ABSTRACT
Introduction
Previous research has identified an association between olfactory impairment (reduced odor sensitivity/ identification/ discrimination) and cognitive impairment in older adults. The present study focused on the relationship between olfactory memory performance and cognitive/affective functioning.
Method
Recognition performance for olfactory and visual stimuli (control condition) was tested through a matching task in older adults (n = 44; Mage = 76 years) and younger adults (n = 56; Mage = 24 years). Additionally, negative affect (anxiety, depression) and cognitive functioning were assessed via validated questionnaires and a neuropsychological test battery.
Results
The older participants performed worse than the younger adults in the olfactory memory task. In older adults, difficulties in remembering odors were associated with reduced odor identification and executive functioning (reduced cognitive flexibility). Affective well-being was not related to olfactory memory performance.
Implication
Olfactory memory impairment in older adulthood might be a marker for cognitive decline in areas related to executive functions.
Introduction
Test batteries for the assessment of olfactory performance typically measure odor sensitivity (i.e., the lowest detectable concentration of odors), odor discrimination (i.e., the ability to differentiate between odors), and odor identification (i.e., the ability to identify/label odors) (Hedner, Larsson, Arnold, Zucco, & Hummel, Citation2010; Hummel, Sekinger, Wolf, Pauli, & Kobal, Citation1997). Another index of olfactory performance, odor memory, is not included in the standard assessment.
Measuring episodic odor memory performance may be particularly useful in older adults as both memory deficits and reductions in olfactory functions are associated with cognitive decline and impending dementia (e.g., Bäckman, Jones, Berger, Laukka, & Small, Citation2005; Murphy, Citation2019; Roberts et al., Citation2016). Hence, the assessment of episodic odor memory may offer the possibility to detect subtle cognitive deficits associated with aging.
So far, studies have tested episodic odor memory performance with delayed matching-to-sample paradigms that included a memorization phase and a recognition phase (Choudhury, Moberg, & Doty, Citation2003; Croy, Zehner, Larsson, Zucco, & Hummel, Citation2015; Zucco & Bollini, Citation2011). In the mentioned investigations, initially presented target odors had to be identified within a group of distractor odors after varying time intervals.
In the present study, another testing method for olfactory memory performance was chosen. We designed an olfactory memory task in the format of the well-known game ‘Concentration,’ where matching stimulus pairs have to be identified in their corresponding spatial locations as quickly as possible. A parallel version with visual stimuli was created as the control condition. This design has a playful character to increase motivation and test compliance. A similar approach has been used by Olofsson et al. (Citation2020). In that study on olfactory memory training, adult participants (aged between 18 and 50 years) were randomly assigned to daily training sessions for 40 days. Participants’ task was either to detect matching odors or visual stimuli. The results showed that engaging the olfactory system in memory training was associated with a transfer effect to a similar visual memory task, as well as to novel olfactory tasks. In contrast, participants who trained with the visual memory task did not show any improvements in the olfactory memory task.
The goal of the present investigation was twofold: First, olfactory (visual) memory performance was compared between younger (M = 24 years) and older adults (M = 76 years). It is well documented that older adults experience a general decrease in olfactory functions (e.g. reduced odor identification/ sensitivity (Oleszkiewicz, Schriever, Croy, Hähner, & Hummel, Citation2019). Age-related impairment also includes memory for odors (Murphy, Cain, Gilmore, & Skinner, Citation1991; Lehrner, Glück, & Laska, Citation1999; Croy, Zehner, Larsson, Zucco, & Hummel, Citation2015; Gilbert, Pirogovsky, Ferdon, Brushfield, & Murphy, Citation2008; Larsson et al., Citation2016). Based on these findings we hypothesized that olfactory memory performance would be reduced in older adults compared to younger adults. Moreover, we expected the olfactory memory task to be more difficult than the visual memory task.
Second, this study used an exploratory regression analysis approach to capture possible associations between cognitive/affective variables and odor memory performance in older adults. Different olfactory performance measures are associated with cognitive/executive functions (Hedner et al., Citation2010) and affective variables (e.g. symptoms of depression (Croy et al., Citation2014; Schienle, Wolf, Tomazic, & Ille, Citation2018; Schienle et al., Citation2018). Olfactory dysfunctions are even discussed as early markers for neurodegenerative disorders (e.g., Murphy, Citation2019; Roberts et al., Citation2016). Odor memory is particularly interesting for the investigation of cognitive-affective functioning because it is reported to be more emotional than memories from other sensory modalities (Herz, Citation2004) and it extends further back in time (Chu & Downes, Citation2002). Although studies have reported altered odor memory performance in groups diagnosed with clinically relevant neurodegenerative disorders (e.g., dementia (Naudin et al., Citation2014; Nordin & Murphy, Citation1998) and affective disorders (e.g. depression (Naudin et al., Citation2014; Sorokowska, Sabiniewicz, & Larsson, Citation2020; Zucco & Bollini, Citation2011), to the best of our knowledge no study has investigated cognitive and affective correlates of episodic odor memory performance in healthy older adults.
The chosen olfactory memory task for the present study very likely puts demands on different cognitive processes. It involves executive functions because a transient representation of a target odor and its spatial location has to be formed. This representation has to be used for comparison and subsequent selection of the matching pairs (e.g., Bowie & Harvey, Citation2006). Further, it includes cognitive processes associated with learning and memory since the task requires the participant to consciously remember odors. Moreover, since it is known that semantic activations contribute to optimal episodic memory functioning, odor labeling abilities might also influence performance in the olfactory memory task (e.g., Menon, Boyett-Anderson, Schatzberg, & Reiss, Citation2002). For example, Kollndorfer, Reichert, Braunsteiner, and Schöpf (Citation2017) reported that performance in different olfactory memory tasks was associated with odor labeling abilities.
In the present investigation, cognitive functioning was assessed with the CERAD test battery (Consortium to Establish a Registry for Alzheimer’s Disease; (Morris et al., Citation1989). The CERAD measures functions associated with learning, memory, and language (e.g., verbal fluency, word/ figural recall). Additional CERAD Plus subtests (e.g., trail-making test) screen for executive dysfunctions. A self-report measure (STADI; Laux, Hock, Bergner-Köther, Hodapp, & Renner, Citation2013) was administered to assess affective symptoms (anxiety/depression).
Method
Sample
We tested 100 participants from two age groups (younger adults, and older adults). Younger adults (n = 56) had a mean age of 23.84 (SD = 2.66; range: 19–32). Most of them were recruited via advertisements at the university campus and were students (77%; average years of education: M = 12.93, SD = 1.52). The group of older adults (n = 44) had a mean age of 75.62 (SD = 7.33; range: 60–89) and had completed on average 12.18 years (SD = 2.05) of education. The older adults were recruited via advertisements at associations for senior citizens and care facilities. On average, the participants reported normal affective functioning (as indicated by the trait scales of the state/ trait anxiety and depression inventory (STADI; (Laux et al., Citation2013). Older participants showed normal overall cognitive functioning (as indicated by the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD)) scores standardized according to age and years of education (Morris et al., Citation1989). Hence, the participants only differed in their tendency for cognitive and affective problems. For sample characteristics see . Furthermore, the older adults did not deviate from the age-related norm in odor threshold (as indicated via the Sniffin sticks threshold test for n-Butanol (Oleszkiewicz et al., Citation2019) (M = 7.19, SD = 2.94, p = .67).
Table 1. Means (M), standard deviations (SD), and norm values for the questionnaire data and the cognitive test battery.
Exclusion criteria for participation were reported chronic and acute diseases affecting the respiratory system (e.g., asthma, infections). All participants provided written informed consent. The study was performed following the Declaration of Helsinki and approved by the ethics committee of the University of Graz (GZ. 39/129 /63 ex 2020/21).
Material
Memory Task
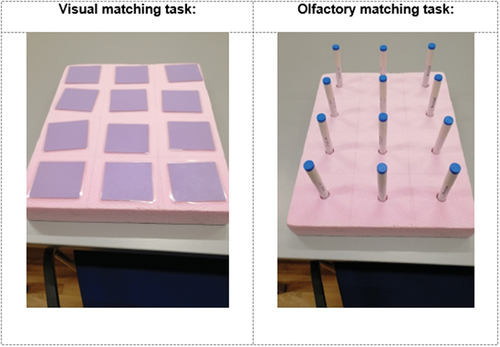
Younger adults and older adults completed the same two matching-pair memory tasks (similar to the game “Concentration”). For the visual task six image pairs (peppermint, shoe leather, rose, licorice, banana, and coffee), printed on cards (size: 8 × 7 cm) and placed face down in a 4 (rows) x 3 (columns) grid (see ), had to be identified. For the olfactory task, six stimulus pairs from the Sniffin’ Sticks Test (Burghardt Ltd instruments, Wedel, Germany) were selected that corresponded with the visual task (peppermint, shoe leather, rose, licorice, banana, and coffee) and were displayed on a 4 × 3 grid (see ). The odorants (Sniffin’ Sticks) are pen-like devices that are easy to use, hygienic and reusable. For all odorants standardized data for olfactory threshold, pleasantness and identifiability exist (Hummel et al., Citation1997), ensuring the homogeneity of the test. All administered odorants had suprathreshold intensities, were on average perceived as pleasant and had above-average familiarity ratings.
The matching task consisted of two parts: first, the experimenter presented the 12 image cards (Sniffin’ Sticks) one after another for 3 s each, with a 15s inter-stimulus interval. The participants were asked to remember the stimuli. Second, the participants were asked to find the matching stimulus pairs as quickly as possible by choosing two stimuli from the grid. Correctly identified pairs were handed over to the experimenter. If the cards/Sniffin’ Sticks did not match, they were returned to their spatial locations on the grid. The task was completed when all pairs were correctly identified or the participant abandoned the task after the time had exceeded 300 s without identifying a single matching stimulus pair. The number of errors (incorrect pair selections), as well as detection time per pair, were recorded. The sequence of the stimuli on the grid was random and the sequence of the visual and olfactory tasks was counterbalanced. After the olfactory memory task, participants were asked to label the to-be-remembered odors.
Questionnaire
Younger and older participants answered German versions of the Trait section of the State-Trait Anxiety and Depression Inventory (STADI; Laux et al., Citation2013) either via an online survey tool (adults) or as a paper-pencil version (older adults). The STADI subscales Depression (αyoung adults = .90; αolder adults = .76) and Anxiety (αyoung adults = .92; αolder adults = .87) have ten items each (e.g. depression: “I am sad”; anxiety: “I worry that something might happen”) that are scored on a four-point Likert scale ranging from 1 (not at all) to 4 (very much). The total score is calculated from the sum of the two subscales (αyoung adults = .93; αolder adults = .84.
Neuropsychological Test Battery
Older participants completed the CERAD test battery (Morris et al., Citation1989). The CERAD assesses impairments in cognitive functioning and is divided into two parts: the CERAD-NP subtests capture cognitive functions associated with learning, memory, and language and the CERAD-Plus subtests screen for neuropsychological impairments in executive functions. The total score of the CERAD-NP (i.e. CERAD_NP_total) is a sum score of the subtests verbal fluency (i.e. listing as many animals as possible for one minute; (Isaacs & Kennie, Citation1973), Modified Boston naming test (i.e. object naming; (Kaplan, Goodglass, & Weintraub, Citation1983), word list learning, word list recall, word list recognition (Atkinson & Shiffrin, Citation1971) and figure recall (Rosen, Mohs, & Davis, Citation1984). The CERAD-NP total score ranges between 0 and 100 and was obtained following (Chandler et al., Citation2005). Additionally, scores were computed for the Trail making tests (TMT) A and B (Army Individual Test Battery, Citation1944) and phonemic fluency (i.e. listing as many s-words as possible in one minute; (Thurstone, L. L. Citation1938) from the CERAD-Plus. In the TMTs, numbers, and letters are distributed over a sheet of paper. Participants are asked to draw lines to connect numbers (i.e. TMT A) and letters in ascending patterns (i.e. TMT B: 1-A-2-B-3-C, etc.). The TMT scores reflect the time (s) needed to complete the tests (A/B); lower scores indicate better performance). The CERAD-Plus total score was calculated via summing up the time (in s) needed in the two Trail making tests and the inverse score (i.e. 24 – number of named s-words) of the phonemic fluency task.
Procedure
In older participants, cognitive functioning (CERAD test battery) and the odor threshold test of the Sniffin’ Sticks test battery (Burghardt Ltd instruments, Wedel, Germany; Hummel et al., Citation1997) to control for general odor sensitivity were assessed first. Since the completion of the CERAD test battery and the Sniffin’ Sticks test take approximately one hour, there was a break of at least one hour before the memory test (the participants were told to relax, however, received no standardized relaxation task). Finally, participants filled out the questionnaire (STADI) to assess affective symptoms.
Statistical Analysis
First, we computed analyses of variance (ANOVAs) to test the effects of MEMORY TASK (visual, olfactory) and AGE GROUP (younger adults, older adults) on memory performance in the matching-pair task (number of errors, time until completion, average detection speed per stimulus pair and error percent). “Number of errors” represents the number of incorrect pair selections. “Time to completion” is defined as the time (in seconds) until all matching stimulus pairs have been correctly identified. “Detection speed per stimulus pair” is the average time for detecting a matching stimulus pair (hit). “Error percent” represents a combined measure of the number of errors and number of correctly detected pairs within 300s. It was calculated via the formula “number of errors”/ (“number of errors” + “number of hits”)*100.
Second, to capture the association between memory performance for odors and the independent variables cognitive functions (language and memory = CERAD_NP_total; executive functions = CERAD_Plus_total), affective symptoms (= STADI_total score), and odor labeling performance in older adults, a multiple linear regression analysis was computed. Since the speed measures “time to completion” and “detection speed per matching stimulus pair” are not only related to olfactory memory performance but also motor skills and olfactory adaptation processes, “error percent” in the olfactory memory task functioned as the dependent variable. It constitutes a combined measure including the number of errors, the number of correct responses (hits), and the detection speed (within a time interval of a maximum of 300s). The model was assessed for multicollinearity (all VIF <10; Tolerance >0.1) and residual distribution (All standardized residuals < 2.5, Cook’s Distance < 1, Durbin Watson >1.5 and <2.5). The analysis was conducted with SPSS version 26 (IBM Corp. 2019).
Results
ANOVAs
The group of younger adults completed the visual and olfactory memory task. In the group of older participants, ten (23%) were not able to complete the olfactory memory task (they abandoned the test after ≥ 5 minutes of testing and not being able to identify a single pair). For these participants, we assigned an error percent of 100. All older adults completed the visual task.
The ANOVAs for the dependent variables “number of errors,” “time to completion,” “detection speed per stimulus pair” and “error percent” showed that for younger adults as well as older adults the visual memory task was easier to solve (fewer errors, faster detection speed, lower error percent) than the olfactory memory task (all p < .001). Overall, younger adults outperformed older adults in all four performance variables (all p < .001) (for descriptives (see ) and F-statistics see (). Differences between the age groups in task performance were more pronounced for the olfactory task than for the visual task (p = .003).
Table 2. Means (M) and standard deviations (SD) of the number of errors, time to task completion, detection speed per stimulus pair, and error percent for the olfactory and visual matching task of young and older adults.
Table 3. F-statistics, p-values and effect sizes (part.η2) of the 2 × 2 ANOVA on the effects of age group and memory task on the memory performance variables.
Years of education were not significantly correlated with any of the olfactory memory performance measures in older adults (all r < .22; all p > .05).
Concerning odor labeling ability, younger adults (M = 3.6, SD = 1.1) and older adults (M = 2.3, SD = 1.6) differed in the number of correctly labeled odors (p < .001). On average, younger adults identified one odor more than older participants.
Multiple Linear Regression Analyses
Means, standard deviations and norm values according to age for affective symptoms (i.e. STADI_total) and cognitive functioning (language and memory (i.e. CERAD_NP_total), executive functions (i.e. CERAD_Plus_total) are presented in . The two subscales assessing cognitive function were significantly intercorrelated (r = −.56; all p < .01). The number of labeled odorants significantly correlated with CERAD_NP_total (r = .39; p = .01). In contrast, affective symptoms did not significantly correlate with any of the cognitive or semantic variables (all p > .05).
The regression equation for the dependent variable error percent in the olfactory memory task with the predictors CERAD_NP_total, CERAD_Plus_total, odor labeling) and STADI_total, was significant (F(4, 39) = 8.77; p < .001; R2 = .50). CERAD_Plus_total as well as odor labeling performance were significant predictors (see ).
Table 4. Results of the multiple linear regression analysis for the association between error % in the olfactory memory task and “odor labeling performance,” “CERAD_Plus_total,” “CERAD_NP_total,” and “STADI_total” in older adults. B-values (B), standard error of B (SE B), 95% confidence interval for B (95% CI for B), beta values (β) and p-values (p), bivariate correlation (r) and partial correlation (sr).
To analyze which of the CERAD_Plus subscales (Trail Making test A, B, or phonemic fluency) were associated with olfactory memory performance, we conducted an additional regression analysis for the criterion error percent in the olfactory memory task and the three CERAD_Plus subscales as predictors. The results revealed a significant regression equation (F(3, 39) = 6.46, R2 = .35, p = .001). Trail Making Test B was a significant predictor (Beta = .54; p = .004), whereas phonemic fluency and Trail making test A were not relevant (all p > .05).
The z-standardized values according to age and level of education for the Trail Making test B were significantly negatively correlated with error% in the olfactory memory task (r = −.378, p = .011). Eight participants attained z-values < −1.3, which reflects slight to moderate impairments in Trail Making test B performance according to (Lezak, Howieson, Bigler, & Tranel, Citation2012). These eight participants had above-average error% (M = 83.10, SD = 25.56) in the olfactory memory task. Five of the eight participants (62.5%) abandoned the olfactory memory task (i.e. error% = 100), which is significantly more participants than in the group who showed no impairments according to z-standardization (13.8%; X2(1) = 8.81, p = .003).
Discussion
The current study compared memory performance for odors (and pictures) between younger and older adults. Olfactory memory performance was assessed with a recognition test where matching stimulus pairs had to be identified in their spatial locations as quickly as possible. Replicating findings by Lehrner et al. (Citation1999), Murphy et al. (Citation1991), Croy et al. (Citation2015), Larsson et al. (Citation2016), and Gilbert et al. (Citation2008) we demonstrated that olfactory memory performance was significantly worse (more errors, slower detection speed) in older adults than younger adults. In the older group, 23% even abandoned the olfactory memory task because they were not able to identify a single odor pair correctly. In contrast, all of the younger adults completed the task. The latter finding points to the pronounced difficulties concerning odor memory in the older participants. Although odor memory is reported to be resistant to forgetting (e.g. Engen & Ross, Citation1973; Lawless & Cain, Citation1975) and extends further back in time (Chu & Downes, Citation2002), the encoding becomes more difficult in older age, particularly in comparison to the encoding of visual information.
The exploratory examination of cognitive/ affective variables associated with olfactory memory performance in older adults revealed that specific areas of cognitive functioning were linked with memory for odors. On the one hand, odor labeling performance was positively correlated with the test scores of the olfactory memory test. Previous studies have shown that odor recognition is facilitated when odor label alternatives are provided during the encoding and retrieval of odors (e.g., Frank, Rybalsky, Brearton, & Mannea, Citation2011). Moreover, Jehl, Royet, and Holley (Citation1997) revealed that the semantic content of odor labels provided during the encoding phase significantly influenced short- and long-term odor recognition. Incomplete semantic activations might compromise the ability to store and retrieve odor representations, leading to a decreased performance in the odor memory task.
On the other hand, the performance in the CERAD-Plus tests, specifically the Trail Making test B (Army Individual Test Battery, Citation1944), was correlated with the performance in the olfactory memory task. Problems in the Trail making test B predicted difficulties in completing the olfactory memory task. Trail-making Test B measures executive functions by asking the participants to connect letters and digits in ascending order. To solve this task, cognitive switching/flexibility is needed.
The association between the performance in the Trail-Making test and the odor memory task could be explained as follows: olfaction compared to vision is less important for humans in everyday life (e.g., San Roque et al., Citation2015). Therefore, a memory task for odors is an unusual, new exercise that requires cognitive flexibility and quick adaptation to a new situation. Previous literature suggests that the implementation of executive functions entails dynamic interactions among neural populations in different areas of the brain that in many regions overlap with brain areas activated in odor processing (e.g., prefrontal/ orbitofrontal lobe, and subcortical areas including the thalamus and limbic system (e.g., Fagundo et al., Citation2015; Gottfried, Citation2006; Reuter-Lorenz, Festini, & Jantz, Citation2021; Wilson, Chapuis, & Sullivan, Citation2015).
To the best of our knowledge, this is the first study showing an association between olfactory memory performance and executive functions (cognitive flexibility) in a group of healthy older adults. Memory performance for odors was already strongly impaired in older individuals with mild cognitive problems. Sixty-three percent of the participants with clinically relevant scores (according to z-standardization) in the Trail making test B abandoned the odor memory task, compared to only 14% of participants with scores in the normal range. Future studies with a longitudinal design should investigate whether olfactory memory tasks might be a promising additional tool for the diagnosis of early stages of cognitive impairment. Executive functions are one of the most vulnerable areas of age-related decline (Murman, Citation2015) and brain regions linked to executive functioning show the earliest adverse effects of aging (Reuter-Lorenz et al., Citation2021). Moreover, training interventions for olfactory memory could be implemented and tested for older adults. For a group of healthy adults (aged between 18 and 50 years), Olofsson et al. (Citation2020) already revealed transfer effects from an odor-memory task to a visual memory task. This finding might reflect cognitive flexibility.
As with any study some limitations need to be acknowledged. It must be noted that our sample comprised more female than male participants, therefore we were not able to analyze sex differences. We recommend replication of the findings with larger sample sizes. For the assessment of olfactory memory performance, we used a specific memory task. Future studies need to test the reliability as well as the validity of this task. Tasks for the breaks between assessments (cognitive/olfactory tests) should be standardized (e.g. introducing a session of relaxation training). Our study had a cross-sectional design. In a future project, it would be interesting to examine the same participants again to find out whether the likelihood of developing dementia is increased in those individuals who had difficulties in the olfactory memory task.
Conclusion
Our results indicate that older adults performed worse in the olfactory memory task compared to younger adults. Difficulties with memory for odors were associated with reduced odor identification and executive functioning. Future longitudinal studies should investigate whether olfactory memory impairments might be an early marker for adverse effects of aging and disproportionate cognitive decline in older adults. Finally, it should be investigated whether cognitive flexibility can be improved via olfactory memory training.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University of Graz and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Additional information
Funding
References
- Army Individual Test Battery. (1944). Manual of direction and Scoring. Washington, DC: War department, Adjutant General’s Office.
- Atkinson, R. C., & Shiffrin, R. M. (1971). The control of short-term memory. Scientific American, 225(2), 82–91. doi:10.1038/scientificamerican0871-82
- Bäckman, L., Jones, S., Berger, A. ‑. K., Laukka, E. J., Jonsson, E., & Small, B. J. (2005). Cognitive impairment in preclinical Alzheimer’s disease: A meta-analysis. Neuropsychology, 19(4), 520. doi:10.1037/0894-4105.19.4.520
- Bowie, C. R., & Harvey, P. D. (2006). Administration and interpretation of the trail making test. Nature Protocols, 1(5), 2277–2281. doi:10.1038/nprot.2006.390
- Chandler, M. J., Lacritz, L. H., Hynan, L. S., Barnard, H. D., Allen, G., Deschner, M., … Cullum, C. M. (2005). A total score for the CERAD neuropsychological battery. Neurology, 65(1), 102–106. doi:10.1212/01.wnl.0000167607.63000.38
- Choudhury, E. S., Moberg, P., & Doty, R. L. (2003). Influences of age and sex on a microencapsulated odor memory test. Chemical Senses, 28(9), 799–805. doi:10.1093/chemse/bjg072
- Chu, S., & Downes, J. J. (2002). Proust nose best: Odors are better cues of autobiographical memory. Memory & Cognition, 30(4), 511–518. doi:10.3758/BF03194952
- Croy, I., Symmank, A., Schellong, J., Hummel, C., Gerber, J., Joraschky, P., & Hummel, T. (2014). Olfaction as a marker for depression in humans. Journal of Affective Disorders, 160, 80–86. doi:10.1016/j.jad.2013.12.026
- Croy, I., Zehner, C., Larsson, M., Zucco, G. M., & Hummel, T. (2015). Test-retest reliability and validity of the Sniffin’ TOM odor memory test. Chemical Senses, 40(3), 173–179. doi:10.1093/chemse/bju069
- Engen, T., & Ross, B. M. (1973). Long-term memory of odors with and without verbal descriptions. Journal of Experimental Psychology, 100(2), 221–227. doi:10.1037/h0035492
- Fagundo, A. B., Jiménez-Murcia, S., Giner-Bartolomé, C., Islam, M. A., La Torre, R. D., Pastor, A., & Fernández-Aranda, F. (2015). Modulation of higher-order olfaction components on executive functions in humans. PloS One, 10(6), e0130319. doi:10.1371/journal.pone.0130319
- Frank, R. A., Rybalsky, K., Brearton, M., & Mannea, E. (2011). Odor recognition memory as a function of odor-naming performance. Chemical Senses, 36(1), 29–41. doi:10.1093/chemse/bjq095
- Gilbert, P. E., Pirogovsky, E., Ferdon, S., Brushfield, A. M., & Murphy, C. [. (2008). Differential effects of normal aging on memory for odor-place and object-place associations. Experimental Aging Research, 34(4), 437–452. doi:10.1080/03610730802271914
- Gottfried, J. A. (2006). Smell: Central nervous processing. Taste and Smell, 63, 44–69.
- Hedner, M., Larsson, M., Arnold, N., Zucco, G. M., & Hummel, T. (2010). Cognitive factors in odor detection, odor discrimination, and odor identification tasks. Journal of Clinical and Experimental Neuropsychology, 32(10), 1062–1067. doi:10.1080/13803391003683070
- Herz, R. S. (2004). A naturalistic analysis of autobiographical memories triggered by olfactory visual and auditory stimuli. Chemical Senses, 29(3), 217–224. doi:10.1093/chemse/bjh025
- Hummel, T., Sekinger, B., Wolf, S. R., Pauli, E., & Kobal, G. (1997). ‘Sniffin’sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chemical Senses, 22(1), 39–52. doi:10.1093/chemse/22.1.39
- Isaacs, B., & Kennie, A. T. (1973). The Set test as an aid to the detection of dementia in old people. The British Journal of Psychiatry: The Journal of Mental Science, 123(575), 467–470. doi:10.1192/bjp.123.4.467
- Jehl, C., Royet, J. P., & Holley, A. (1997). Role of verbal encoding in short and long-term odor recognition. Perception & Psychophysics, 59(1), 100–110. doi:10.3758/BF03206852
- Kaplan, E., Goodglass, H., & Weintraub, S. (1983). The Boston Naming Test. Philadelphia: Lea & Febiger. City
- Kollndorfer, K., Reichert, J., Braunsteiner, J., & Schöpf, V. (2017). Assessment of olfactory memory in olfactory dysfunction. Perception, 46(3–4), 516–529. doi:10.1177/0301006616683201
- Larsson, M., Hedner, M., Papenberg, G., Seubert, J., Bäckman, L., & Laukka, E. J. (2016). Olfactory memory in the old and very old: Relations to episodic and semantic memory and APOE genotype. Neurobiology of Aging, 38, 118–126. doi:10.1016/j.neurobiolaging.2015.11.012
- Laux, L., Hock, M., Bergner-Köther, R., Hodapp, V., & Renner, K. ‑. H. (2013). Das State-Trait-Angst-Depressions-Inventar: STADI; Manual.
- Lawless, H. T., & Cain, W. S. (1975). Recognition memory for odors. Chemical Senses, 1(3), 331–337. doi:10.1093/chemse/1.3.331
- Lehrner, J. P., Glück, J., & Laska, M. (1999). Odor identification, consistency of label use, olfactory threshold and their relationships to odor memory over the human lifespan. Chemical Senses, 24(3), 337–346. doi:10.1093/chemse/24.3.337
- Lezak, M. D., Howieson, D. B., Bigler, E. D., & Tranel, D. (2012). Neuropsychological Assessment. New York: Oxford University Press.
- Menon, V., Boyett-Anderson, J. M., Schatzberg, A. F., & Reiss, A. L. (2002). Relating semantic and episodic memory systems. Cognitive Brain Research, 13(2), 261–265. doi:10.1016/S0926-6410(01)00120-3
- Morris, J. C., Heyman, A., Mohs, R. C., Hughes, J. P., van Belle, G., Fillenbaum, G., … Clark, C. (1989). The consortium to establish a registry for alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of alzheimer’s disease. Neurology, 39(9), 1159. doi:10.1212/WNL.39.9.1159
- Murman, D. L. (2015). The Impact of Age on Cognition. Seminars in Hearing, 36(3), 111–121. doi:10.1055/s-0035-1555115
- Murphy, C. (2019). Olfactory and other sensory impairments in Alzheimer disease. Nature Reviews. Neurology, 15(1), 11–24. doi:10.1038/s41582-018-0097-5
- Murphy, C., Cain, W. S., Gilmore, M. M., & Skinner, R. B. (1991). Sensory and semantic factors in recognition memory for odors and graphic stimuli: Elderly versus young persons. The American Journal of Psychology, 104(2), 161. doi:10.2307/1423153
- Naudin, M., Mondon, K., El-Hage, W., Desmidt, T., Jaafari, N., Belzung, C., … Atanasova, B. (2014). Long-term odor recognition memory in unipolar major depression and Alzheimer׳s disease. Psychiatry Research, 220(3), 861–866. doi:10.1016/j.psychres.2014.08.050
- Nordin, S., & Murphy, C. (1998). Odor memory in normal aging and Alzheimer’s disease. Annals of the New York Academy of Sciences, 855, 686–693. doi:10.1111/j.1749-6632.1998.tb10646.x
- Oleszkiewicz, A., Schriever, V. A., Croy, I., Hähner, A., & Hummel, T. (2019). Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. European Archives of Oto-Rhino-Laryngology: Official Journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): Affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery, 276(3), 719–728. doi:10.1007/s00405-018-5248-1
- Olofsson, J. K., Ekström, I., Lindström, J., Syrjänen, E., Stigsdotter-Neely, A., Nyberg, L., … Larsson, M. (2020). Smell-based memory training: Evidence of olfactory learning and transfer to the visual domain. Chemical Senses, 45(7), 593–600. doi:10.1093/chemse/bjaa049
- Reuter-Lorenz, P. A., Festini, S. B., & Jantz, T. K. (2021). Chapter 5 - Executive functions and neurocognitive aging. In K. W. Schaie & S. L. Willis (Eds.), Handbook of the psychology of aging (ninth edition): Handbooks of aging (pp. 67–81). London, Oxford, Boston, New Yor, San Diego: Academic Press. doi:10.1016/B978-0-12-816094-7.00019-2
- Roberts, R. O., Christianson, T. J. H., Kremers, W. K., Mielke, M. M., Machulda, M. M., Vassilaki, M., … Petersen, R. C. (2016). Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer's disease dementia. JAMA Neurology, 73(1), 93–101. doi:10.1001/jamaneurol.2015.2952
- Rosen, W. G., Mohs, R. C., & Davis, K. L. (1984). A new rating scale for Alzheimer’s disease. The American Journal of Psychiatry, 141(11), 1356–1364. doi:10.1176/ajp.141.11.1356
- San Roque, L., Kendrick, K. H., Norcliffe, E., Brown, P., Defina, R., Dingemanse, M., … Hammond, J. (2015). Vision verbs dominate in conversation across cultures, but the ranking of non-visual verbs varies. Cognitive Linguistics, 26(1), 31–60. doi:10.1515/cog-2014-0089
- Schienle, A., Wolf, A., Tomazic, P. V., & Ille, R. (2018). Affective personality traits in olfactory dysfunction: The role of dysthymia and arousal. Chemosensory Perception, 11(2), 72–76. doi:10.1007/s12078-017-9242-6
- Sorokowska, A., Sabiniewicz, A., & Larsson, M. (2020). Tom-32-An extended test for the assessment of olfactory memory. Journal of Neuroscience Methods, 344, 108873. doi:10.1016/j.jneumeth.2020.108873
- Thurstone, L. L. . (1938). Primary Mental Abilities (rev.). Chicago: University of Chicago Press.
- Wilson, D. A., Chapuis, J., & Sullivan, R. M. (2015). Cortical olfactory anatomy and physiology. Handbook of Olfaction and Gustation, Ed, 3, 209–225.
- Zucco, G. M., & Bollini, F. (2011). Odour recognition memory and odour identification in patients with mild and severe major depressive disorders. Psychiatry Research, 190(2–3), 217–220. doi:10.1016/j.psychres.2011.08.025