the sensory profiles of 14 commercial whisky products and identified whiskies elevated in the target mango trait. Further analysis of product volatile compound composition allowed for the shortlisting of candidate compounds potentially contributory to this trait. Spiking of candidate compounds into a base whisky identified a potential role for several common whisky components in the development of mango aroma. In particular, aldehyde and acetal components (such as isobutyraldehyde, isovaleraldehyde, and isovaleraldehyde diethyl acetal) were found to positively impact the sensory reporting of mango aroma in whisky. These compounds are not atypical to whisky, and their production pathways and precursors are previously identified; this provides scope for their control without substantial process modification.
Introduction
There is increasing interest across the global whisky sector for the development of novel and diverse aroma properties in mature spirit products.[Citation1] The development of distinctive product characteristics aids brand recognition, which is of particular importance in growing and crowded markets.[Citation2] There has been a consequent demand amongst spirit producers for an increased understanding of the factors influencing product aroma profile in order to aid the development of new single-malt and blended products.[Citation1] The specific terminology for the description of aroma/flavour properties in whisky products might vary across producers. Still, there are common descriptors that are frequently used, such as those detailed in published flavour wheel diagrams.[Citation3] Within a given lexicon, the descriptors used to describe whisky aroma are typically grouped under umbrella terms such as ‘peaty’, ‘grainy’, and ‘woody’. Within a given grouping, descriptors are generally related but can be reasonably diverse. For example, the ‘woody’ grouping might address descriptors ranging from green bark through treacle and coffee-like aroma properties.[Citation3] The descriptors housed within the common grouping ‘fruity’ can be associated with fresh and dried fruit-type aromas as well as citrus and artificial fruit flavourings.[Citation3] Alongside apple, pear, and banana, ‘tropical fruit’ is used as a catch-all for more exotic fruits including pineapple and melon, as well as mango, which has been used specifically in commercial product descriptors and published literature.[Citation3–5]
There has been considerable research of the development of fruit-type aromas in whisky, with much of the work focussing on the impact of esters and higher alcohols.[Citation3,Citation6] Acetate esters such as isoamyl acetate have been linked to the properties of banana and pear-drop, and ethyl esters (of varying chain length) are often associated with fresh fruit aromas.[Citation3] For example, ethyl hexanoate is typically described as contributing apple-like aroma to spirit,[Citation7] whilst ethyl octanoate has been linked to increasing pineapple aroma properties.[Citation8] Whilst there is currently little published research around tropical fruit aroma in distilled spirits, there have been studies investigating this trait in wine and beer. Passion fruit and mango aromas have been described in white wine and were linked to specific grape cultivars,[Citation9,Citation10] and in beer, a role for hop-derived thiols has been identified.[Citation11] Analysis of mango fruit itself identifies a range of volatiles that are contributory to its aroma, these include terpenes (e.g. α-pinene, β-myrcene, terpinolene, limonene, δ-3-carene, and β-caryophyllene), ketones (e.g. (E)-β-damascenone), esters (e.g. ethyl butanoate and ethyl 2-methyl propanoate), and aldehydes (e.g. nonanal, hexanal, and isovaleraldehyde).[Citation12–14] Whilst some volatiles associated with mango fruit have previously been identified in whisky many are not typical, and the spirit components contributing to mango-type aroma in whisky products remain largely unknown.
In the present research, the contribution of volatile compounds to mango-type aroma in mature whisky products was investigated. Sensory and volatile compound profiles were developed for 14 commercially available mature whisky products (from Scotland and Ireland). Through the use of Principal Component Analysis (PCA), whisky products elevated in mango aroma were identified, and a pool of compounds possibly contributing to the mango aroma descriptor was established by correlation analysis. Sensory analysis of whisky samples spiked with candidate compounds was used to investigate the contribution of individual and grouped volatile compound contribution to mango-type aroma in a whisky matrix.
Materials and methods
Chemicals
All chemicals were obtained from the following commercial sources at analytical grade purity: acetaldehyde (≥99.5%), acetaldehyde diethyl acetal (Analytical Standard), acetaldehyde diethyl acetal (Natural, FG, ≥97%), dichloromethane (>99%), ethyl decanoate (>99%), 2-heptanol (Analytical Standard), 2-heptanol (FG, ≥97%), 2-heptanone (Analytical Standard), isoamyl decanoate (≤100%), isoamyl octanoate (≥98%), isobutyraldehyde (Analytical Standard), isobutyraldehyde (FG, ≥98%), isobutyraldehyde diethyl acetal (≤100%), isovaleraldehyde (Analytical Standard), isovaleraldehyde (FG, ≥97%), isovaleraldehyde diethyl acetal (FG, ≥98%), 4-methyl-3-penten-2-one (Analytical Standard), 1,1,3-triethoxypropane (≥95%) from Sigma-Aldrich (Dorset, UK); ethanol (≥99.8%), sodium chloride (>95%) from Fisher Chemicals (Loughborough, UK); 3-heptanone (≥98%) from Thermo Scientific Chemicals (Waltham, U.S.A.); isovaleraldehyde diethyl acetal (≥95%) from BOC Sciences (New York, U.S.A.); Grain Neutral Spirit (96% ABV) from Kimia Fine Alcohols (Witham, UK).
Sensory characterisation of commercial whisky products
To assess the aroma properties of commercial whisky products, a sensory evaluation using a Quantitative Descriptive Analysis (QDA) approach was conducted using whisk(e)y (henceforth described as whisky) products from across Scotland and Ireland (). Whisky samples were collected from the UK market during 2021 and 2022, except Whiskies 1 and 2, sourced in Japan in 2020.
Table 1. The production region and additional properties of the investigated single malt whisky products. Production regions are defined using nomenclature of territorial units for statistics (NUTS) descriptors[32] or Scotch whisky producing regions as described by the Scotch whisky regulations 2009.[33]
The sensory evaluation methodology was based on the approach previously described by Jack,[Citation15] and the evaluation sheets were modified from the work of Lawless and Heymann.[Citation16] Ethics for the trial (Project ID 2916) were assessed and approved by the Engineering and Physical Sciences Ethics Committee at Heriot-Watt University. Participation was voluntary and written consent was sought from all panellists following a briefing and before experimental work commenced. Whisky samples were diluted to 20% ABV with distilled water, and then 30 mL was poured into 130 mL standard tulip-shaped glasses with a glass lid. Samples were prepared fresh for each panellist and were not reused. Samples were used within 6 h of preparation as recommended by Jack.[Citation15] The panellists evaluated the products under red light and in random order across a 1 h assessment period. The tests were duplicated in a follow-up session.
Samples were evaluated by eight trained panellists working in blender or researcher positions in the Whisky and Spirits section at the Institute for Future Beverages in the Research and Development Division of Kirin Holdings Company (Yokohama, Japan). The panellists had received prior training for the aroma properties investigated. Panellists were asked to evaluate the aroma properties of each sample by orthonasal assessment with aroma intensity indicated by placing a mark against a 150 mm horizontal bar for each lexicon term. The bars were labelled at 15, 75, and 135 mm (from left to right) with the respective descriptors of Weak, Moderate, and Strong. Following the evaluation, panellist scores were converted to percentage values for further analysis. Panellists used 16 sensory descriptors to characterise the samples: floral, green, smoky, cereal, sulphury, banana, apple, peach, orange, tropical, mango, cream, caramel, oily, alcohol, and woody. Data was processed using Principal Component Analysis (PCA) in XLSTAT (ver. 2022.1.1.1248; Addinsoft, Paris, France) to identify correlations between each product and the sensory properties evaluated.
Analysis of whisky aroma volatile composition
Whisky samples were prepared according to the method described by Marčiulionytė et al.[Citation17] Triplicate samples were diluted with distilled water to 15 mL at 20% ABV and placed in a 50 mL centrifuge glass tube. An internal standard (100 µL of 3-heptanone in ethanol; 4000 mg/L) and 0.5 mL saturated sodium chloride solution were added to diluted samples. Dichloromethane (DCM; 0.5 mL) was added to the sample mixtures, followed by agitation for 30 s using a vortex mixer and centrifugation for 3 min at 1000 rpm. The DCM layer was recovered and was transferred to 2 mL GC vials with glass inserts (100 µL). Extracts of 1 µL were injected directly into a Shimadzu QP2010 Ultra GC–MS coupled to an AOC-5000 for analysis (Shimadzu, Kyoto, Japan). The injection port was held at 230 °C (split ratio 1:1), and samples were separated on a DB-WAX Ultra Inert GC Column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies, California, U.S.A.). The temperature profile was as follows: 40 °C for 5 min, increased at 3 °C/min to 250 °C, holding for 5 min at the final temperature. Helium was used as carrier gas with a constant velocity of 29 cm/s. The MS transfer line and ion source temperature were held at 250 °C and 200 °C, respectively. GCMSsolution software (version 2.61; Shimadzu) was used for peak integration and sample analysis, and the detected peaks in SCAN mode were identified preliminarily using NIST08s library software (The National Institute of Standards and Technology, Maryland, U.S.A.). Using each compound peak area ratio to the internal standard and the sensory evaluation scores, Pearson’s correlation coefficient was calculated by XLSTAT to identify which volatile components correlated most strongly to mango aroma in the 14 whiskies investigated.
Quantification of candidate compounds
Volatile compounds provisionally identified as correlating to mango-type aroma in whisky were quantified in triplicate compared to reference compounds. Whisky samples were diluted with distilled water to 40% ABV, and 10 mL was added to a crimp neck 20 mL glass vial. Internal standard (100 µL) of 3-heptanone in ethanol (4000 mg/L) was added to the sample. Aldehydes and acetals were analysed with a GC-MS-QP-2010 Ultra coupled to a CPL AOC-5000 with Solid-Phase Microextraction (SPME) using a Polydimethylsiloxane/Divinylbenzene (PDMS/DVB) fibre (65 µm; Supleco, Pennsylvania, U.S.A.). Prior to extraction, samples were heated at 50 °C for 1 min whilst agitating at 500 rpm in a Heatex Stirrer (Axel Semrau GmbH, Sprockhövel, GB). Samples were extracted for 1 min and then desorbed to the GC injection port (250 °C) for 1 min (split ratio was set to 0) then separated using a HP-5ms capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies) operated with the following temperature profile: hold at 28 °C for 15 min, ramp 2 °C/min to 70 °C, ramp 3 °C/min to 100 °C, ramp 10 °C/min to 200 °C, ramp 20 °C/min to 300 °C and hold at temperature for 3 min. Helium was used as carrier gas with a constant velocity of 29 cm/s. The MS transfer line and ion source temperature were held at 250 °C and 200 °C, respectively. The MS was operated in SIM mode, and the samples were quantified against calibration curves for the target compounds. The following m/z values were monitored: acetaldehyde (42, 43, 44); isobutyraldehyde (72, 41, 39); isovaleraldehyde (44, 58, 71, 41); acetaldehyde diethyl acetal (73, 103, 47, 29); isobutyraldehyde diethyl acetal (103, 101, 75, 73, 55); isovaleraldehyde diethyl acetal (103, 115, 75, 47, 69, and 71); 3-heptanone (57, 85, 41).
The alcohols, ketones, and fatty acid esters were analysed by direct injection to GC-MS. Samples were analysed in triplicate with DCM extraction as previously described. Extracts of 1 µL were injected directly into the GC–MS (split ratio was 1:0), followed by HP-5ms column separation with helium carrier gas. Analysis of 2-heptanol and 2-heptanone used the following temperature profile: hold 40 °C for 10 min, ramp 10 °C/min to 65 °C, hold for 10 min, ramp 10 °C/min to 90 °C, hold for 10 min, ramp 10 °C/min to 120 °C, hold for 10 mins, ramp 30 °C/min to 320 °C and hold for 5 min. For the analysis of 4-methyl-3-penten-2-one and ethyl decanoate, samples were separated using a temperature profile of: hold 40 °C for 10 min, ramp 3 °C/min to 180 °C, ramp 20 °C/min to 320 °C with a 5 min hold. Analysis of isoamyl octanoate and isoamyl decanoate used the following modified temperature profile: hold 50 °C for 10 min, ramp 10 °C/min to 85 °C, hold for 10 min, ramp 10 °C/min to 170 °C hold for 10 min, ramp 30 °C/min to 320 °C and hold for 5 min. Helium was used as carrier gas with a constant velocity of 29 cm/s. The MS transfer line and ion source temperature were held at 250 °C and 200 °C, respectively. The MS was operated in SIM mode, and the samples were quantified against calibration curves for the compounds: the software GCMSsolution was used for peak integration and sample analysis. The following m/z values were monitored: 2-heptanol (45, 55, 83); 2-heptanone (58, 59, 71); 4-methyl-3-penten-2-one (55, 83, 43); ethyl decanoate (88, 101, 41); isoamyl octanoate (70, 43, 127); isoamyl decanoate (70, 43, 55); 3-heptanone (57, 85, 41). For statistical analysis, compounds returning undetected or out of calibration range were treated as a peak area 0.
Sensory analysis of volatile impact on mango-type aroma in whisky
To confirm the contribution of volatile compounds to mango-type aroma in whisky, candidate compounds were introduced into a sample previously found to present with only low mango sensory properties (Whisky 13) with subsequent sensory analysis. Trial ethics were assessed and approved (Project ID 4580) as described previously. Participation was voluntary and written consent was sought from all panellists following a trial briefing. Selection of candidate compounds was informed by correlation to mango aroma observed in commercial whisky samples, odour activity values (OAVs; calculated using a volatile concentration in Whisky 2 at 20% ABV), and availability of food-grade standards. Each stock solution of candidate compounds was prepared using grain neutral spirit (GNS) and added to Whisky 13 such that the volatile concentration in Whisky 2 was replicated. Sensory analysis was conducted at Heriot-Watt University (Edinburgh, UK) by a total of 21 unique panellists across duplicate trials (16 panellists at each trial). Panellists were all students or staff at Heriot-Watt University and were familiar with the sensory properties of whisky. To reduce the risk of general fruity aroma properties presenting as mango aroma, panellists assessed four sensory properties capturing a range of fruit-type aroma descriptors: mango, peach, apple, and orange. Prior to the analysis of experimental samples, panellists were trained on each sensory property using commercial food flavourings added into a 20% ABV whisky. Training samples were produced by the addition of flavourings at two concentrations (termed ‘moderate’ and ‘strong’) into Whisky 13, with an additional unaltered Whisky 13 acting as a ‘weak’ reference. The following food flavourings were used to prepare reference samples for recognition training: Double Apple High Strength Professional Flavouring (moderate addition rate: 0.021% v/v, strong addition rate: 0.042% v/v), Juicy Peach High Strength Professional Flavouring (moderate: 0.005% v/v, strong: 0.010% v/v), and Sweet Mango High Strength Professional Flavouring (moderate: 0.0009% v/v, strong: 0.0017% v/v) from GALAX-E-JUICE Ltd. (Norfolk, UK), Natural Orange Flavour (moderate: 0.0005% v/v, strong: 0.0010% v/v) from Foodie Flavours Ltd. (Hertfordshire, UK). To test recognition training, panellists were asked to identify anonymised reference samples prior to exposure to experimental whiskies. Analysis of experimental samples was completed under red light using the same sensory approach as previously described for analysis of samples by the trained industry panel (orthonasal assessment).[Citation15,Citation16] Samples were presented to panellists in random order. Sensory data was processed using Analysis of Variance (ANOVA) with Tukey’s HSD (using XLSTAT). A significance threshold of p < 0.05 was used to define significant differences.
Results and discussions
Sensory analysis of the 14 whisky samples (representing a range of Scottish and Irish whisk(e)y producing regions) by expert panellists revealed significant differences in the sensory properties of the samples, which were primarily found in the fruity-type aroma descriptors, although some separation was also evident for green, smoky, and sulphury terms (). With regards to fruity-type aroma properties, the five non-chill filtered (as identified by product packaging) samples scored highest (average score of banana, apple, peach, orange, tropical, and mango; 41.2–50.5) of the assessed products. There is also a general trend of increased reporting of mango aroma in whisky samples packaged at higher alcohol strength. The contribution of ethyl esters to fruit aroma in distilled spirits is well-established, and long-chain ethyl esters are able to precipitate to form haze.[Citation18] Several studies have identified a significant reduction in ethyl ester content in distilled spirits following chill filtration that might explain the trends observed here[Citation19,Citation20] although specific filtration methodology is likely important. Within the whisky industry chill filtration conditions vary considerably, with use of varying temperatures (–10 °C to 10 °C) and filtration media.[Citation21] The filtration conditions used during production of samples here are unknown outside of label declarations. Whisky 2 (a non-chill filtered whisky from the Ireland Dublin region), in particular, scored highly for overall fruity character (50.5) compared to other products and returned the highest score for all individual fruit-type descriptors. For the mango descriptor, scores ranged from 54.7 (Whisky 2) to 21.6 (Whisky 5), and similarly to overall fruity character, the samples returning the highest values trended towards non-chill filtered production processes and elevated packaged ABV. ANOVA revealed three overlapping Tukey’s HSD post-hoc groups within assessment of mango aroma (), with Whisky 2 fully separated from Whisky 5, 9, 10, and 13. Principal Component Analysis (PCA) was used to identify overall associations of whisky samples and sensory properties (). PCA confirmed strong associations of Whisky 2 with mango and tropical aroma properties and of Whisky 1, 11, 12, and 14 with overall fruity descriptors () through a positive loading to PC1. The close association of tropical aroma with that of mango perhaps indicates that panellists interpret these two descriptors similarly. Those samples trending furthest from fruit-type aromas though negative PC1 associations (Whisky 5 and 10) were generally associated with elevated sensory scores for smoky (respectively, 82.1 and 51.1), cereal (38.7 and 36.2), and sulphury (40.3 and 59.1) character as compared to other products, and at least one of these samples (Whisky 5) was produced using peated malt. Previous research has identified similarly inverse reporting of fruity/floral traits against cereal, sulphury, and oily terms in new make spirit samples.[Citation22]
Figure 1. PCA plot displaying the aroma characteristics of 14 whisky samples assessed using sensory evaluation by eight trained panellists (PC1 and PC2 70.16%).
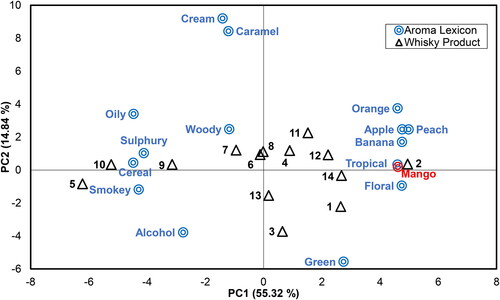
Table 2. The average sensory scores of 16 lexicon terms in 14 commercial whiskies as determined by an industry panel. Traits scored on a scale of 0–100.
Preliminary analysis of whisky samples by GC-MS provisionally identified a range of aroma volatiles present in each whisky sample. Pearson’s correlation of provisionally identified volatiles against each sensory trait was used to shortlist those volatile components potentially associated with a positive contribution to mango-type aroma. Pearson’s correlation values of >0.4 have previously been described as indicating a moderate/fair-strong positive correlation, with >0.7 and >0.8 indicating moderate-very strong and strong-very strong correlations, respectively.[Citation23] In the present study, a Pearson’s correlation value of 0.5 was used as a minimum threshold for shortlisting candidate volatiles, and 10 compounds were identified with significant (p < 0.05) correlation values that met this threshold for the mango trait (). All 10 compounds displaying a significant correlation to mango aroma also returned a significant positive correlation to the tropical aroma trait, again indicating a potential overlap in the reporting of these sensory characteristics. Isoamyl decanoate and isoamyl octanoate both returned significant positive correlations to all of the fruit-type aromas evaluated (mango, tropical, apple, orange, banana, peach). This is in agreement with previous reports of these components contributing to fruity/floral-type aromas in distilled spirits.[Citation24,Citation25] As for PCA of whisky product sensory traits (), an abundance of the volatile compounds that correlated strongly to fruit-type aroma was often associated with a negative correlation to the properties of cereal, sulphury, and oily. Interestingly, four of the compounds identified to correlate most strongly to mango and tropical aroma in the whisky samples were acetal compounds. Acetals have previously been identified in distilled spirits, including whisky and have been associated with fruity-type aroma properties,[Citation1,Citation26] such as apple, cherry, and pineapple.[Citation27]
Table 3. Pearson’s correlation assessing the relationship between sensory evaluation score by eight panellists (of 14 commercial whiskies) and peak area of whisky component compounds measured using GC-MS.
Quantification of provisionally identified candidate compounds () confirmed their presence in the whisky samples and indicated substantial differences in concentration between the various products. All volatiles were generally present in high abundance in Whisky 2, and the acetal compounds and 2-heptanol and 2-heptanone, were particularly elevated here as compared to the other whisky samples. Both 4-methyl-3-penten-2-one and 2-heptanol were only detected in Whiskies 1 and 2, and 2-heptanone was only detected in Whiskies 1-4; these were all produced in Ireland-based distilleries. Odour Activity Values (OAV) are often used as an indicator of how likely a food/beverage component may be to influence product aroma, and these values are based on reported sensory threshold values for a given component and its concentration in the product of interest. Components with an OAV of <1 are generally not expected to contribute substantially to product aroma.[Citation28] OAVs (reported based on Whisky 2 composition) for the compounds of interest in the present trial () highlighted acetaldehyde diethyl acetal (OAV = 16), isobutyraldehyde diethyl acetal (OAV = 12), ethyl decanoate (OAV = 11), and isovaleraldehyde diethyl acetal (OAV = 6) to return OAVs >1. Likewise, the aldehyde precursors to isobutyraldehyde diethyl acetal and isovaleraldehyde diethyl acetal, isobutyraldehyde and isovaleraldehyde, returned strong OAVs (442 and 189, respectively). Concentration of acetals formed from aldehydes and alcohols in solution is affected by ABV and pH.[Citation29] For example, previous research has described a solution of pH 3.0 and 40% ABV reaching an equilibrium state between aldehyde and acetal in 16 h, whilst an equivalent sample at pH 4.0 took 3 days.[Citation30] In the present work, whisky samples had a pH of 3.7-4.2, and sensory evaluations were examined within 6 h after adding candidate compounds to minimise conversion before analysis.
Table 4. The concentration of compounds identified to correlate positively with mango aroma (with three associated aldehydes) in 14 whisky samples. Results are presented as the mean of triplicate analyses ± standard deviation.
Table 5. Odour activity values (OAV) for whisky components. Values are based on previously reported aroma thresholds and compound concentration in Whisky 2 at 20% ABV.
In order to confirm the contribution of candidate chemical types to mango aroma in whisky, volatile compounds were spiked into a base whisky (to replicate the concentration observed in Whisky 2) and presented to a sensory panel for orthonasal analysis. Five candidate compounds were selected for further testing according to evidence of elevated concentration in Whisky 2 (the sample correlating most closely to strong mango aroma; ), correlation to mango-type aroma, OAVs (), and availability of food-grade standards. To avoid panellist fatigue, sample numbers were limited by grouping of candidate volatiles with sequential omission of each candidate component in reverse OAV order ( and ). Whisky 13 was selected as a base spirit as, besides a notably lower score for mango aroma by industry panel analysis, it was previously judged otherwise similar in sensory profile to Whisky 2 for most other non-fruit-type sensory properties. Initial analysis of commercial whiskies () indicated that some samples scored highly across all/most descriptors under the umbrella term fruity. In order to ensure panellists were correctly identifying the intended mango trait rather than a general fruit-type aroma, panellists underwent recognition training prior to the nosing of spiked samples and were asked to score four distinct fruit aroma descriptors (mango, peach, orange, and apple). Assessment of mango aroma in as-is Whiskies 2 and 13 (scored 51.7 and 32.3 respectively; ) was similar to that of previous industry panel analysis (); Whisky 2 was scored significantly (p < 0.05) higher than Whisky 13 for mango aroma and awarded values were generally in agreement across the two panels. Addition of any of the selected compounds into Whisky 13 resulted in an increased average mango aroma score and movement across post-hoc groupings. Where volatiles were added to Whisky 13, mango aroma scores increased to 46.9-48.8, and samples moved into a shared post-hoc group with Whisky 2. Addition of isobutyraldehyde in isolation resulted in a change to post-hoc grouping; still, in terms of the mango sensory score reported, the value was only slightly higher than that of Whisky 13. Interestingly, some panellists anecdotally reported difficulty distinguishing mango and peach aromas. Still, the whisky additives used in the present study did not cause a significant change to the reporting of any of the fruity descriptors assessed besides mango. This suggests that (for the sensory properties assessed) the impact of the additive volatiles was generally limited to the mango aroma trait under the test conditions used in this trial (). Some previous trials have indicated a role for acetal compounds in pleasant fruity and apple-like aromas in distilled spirits.[Citation27,Citation31] Neither of the two acetals investigated here (acetaldehyde diethyl acetal and isovaleraldehyde diethyl acetal) resulted in a significant increase to reported apple aroma observed upon addition to the base whisky. Given the high mango value returned for the addition of only isobutyraldehyde and isovaleraldehyde into the base liquid during omission testing (), a follow-on trial was used to separate the individual and grouped impacts of these aldehydes with and without acetal addition (). Follow-on testing displayed reasonable consistency with previous trials with regard to the base whisky, but there was some panel-to-panel variation. For instance, upon spiking of isobutyraldehyde to the base spirit, the observed mango aroma value was notably higher in the second sensory trial ( and ). As in the previous trial, there was no significant impact of any of the spirit additives on the reporting of peach, orange, or apple aroma descriptors. Individual addition of isobutyraldehyde, isovaleraldehyde, and isovaleraldehyde diethyl acetal into the base spirit resulted in an increase in returned mango values (38.7–44.9) as compared to the base spirit (34.0) and movement into a new post-hoc grouping. There is also some evidence of a cumulative impact of these compounds on mango aroma, with all grouped additions scoring higher (although only subtly in some cases) than individual compound additions. Simultaneous addition of isobutyraldehyde, isovaleraldehyde, and isovaleraldehyde diethyl acetal into the base spirit returned the highest overall mango value (53.3) and full post-hoc separation from the base spirit ().
Table 6. Impact of spiking five candidate compounds on reporting of fruit-type aroma descriptors in mature whisky product. Results are presented as the average scores of 16 panellists across two duplicate sensory sessions.
Table 7. Impact of spiking isobutyraldehyde, isovaleraldehyde, and isovaleraldehyde diethyl acetal on reporting of fruit-type aroma descriptors in mature whisky product. Results are presented as the average scores of 16 panellists across two duplicate sensory sessions.
Conclusions
The present work has assessed the sensory and volatile profiles of 14 commercially available whiskies and identified substantial differences in the reporting of mango and tropical-type aromas and overall volatile composition across the product range investigated. Several volatiles of varying chemical groups (acetals, esters, ketones, and alcohols) were found to correlate positively with the reporting of mango and tropical aroma descriptors in commercial whiskies. A role for some aldehydes and acetals to positively contribute to mango-type aroma in a whisky matrix was confirmed by sensory analysis of a base spirit spiked with candidate aroma compounds. Addition of individual aldehydes (isobutyraldehyde and isovaleraldehyde) and acetal compounds (isovaleraldehyde diethyl acetal) to a base whisky presenting low mango aroma resulted in an increase in reported mango aroma and movement of experimental samples into a different Tukey post-hoc grouping to the base whisky. Addition of isobutyraldehyde, isovaleraldehyde, and isovaleraldehyde diethyl acetal together into the base whisky resulted in the complete post-hoc separation of the experimental sample from the base whisky and significantly increased reporting of mango-type aroma in the sample. Analysis of some candidate compounds identified by correlation analysis was limited by the availability of food-grade standards, and these warrant further investigation. The volatile compounds identified in the present trial as contributing to mango-type aroma in mature whisky products are not newly identified in whisky, and known mechanisms of control for these volatiles exist within common industrial process steps. It is therefore likely that distillers wishing to increase or decrease the novel mango/tropical aroma trait in their products already possess the tools to do so within current distillery practices.
| Abbreviations | ||
| ABV | = | alcohol by volume |
| ACD | = | acetaldehyde diethyl acetal |
| ANOVA | = | analysis of variance |
| DCM | = | dichloromethane |
| GC-MS | = | gas chromatography-mass spectrometry |
| GNS | = | grain neutral spirit |
| 2HN | = | 2-heptanol |
| IBA | = | isobutyraldehyde |
| IVA | = | isovaleraldehyde |
| IVD | = | isovaleraldehyde diethyl acetal |
| NIST | = | National Institute of Standards and Technology |
| OAVs | = | odour activity values |
| PCA | = | principal component analysis |
| SIM | = | selected ion monitoring |
| SPME | = | solid phase microextraction. |
Acknowledgements
The authors would like to thank Mr. Maarten Gorseling of the International Centre for Brewing and Distilling for his technical contribution to this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Kelly, T. J.; O’Connor, C.; Kilcawley, K. N. Sources of Volatile Aromatic Congeners in Whiskey. Beverages 2023, 9(3), 64. DOI: 10.3390/beverages9030064.
- Brooker, A. Brands Must Focus on Distinctiveness to Survive. 2023. https://www.thespiritsbusiness.com/2023/01/brands-must-focus-on-distinctiveness-to-survive/. (accessed Aug 5, 2023).
- Lee, K.-Y. M.; Paterson, A.; Piggott, J. R.; Richardson, G. D. Origins of Flavour in Whiskies and Revised Flavour Wheel: Review. J. Inst. Brew. 2001, 107(5), 287–313. DOI: 10.1002/j.2050-0416.2001.tb00099.x.
- Murray, J. Jim Murray’s Whisky Bible 2017. Litchborough, UK: Dram Good Books; 2016; p. 225.
- Waterbury, M.; Bryson, L. Scotch: A Complete Introduction to Scotland’s Whiskies. New York City, USA: Sterling Epicure; 2020; p. 245.
- Nykänen, L.; Nykänen, I. Distilled Beverages. In Volatile Compounds in Foods and Beverages, Maarse, H., Editor. Routledge: New York City, USA; 1991; pp. 547–580.
- Lee, K.-Y. M.; Paterson, A.; Piggott, J. R.; Richardson, G. D. Perception of Whisky Flavour Reference Compounds by Scottish Distillers. Inst. Brew. Distill. 2000, 106(4), 203–208. DOI: 10.1002/j.2050-0416.2000.tb00058.x.
- Christoph, N.; Bauer-Christoph, C. Flavour of Spirit Drinks: Raw Materials, Fermentation, Distillation, and Ageing. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability, Berger, R.G., Editor. 2007, Springer: Berlin, Heidelberg, Germany. pp. 219–239.
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of New Volatile Thiols in the Aroma of Vitis vinifera L. var. Sauvignon blanc Wines. Flavour Fragr. J. 1998, 13(3), 159–162. DOI: 10.1002/(SICI)1099-1026(199805/06)13:3<159::AID-FFJ709>3.0.CO;2-7.
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; Benito, S. Effects on Varietal Aromas During Wine Making: A Review of the Impact of Varietal Aromas on the Flavor of Wine. Appl. Microbiol. Biotechnol. 2019, 103(18), 7425–7450. DOI: 10.1007/s00253-019-10008-9.
- Holt, S.; Miks, M. H.; Carvalho, B.; Foulquié-Moreno, M. R.; Thevelein, J. M. The Molecular Biology of Fruity and Floral Aromas in Beer and Other Alcoholic Beverages. FEMS Microbiol. Rev. 2019, 43(3), 193–222. DOI: 10.1093/femsre/fuy041.
- Pandit, S. S.; Chidley, H. G.; Kulkarni, R. S.; Pujari, K. H.; Giri, A. P.; Gupta, V. S. Cultivar Relationships in Mango Based on Fruit Volatile Profiles. Food Chem. 2009, 114(1), 363–372. DOI: 10.1016/j.foodchem.2008.09.107.
- Liu, H.; An, K.; Su, S.; Yu, Y.; Wu, J.; Xiao, G.; Xu, Y. Aromatic Characterization of Mangoes (Mangifera indica L.) Using Solid Phase Extraction Coupled with Gas Chromatography-Mass Spectrometry and Olfactometry and Sensory Analyses. Foods 2020, 9(1), 75. DOI: 10.3390/foods9010075.
- Pino, J. A. Odour-Active Compounds in Mango (Mangifera indica L. cv. Corazón). Int. J. Food Sci. Tech. 2012, 47(9), 1944–1950. DOI: 10.1111/j.1365-2621.2012.03054.x.
- Jack, F. Development of Guidelines for the Preparation and Handling of Sensory Samples in the Scotch Whisky Industry. J. Inst. Brew. 2003, 109(2), 114–119. DOI: 10.1002/j.2050-0416.2003.tb00139.x.
- Lawless, H. T.; Heymann, H. Chapter 7 Scaling. In Sensory Evaluation of Food, Principles and Practices. Springer: New York, NY; 2010.
- Marčiulionytė, R.; Johnston, C.; Maskell, D. L.; Mayo, J.; Robertson, D.; Griggs, D.; Holmes, C. P. Roasted Malt for Distilling: Impact on Malt Whisky New Make Spirit Production and Aroma Volatile Development. J. Am. Soc. Brew. Chem. 2022, 80(4), 329–340. DOI: 10.1080/03610470.2022.2034133.
- Pryde, J.; Conner, J.; Jack, F.; Lancaster, M.; Meek, L.; Owen, C.; Paterson, R.; Steele, G.; Strang, F.; Woods, J. Sensory and Chemical Analysis of ‘Shackleton’s’ Mackinlay Scotch Whisky. Journal of the Institute of Brewing 2011, 117(2), 156–165. DOI: 10.1002/j.2050-0416.2011.tb00455.x.
- Puškaš, V.; Miljić, U.; Vasić, V.; Jokić, A.; Manović, M. Influence of Cold Stabilisation and Chill Membrane Filtration on Volatile Compounds of Apricot Brandy. Food Bioprod. Process. 2013, 91(4), 348–351. DOI: 10.1016/j.fbp.2012.12.005.
- Muñoz-Redondo, J. M.; Puertas, B.; Valcárcel-Muñoz, M. J.; Rodríguez-Solana, R.; Moreno-Rojas, J. M. Impact of Stabilization Method and Filtration Step on the Ester Profile of “Brandy de Jerez”. Appl. Sci. 2023, 13(6), 3428. DOI: 10.3390/app13063428.
- Piggott, J.R., 15 - Whisky, in Current Developments in Biotechnology and Bioengineering, Pandey, A.; et al. Editors. Elsevier: Amsterdam; 2017; pp. 435–450.
- Daute, M.; Jack, F.; Baxter, I.; Harrison, B.; Grigor, J.; Walker, G. Comparison of Three Approaches to Assess the Flavour Characteristics of Scotch Whisky Spirit. Appl. Sci. 2021, 11(4), 1410. DOI: 10.3390/app11041410.
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18(3), 91–93. DOI: 10.1016/j.tjem.2018.08.001.
- Nascimento, E. S. P.; Cardoso, D. R.; Franco, D. W. Quantitative Ester Analysis in Cachaça and Distilled Spirits by Gas Chromatography − Mass Spectrometry (GC − MS). J. Agric. Food Chem. 2008, 56(14), 5488–5493. DOI: 10.1021/jf800551d.
- Wang, X.; Guo, W.; Sun, B.; Li, H.; Zheng, F.; Li, J.; Meng, N. Characterization of Key Aroma-Active Compounds in Two Types of Peach Spirits Produced by Distillation and Pervaporation by Means of the Sensomics Approach. Foods 2022, 11(17), 2598. DOI: 10.3390/foods11172598.
- MacNamara, K.; Wyk, C.; Augustyn, O. P. H.; Rapp, A. Flavour Components of Whiskey. II Ageing Changes in the High-Volatility Fraction. SAJEV. 2017, 22(2), 75–81. DOI: 10.21548/22-2-2196.
- Kyraleou, M.; Herb, D.; O’Reilly, G.; Conway, N.; Bryan, T.; Kilcawley, K. N. The Impact of Terroir on the Flavour of Single Malt Whisk(e)y New Make Spirit. Foods 2021, 10(2), 443. DOI: 10.3390/foods10020443.
- Poisson, L.; Schieberle, P. Characterization of the Key Aroma Compounds in an American Bourbon Whisky by Quantitative Measurements, Aroma Recombination, and Omission Studies. J. Agric. Food Chem. 2008, 56(14), 5820–5826. DOI: 10.1021/jf800383v.
- Misselhorn, K. Formation of Acetals in Rum: A Kinetic Study. Annales de Technologie Agricole 1975, 24(3-4), 371–381.
- Suomalainen, H., Kauppila, O., Nykänen, L., and Peltonen, R.J., Branntweine. In Alkoholische Genussmittel, Bergner, K.-G.; et al. Editors. Springer: Berlin, Heidelberg; 1968; p. 496–653.
- González-Robles, I. W.; Cook, D. J. The Impact of Maturation on Concentrations of Key Odour Active Compounds which Determine the Aroma of Tequila. J. Inst. Brew. 2016, 122(3), 369–380. DOI: 10.1002/jib.333.
- Regulation (EC) No 1059/2003 of the European Parliament and of the Council of 26 May 2003 on the Establishment of a Common Classification of Territorial Units for Statistics (NUTS). Off. J. 2003, 154, 0001–0041.
- The Scotch Whisky Regulations 2009 (SI 2009/2890). 2009. Available from https://www.legislation.gov.uk/uksi/2009/2890/contents/made (Accessed Jan 23, 2024).
- Masuda, M.; Sugibayashi, K. Uisuki- no kaori. J. Brew. Soc. Japan 1980, 75(6), 480–484. (in Japanese).
- Meilgaard, M. C. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. Master Brew Assoc. Am. Techn. Q. 1975, 12(3), 151–168.
- Salo, P.; Nykänen, L.; Suomalainen, H. Odor thresholds and relative intensities of volatile aroma components in an artificial beverage imitating whisky. J. Food Sci. 1972, 37(3), 394–398. DOI: 10.1111/j.1365-2621.1972.tb02647.x.
