ABSTRACT
To evaluate the clinical features, molecular subtypes, therapeutic strategies, and prognostic factors of occult breast cancer (OBC). Patients with T0-3/N1-3/M0 breast cancer diagnosed in 2010–2018 (n = 114,303, including 691 with OBC) were retrieved from the Surveillance, Epidemiology, and End-Results (SEER) database. The endpoints were overall survival (OS) and breast cancer-specific survival (BCSS). Compared with non-OBC, OBC presented significantly more adverse clinicopathological prognostic features. More patients with OBC underwent breast-conserving treatment (BCT) and less had axillary lymphadenectomy (ALD). Outcomes were more favorable in OBC cases compared with non-OBC cases (p = .002 for OS, p = .002 for BCSS). Triple-negative (TNBC) and HER2-enriched were the subtypes with the worst prognosis in OBC (p < .05). Prognosis was better for triple-negative OBC compared with the same subtype of non-OBC. N-stage was not a strong prognostic indicator of OBC (p > .05 for OS). Cases who underwent systemic chemotherapy alone without surgery had the worst prognosis among OBC patients. For locoregional therapy, mastectomy and radiotherapy could confer survival advantage; standard axillary lymph node dissection (ALND) and positive lymph node dissection (PLND) contributed notably to OS in OBC patients. Both OS and BCSS were better in OBC cases compared with non-OBC. Systemic chemotherapy alone without surgery is not appropriate for OBC treatment, and mastectomy plus standard axillary surgery is recommended. Patients with hormone receptor-positive and low burden of axillary lymph node metastasis may be spared from radiotherapy after undergoing standard axillary lymphadenectomy.
Introduction
Occult breast cancer (OBC) usually presents as axillary lymph node (ALN) metastasis without evidence of primary breast cancer on clinical examination or mammography (Baron et al. Citation1990; Patel et al. Citation1981; Rosen Citation1980). OBC is a rare disease presentation, representing 0.3%–1.0% of all breast cancer cases (Baron et al. Citation1990; Patel et al. Citation1981; Rosen Citation1980). Breast magnetic resonance imaging (MRI) has emerged as the most sensitive imaging modality in the detection and evaluation of breast lesions, and could help decrease the current prevalence of OBC (de Bresser et al. Citation2010). The rarity of OBC precludes related randomized controlled studies and, therefore, the diagnosis, treatment, and prognosis of OBC remain elusive. Although studies suggested that cancer outcomes in patients with OBC are similar to or more favorable than those of stage II-III, T1N1, and small invasive breast cancer (pT1) cases, many authors support the opposite (Montagna et al. Citation2011; Pentheroudakis, Lazaridis, and Pavlidis Citation2010; Ping et al. Citation2014; Rosen and Kimmel Citation1990; Sohn et al. Citation2014). Hence, the outcomes of patients with OBC are still controversial.
So far, several population-based studies based on large databases, such as the Surveillance, Epidemiology and End Results (SEER) database and National Cancer Database (NCDB) analyzed the clinicopathological characteristics, treatments, and prognosis of OBC (Ge et al. Citation2018; Hessler et al. Citation2017; Kim, Kwon, and Kim Citation2018; Sohn et al. Citation2014; Walker et al. Citation2010; Wu et al. Citation2017). These studies revealed that OBC has unique clinicopathological characteristics and different prognosis compared with non-OBC. They also showed that breast-conserving surgery (BCS) plus ALN dissection (ALND) and radiotherapy (RT) confer similar or even superior survival benefits compared to modified radical mastectomy (MRM) in patients with OBC (Ge et al. Citation2018; Hessler et al. Citation2017; Kim, Kwon, and Kim Citation2018; Sohn et al. Citation2014; Walker et al. Citation2010; Wu et al. Citation2017). Nevertheless, the diagnosis years of OBC in the latter studies varied from 1983 to 2014 (Ge et al. Citation2018; Hessler et al. Citation2017; Kim, Kwon, and Kim Citation2018; Sohn et al. Citation2014; Walker et al. Citation2010; Wu et al. Citation2017), leading to a prognosis bias due to the locoregional and systemic therapies available at each diagnosis year. For example, the development and approval of trastuzumab was a game-changer for patients with HER2-positive breast cancer, changing a disease with a poor prognosis into one with a good prognosis. The HER2 status was included in the SEER database in 2010. In addition, according to 2011 St. Gallen’s recommendations, the molecular subtypes of breast cancer were defined as follows: Luminal A was ER-positive or PR-positive with HER2-negative and Ki67 < 14 percent; Luminal B was ER-positive or PR-positive and HER2-positive or Ki67 ≥ 14 percent; HER2 enriched (HER2+) was ER-negative, PR-negative, and HER2-positive; triple-negative was ER-negative, PR-negative, and HER2-negative. Molecular subtypes are known to have different prognoses (Fragomeni, Sciallis, and Jeruss Citation2018), were not examined in previous studies. Recently, Ge et al. (Citation2018) examined the clinical characteristics, local treatments, and prognosis of occult invasive duct carcinoma (IDC) diagnosed from 2004 to 2014. Still, when searching the tumor reporting database, it was found that the histological type of OBC was not limited to “8500/3: Infiltrating duct carcinoma,” but also included “8520/3: Lobular carcinoma,” and “8140/3: Adenocarcinoma, NOS,” among others. In addition, patients with 1–5 involved lymph nodes were considered to undergo sentinel lymph node dissection as an inclusion criterion of the Z0011 trial (Giuliano et al. Citation2017). Still, OBC cases do not meet the criterion of no clinical evidence of ALN metastasis, and not all OBC patients with low lymph node metastasis burden are eligible for radiotherapy either.
Therefore, further large-sample studies are warranted to determine the impact of molecular subtypes and therapeutic strategies on patient survival in OBC. In the present study, we retrieved OBC cases from the SEER database, with large and comprehensive samples, aiming to evaluate the clinical features, molecular subtypes, locoregional treatment strategies and prognostic factors of OBC.
Materials and methods
Data source and patient selection
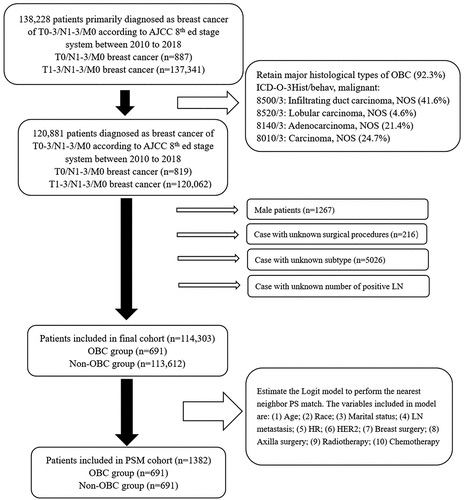
The SEER Program is a primary source of cancer statistics from The National Cancer Institute (USA) (Duggan et al. Citation2016). In this study, the research data file retrieved in SEER was Stat Database: Incidence – SEER Research Plus Data, 18 Registries, Nov 2020 Sub (2000–2018) - Linked to County Attributes – Total U.S., 1969–2019 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2021, based on the November 2020 submission. The data of breast cancer patients (Site and Morphology. Site recode ICD-O-3/WHO 2008 = “Breast”) with T0-3/N1-3/M0 (AJCC 8th edition) were extracted. The data were obtained from 2010 to 2018 (years of diagnosis) because the molecular subtype of breast cancer is available since 2010 in the SEER database. We retrieved 887 records of patients with T0/N1-3/M0 breast cancer and 137,341 of T1-3/N1-3/M0 breast cancer cases. Due to the absence of OBC primary lesions, to include the major histological types of OBC, the following variables in ICD-O-3Hist/behave malignant were retained in the total sample: “8500/3: Infiltrating duct carcinoma, NOS” (369/887 OBC, 41.6%); “8520/3: Lobular carcinoma, NOS” (41/887 OBC, 4.6%); “8140/3: Adenocarcinoma, NOS” (190/887 OBC, 21.4%); and “8010/3: Carcinoma, NOS” (219/887 OBC, 24.7%). After omitting the minor histological types (except the above 4 major histological types) and indeterminable data, we retrieved 691 records of patients with OBC. Finally, 114,303 patients were included in the analysis ().
Data collection
The demographic features (age, race, and marital status), clinicopathological characteristics (LN metastasis, pTNM, hormone receptor, HER-2, and subtype), therapeutic strategies (breast surgery, axilla surgery, chemotherapy, and radiotherapy), and prognosis data (overall survival [OS] and breast cancer-specific survival [BCSS]) were collected from the database. OS was defined as the time from admission to death from any cause. BCSS was defined as the time from surgery to death from breast cancer. The breast surgery mode and radiotherapy are indicated in the SEER database (Duggan et al. Citation2016), but the type of ALN surgery is not specified. The axillary operation was identified based on two SEER variables, including the number of lymph nodes examined and the number of positive lymph nodes. Patients with no lymph nodes examined but >0 positive lymph nodes were considered to have been confirmed by aspiration biopsy and classified as cases with no axillary surgery. Patients with 1–4 lymph nodes examined and less positive lymph nodes than examined were considered as having been confirmed by surgical biopsy and classified as positive lymph node dissection (PLND) cases. Patients with ≥5 lymph nodes examined were classified as ALND cases (Macedo et al. Citation2016).
For survival analysis, breast locoregional therapies in patients with OBC were defined as breast surgery (no surgery, breast-conserving surgery, and mastectomy), axilla surgery (no surgery, PLND and ALND) and radiotherapy (no or unknown and yes).
Statistical analysis
The chi-square and Wilcoxon rank-sum tests were performed to analyze differences in demographic and clinicopathological features between the OBC and non-OBC groups. Propensity score matching (PSM) was performed to mitigate biases due to demographic and clinicopathological characteristics (age, race, marital status, LN metastasis, hormone receptor, HER2, breast surgery, axilla surgery, chemotherapy, and radiotherapy) between the two groups. To estimate the PS score, we followed the standard approach (Becker and Ichino Citation2002; Dehejia and Wahba Citation2002) and used the Logit model with the following steps. We started with estimating probabilities using the Logit model to select independent variables. Then, we calculated the propensity score (PS) which was the predicted values of the Logit model. The nearest neighbor matching method (“psmatch2” in STATA) was to search the closest control sample, both backward and forward, from the estimated PS values of the OBC group. After identifying the matching samples using nearest neighbor matching, we used “pstest” to compares the extent of balancing between the two samples before and after having performed matching. All survival analyses were performed by the Kaplan–Meier method, and differences were examined by the log-rank test. A multivariable Cox proportional hazard model was used to examine prognostic factors independently associated with OS and BCSS. Two-sided p < .05 was considered statistically significant. STATA 15.1 (StataCorp LP, College Station, TX, USA) was used for statistical analysis.
Results
Demographic and clinicopathological features of OBC and non-OBC cases
After omitting censored data and excluding minor histological types of breast cancer (7.7%), 114,303 patients were included. Of these, 691 (0.60%) were diagnosed with T0/N1-3/M0 breast cancer (OBC group), and 113,612 (99.40%) were staged as T1-3/N1-3/M0 (non-OBC group). The clinicopathological features of both groups are shown in . Patients with OBC were older than non-OBC cases (z = −5.71, p = .001, Wilcoxon rank-sum test). Compared with non-OBC, OBC had enhanced adverse prognostic features such as advanced N stage (17.51% vs. 7.73 percent N3, p = .001), reduced positive rate of hormone receptor (65.27% vs. 82.43 percent, p = .001), and elevated rate of positive HER2 expression (29.81% vs. 18.93 percent, p = .001). Triple-negative breast cancer (TNBC) and HER2-enriched were more common in OBC. Less patients with OBC underwent mastectomy (33.72% vs. 55.24 percent, p < .05) and aggressive axillary surgery (84.80% vs. 93.96 percent ALND + PLND, p < .05), but more had systemic treatments (81.33% vs. 68.91 percent chemotherapy, p < .05).
Table 1. Clinicopathological characteristics of OBC and non-OBC patients.
Survival of OBC and non-OBC cases
The median follow-up was 54 months (range, 1 to 107 months) for the 114,303 patients with T0-3/N1-3/M0 breast cancer. By the end of the follow-up period, 110 of the 691 patients with OBC had died (including 72 of breast cancer); meanwhile, 16,832 of the 113,612 patients in the non-OBC group had died (including 10,556 of recurrent or metastatic breast cancer). In all patients, OS and BCSS had no significant differences between the OBC and non-OBC groups (p = .253 for OS and p = .197 for BCSS, log-rank test) (Supplementary Figure S1a,b).
Survival of matched OBC and non-OBC cases
After PSM, the general features of the OBC and non-OBC groups (691 patients in each group) were similar (Supplementary Figure S1c,d, Supplementary Table S1). After PSM, 143 of the 691 patients in the non-OBC group had died (including 107 of breast cancer). OS curves for the OBC and non-OBC groups were significantly different (p = .002, log-rank test; Supplementary Figure S1e). BCSS was lower in the non-OBC group compared with the OBC group (p = .002, log-rank test; Supplementary Figure S1f). Hence, OBC was likely to induce low breast cancer mortality.
Clinical outcomes of OBC and non-OBC according to subtypes
In the non-OBC group, TNBC was the breast cancer subtype with the worst prognosis (p = .001, log-rank test; Supplementary Figure S2a,b). OS was reduced in the HER2-enriched type compared with the luminal subtypes, as well as BCSS (p < .001, log-rank test; Supplementary Figure S2a,b). In the OBC group, prognosis was still worse in non-luminal subtypes compared with luminal subtypes (p < .05, log-rank test). There was no significant difference in survival between TNBC and HER2-enriched subtype (all p > .05, log-rank test; Supplementary Figure S2c,d). In the matched non-OBC sample, the 5-year survival rate of the TNBC subtype was even <60%, which was far less than that of the OBC group (Supplementary Figure S2e).
Clinical outcomes of OBC and non-OBC according to lymph node metastasis burden
In the non-OBC group, the patient prognosis worsened with increasing N stage (p = .001, log-rank test; Supplementary Figure S3a,b). The same trend could be found in the matched non-OBC population (Suplementary Figure S3e,f). In the OBC group, lymph node metastasis only showed a negative trend for OS (p = .080, log-rank test; Supplementary Figure S3c,d).
Prognostic factors of OS and BCSS in multivariate analysis of non-OBC and OBC patients
The characteristics used in PSM were included in multivariate analysis. In the non-OBC group, demographic factors such as older age, black race, and unmarried status were poor prognostic factors of breast cancer; clinicopathological features such as elevated lymph node metastasis, hormone receptor-negativity and HER2-negativity were related to poor prognosis in breast cancer, and standard breast and axillary surgeries and active systemic therapy conferred survival benefits to the patients (all p = .001, Supplementary Table S2). In the OBC group, age, race, and marital status did not affect patient survival. Meanwhile, hormone receptor expression was the key factor affecting patient prognosis (p = .001) and HER2 expression also tended to confer OS benefit (HR = 0.68, p = .086). BCS or no breast surgery had similar therapeutic effects but were both inferior to mastectomy in OBC patients (HR = 0.50 for BCSS, p = .025; HR = 0.58 for OS, p = .029). Chemotherapy and radiotherapy did not contribute to BCSS (all p > .05), but radiotherapy significantly improved OS in patients with OBC (HR = 0.66, p = .043). Advanced lymph node stage predicted poor prognosis in OBC; meanwhile, standard ALND or PLND could extend patient survival (PLND HR = 0.47 for BCSS, p = .046; HR = 0.42 for OS, p = .004; ALND HR = 0.40 for BCSS, p = .002; HR = 0.35 for OS, p = .001).
Clinical outcomes of OBC in different locoregional treatment strategies
After excluding unconventional treatments, including breast surgery without axillary surgery (40 of 691 cases), locoregional therapy strategies were divided into four groups to explore the best combination of locoregional treatment: 1) breast-conserving treatment (BCT) (no breast surgery and BCS) plus axillary lymphadenectomy (ALD) (PLND and ALND) without RT, 122 patients (18.7%); 2) BCT + ALD with RT, 244 patients (37.5%); 3) mastectomy plus ALD without RT, 101 patients (15.5%); 4) mastectomy plus ALD with RT, 119 patients (18.3%). We still kept the observation group as control: 65 patients (10.0%) were treated with systemic chemotherapy without surgery or RT. The clinical outcomes of these five groups were evaluated using the Kaplan–Meier method. As shown in Supplementary Figure S4, except for the observation group, the four locoregional therapy strategies played similar roles in improving breast cancer-specific survival in OBC patients, but BCSS was slightly worse in the BCT group compared with the mastectomy group (mastectomy plus ALD without RT group vs. BCT plus ALD ± RT group, log-rank test p = .024). In terms of overall survival, a trend toward better survival was observed in the mastectomy plus ALD without RT group compared to the BCT plus ALD without RT group (p = .007, log-rank test).
Cox models for OS and BCSS in patients with OBC
According to the clinical significance, locoregional treatment strategies along with the characteristics used in PSM were included in Cox models for OS and BCSS in patients with OBC. As shown in , the prognosis of stage III OBC patients was worse than that of low-stage cases (HR = 2.57 for BCSS, p = .001; HR = 1.91 for OS, p = .002). Except for TNBC, which had the worst prognosis (HR = 3.87 for BCSS, p = .003; HR = 2.96 for OS, p = .003), prognosis was worse for HER2-enriched compared with the other two OBC subtypes. The strategy of locoregional therapy had the greatest influence on OBC prognosis. Patients with OBC could benefit from any standardized locoregional therapy (all p > .05 for HR) but not chemotherapy.
Table 2. Multivariable analysis of OS and BCSS in OBC patients (n = 651).
Discussion
The results strongly suggested that OBC has unique clinicopathological features and a favorable prognosis compared with non-OBC. Triple-negative was the subtype with the worst prognosis in OBC. The present study firstly included contemporary patients in the HER2 and trastuzumab era and analyzed the molecular subtypes and locoregional therapy strategies for OBC.
In the present study, although patients with OBC presented poor clinicopathological features, e.g., enhanced lymph node involvement, reduced positive rate of hormone receptor and more common TNBC and HER2-enriched subtypes, less patients with OBC underwent mastectomy and aggressive axillary surgery, but in all patients, there was no clear detectable differences in OS and BCSS between the OBC and non-OBC groups. Nevertheless, after PSM, OS was longer in OBC cases compared with the non-OBC group, as well as BCSS. These results were consistent with previous studies (Ge et al. Citation2018; Montagna et al. Citation2011). At present, molecular subtype is an important prognostic factor of breast cancer. Montagna et al. (Montagna et al. Citation2011) reported that TNBC has the worst prognosis in OBC. In this study, the prognosis of the TNBC subtype of OBC was worse than those of luminal subtypes but not different from that of the HER2-enriched subtype. Meanwhile, the prognosis of the TNBC subtype of OBC was superior to that of non-OBC cases. Due to the high proportion of TNBC subtype in OBC, the survival advantage of OBC may derive from these patients. In this study, HER2 expression showed a beneficial trend for OS, corroborating previous findings (Huang et al. Citation2020), and in accordance with the achievements of anti-HER2 therapy. The results also did not contradict the poor prognosis of HER2-enriched subtype in subsequent comprehensive multivariate analysis. Nevertheless, due to the lack of primary breast tumors, the treatment strategy for OBC was determined only based on ALN immunohistochemistry. Kinoe et al. (Kinoe et al. Citation2018) reported discordance rates between primary breast cancer and synchronous ALN metastasis of 28.8% for ER (positive in the primary → negative in ALN/negative → positive 22.1%/6.7%), 31.7% for PR (26.9%/4.8%), and 13.5% for HER2 (12.5%/1.0%). Due to such discordance, the molecular subtype of patients with OBC might be underestimated so that treatment decisions and prognosis could be affected. In the future, gene chips may be applied to diagnose primary tumors and specify the molecular subtype of breast cancer to develop appropriate treatment strategies.
Appropriate local treatment strategies for patients with OBC remain controversial. In previous studies with small sample sizes, compared with ALND, MRM ± RT and BCS + ALND + RT showed advantages for recurrence-free survival and local recurrence-free survival (He et al. Citation2012), with no OS benefits (Sohn et al. Citation2014; Wang et al. Citation2013). In other studies, compared with the ALND and observation groups, MRM ± RT and ALND + RT improved cancer-specific survival (CSS) and OS in patients with OBC (Walker et al. Citation2010; Wu et al. Citation2017). ALND is considered an independent factor in improving CSS and OS in both univariable and multivariable analyses (Wu et al. Citation2017), and ALND + RT a favorable prognosis factor of OS (Hessler et al. Citation2017). In the PSM analysis, ALND + RT could prolong OS compared with ALND, and the scope of breast operation did not affect OS (Macedo et al. Citation2016). In these studies, ALND was defined as the excision of more than four lymph nodes. Our results indicated that it was inadequate to infer the scope of lymph node dissection from the number of lymph nodes excised alone. This corroborated a previous meta-analysis, in which the definition of ALND included standard ALND and PLND (Macedo et al. Citation2016). It could be concluded that ALD contributes notably to OS, both standard ALND or PLND. Mastectomy and RT could confer a survival benefit to OBC patients. However, less than 60% of patients in the study underwent radiotherapy definitively. Considering that radiotherapy is not an absolute indication for OBC patients receiving BCT, improved survival was achieved in the mastectomy plus ALD without RT group compared with the BCT plus ALD without RT group in the analysis of local treatment combinations. Mastectomy plus standard axillary surgery is recommended for OBC patients, especially for TNBC or HER2-enriched subtypes with a high risk of recurrence. Some patients with hormone receptor-positive and low axillary lymph node metastatic burden may be spared from radiotherapy after standard axillary surgery.
The present study had limitations inherent to the SEER database, which does not provide the scopes of RT and endocrine therapy, or the details of chemotherapy regimens; the T stage of the tumor was not stated (e.g., pathological versus clinical). Secondly, treatment strategies could lead to different outcomes in patients. Moreover, there are no consensus and strategy for standardized treatment of OBC. The current results may be more suitable for the actual situation under current treatment strategies. Further research and analysis based on more detailed data is warranted.
Conclusion
With a lower incidence than common breast cancer, OBC has unique clinical features. Less patients with OBC underwent mastectomy and aggressive axillary surgery but more underwent systemic treatments. Both OS and BCSS were increased in OBC compared with non-OBC. TNBC and HER2-enriched were the subtypes with worst prognoses in OBC. Systemic chemotherapy alone without surgery is not appropriate for OBC patients. In addition to the obvious contribution of ALD to OS, mastectomy and RT could confer survival benefit to OBC patients. Mastectomy plus standard axillary surgery is recommended for OBC patients. Patients with hormone receptor-positive and low burden of axillary lymph node metastases may be spared from radiotherapy after undergoing standard axillary surgery.
Author contributions
Literature search: XY, LY, QH, JW, and XYL. Study design: CX and RRC. Methodology: XY, LY, and CX. Writing: XY, RRC, and CX. Review and editing: CX and XY. All authors have critically reviewed the final version of the manuscript and approved its content. The corresponding author had the final responsibility for the decision to submit for publication.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Availability of data and materials
The data generated in the present study are included in the figures and/or tables of this article.
Supplementary Materials
Download PDF (1.2 MB)Acknowledgments
The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [XC], upon reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/03630242.2022.2158415.
Additional information
Funding
References
- Baron, P. L., M. P. Moore, D. W. Kinne, F. C. Candela, M. P. Osborne, and J. A. Petrek. 1990. Occult breast cancer presenting with axillary metastases. Updated management. Archives of Surgery 125 (2):210–14. doi:10.1001/archsurg.1990.01410140088014.
- Becker, S. O., and A. Ichino. 2002. Estimation of average treatment effects based on propensity scores. The Stata Journal 2 (4):358–77. doi:10.1177/1536867X0200200403.
- de Bresser, J., B. de Vos, F. van der Ent, and K. Hulsewe. 2010. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: A systematic review. European Journal of Surgical Oncology (EJSO) 36 (2):114–19. doi:10.1016/j.ejso.2009.09.007.
- Dehejia, R. H., and S. Wahba. 2002. Propensity score matching methods for non-experimental causal studies. The Review of Economics and Statistics 84 (1):151–61. doi:10.1162/003465302317331982.
- Duggan, M. A., W. F. Anderson, S. Altekruse, L. Penberthy, and M. E. Sherman. 2016. The Surveillance, Epidemiology, and End Results (SEER) program and pathology: Toward strengthening the critical relationship. The American Journal of Surgical Pathology 40 (12):e94–102. doi:10.1097/PAS.0000000000000749.
- Fragomeni, S. M., A. Sciallis, and J. S. Jeruss. 2018. Molecular subtypes and local-regional control of breast cancer. Surgical Oncology Clinics of North America 27 (1):95–120. doi:10.1016/j.soc.2017.08.005.
- Ge, L. P., X. Y. Liu, Y. Xiao, Z. C. Gou, S. Zhao, Y. Z. Jiang, and G. H. Di. 2018. Clinicopathological characteristics and treatment outcomes of occult breast cancer: A SEER population-based study. Cancer Management and Research 10:4381–91. doi:10.2147/CMAR.S169019.
- Giuliano, A. E., K. V. Ballman, L. McCall, P. D. Beitsch, M. B. Brennan, P. R. Kelemen, D. W. Ollila, N. M. Hansen, P. W. Whitworth, P. W. Blumencranz, et al. 2017. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (alliance) randomized clinical trial. JAMA 318 (10):918–26. doi:10.1001/jama.2017.11470.
- He, M., L. C. Tang, K. D. Yu, A. Y. Cao, Z. Z. Shen, Z. M. Shao, and G. H. Di. 2012. Treatment outcomes and unfavorable prognostic factors in patients with occult breast cancer. European Journal of Surgical Oncology (EJSO) 38 (11):1022–28. doi:10.1016/j.ejso.2012.08.022.
- Hessler, L. K., J. K. Molitoris, P. Y. Rosenblatt, E. C. Bellavance, E. M. Nichols, K. H. R. Tkaczuk, S. J. Feigenberg, S. M. Bentzen, and S. B. Kesmodel. 2017. Factors influencing management and outcome in patients with occult breast cancer with axillary lymph node involvement: Analysis of the National cancer database. Annals of Surgical Oncology 24 (10):2907–14. doi:10.1245/s10434-017-5928-x.
- Huang, K. Y., J. Zhang, W. F. Fu, Y. X. Lin, and C. G. Song. 2020. Different clinicopathological characteristics and prognostic factors for occult and non-occult breast cancer: Analysis of the SEER database. Frontiers in Oncology 10:1420. doi:10.3389/fonc.2020.01420.
- Kim, B. H., J. Kwon, and K. Kim. 2018. Evaluation of the benefit of radiotherapy in patients with occult breast cancer: A population-based analysis of the SEER database. Cancer Research and Treatment 50 (2):551–61. doi:10.4143/crt.2017.189.
- Kinoe, H., K. Yamanouchi, S. Kuba, M. Morita, C. Sakimura, M. Kanetaka, K. Abe, K. Takatsuki, N. Hayashida, and S. Eguchi. 2018. Discordance of hormone receptor, human epidermal growth factor receptor-2, and Ki-67 between primary breast cancer and synchronous axillary lymph node metastasis. Journal of the American College of Surgeons 23 (4):60–66. doi:10.1016/j.jamcollsurg.2018.08.012.
- Macedo, F. I., J. J. Eid, J. Flynn, M. J. Jacobs, and V. K. Mittal. 2016. Optimal surgical management for occult breast carcinoma: A meta-analysis. Annals of Surgical Oncology 23 (6):1838–44. doi:10.1245/s10434-016-5104-8.
- Montagna, E., V. Bagnardi, N. Rotmensz, G. Viale, G. Cancello, M. Mazza, A. Cardillo, R. Ghisini, V. Galimberti, P. Veronesi, et al. 2011. Immunohistochemically defined subtypes and outcome in occult breast carcinoma with axillary presentation. Breast Cancer Research and Treatment 129 (3):867–75. doi:10.1007/s10549-011-1697-6.
- Patel, J., T. Nemoto, D. Rosner, T. L. Dao, and J. W. Pickren. 1981. Axillary lymph node metastasis from an occult breast cancer. Cancer 47 (12):2923–27. doi:10.1002/1097-0142(19810615)47:12<2923:AID-CNCR2820471231>3.0.CO;2-N.
- Pentheroudakis, G., G. Lazaridis, and N. Pavlidis. 2010. Axillary nodal metastases from carcinoma of unknown primary (CUPAx): A systematic review of published evidence. Breast Cancer Research and Treatment 119 (1):1–11. doi:10.1007/s10549-009-0554-3.
- Ping, S., W. H. Ming, S. H. Bin, W. D. Wen, Q. Cheng, C. C. Jun, H. Y. Bin, and C. Z. Jian. 2014. Comparison of clinical characteristics between occult and non-occult breast cancer. J BUON 19 (3):662–66.
- Rosen, P. P. 1980. Axillary lymph node metastases in patients with occult noninvasive breast carcinoma. Cancer 46:1298–306. doi:10.1002/1097-0142/19800901/46:5<1298:aid-cncr2820460535>3.0.co;2-2.
- Rosen, P. P., and M. Kimmel. 1990. Occult breast carcinoma presenting with axillary lymph node metastases: A follow-up study of 48 patients. Human Pathology 21 (5):518–23. doi:10.1016/0046-8177(90)90008-s.
- Sohn, G., B. H. Son, S. J. Lee, E. Y. Kang, S. H. Jung, S. H. Cho, S. Baek, Y. R. Lee, H. J. Kim, B. S. Ko, et al. 2014. Treatment and survival of patients with occult breast cancer with axillary lymph node metastasis: A nationwide retrospective study. Journal of Surgical Oncology 110 (3):270–74. doi:10.1002/jso.23644.
- Walker, G. V., G. L. Smith, G. H. Perkins, J. L. Oh, W. Woodward, T. K. Yu, K. K. Hunt, K. Hoffman, E. A. Strom, and T. A. Buchholz. 2010. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer 116 (17):4000–06. doi:10.1002/cncr.25197.
- Wang, J., Y. F. Zhang, X. Wang, J. Wang, X. Yang, Y. Q. Gao, and Y. Fang. 2013. Treatment outcomes of occult breast carcinoma and prognostic analyses. Chinese Medical Journal 126 (16):3026–29.
- Wu, S. G., W. W. Zhang, J. Y. Sun, F. Y. Li, H. X. Lin, Y. X. Chen, and Z. Y. He. 2017. Comparable survival between additional radiotherapy and local surgery in occult breast cancer after axillary lymph node dissection: A population-based analysis. Journal of Cancer 8 (18):3849–55. doi:10.7150/jca.21217.