Abstract
The proband was a male in his seventies who came to our facility because of shortness of breath. He was not anemic but presented dissociation between oxygen saturation (SpO2) and partial pressure of oxygen (PaO2) by blood gas analysis, and also demonstrated hemoglobinopathy after measurement of Hb A1c using high performance liquid chromatography (HPLC). Twenty-three percent of unknown hemoglobin (Hb) bands were detected. After sequencing the β-globin gene, we noted a missense mutation at codon 74 (GGC>CGC) (Gly→Arg) of the β-globin chain and he was diagnosed with Hb Aalborg (HBB: c.223G>C). One of the proband’s siblings was diagnosed to have a low SpO2 level and also diagnosed to carry Hb Aalborg; she was also mildly anemic. This is the first known familial case of Hb Aalborg in Japan. In addition to Hb Aalborg, our case had underlying chronic obstructive pulmonary disease (COPD). Herein we present this case as a rare addition to the hematological literature.
Hemoglobinopathies are classified by the structural abnormality of the globin chain and the thalassemia, due to either synthetic α chain β chain failure [Citation1]. Unstable hemoglobin (Hb) is one of the hemoglobinopathies becoming a problem in the Japanese population. In Japan, approximately 210 abnormal Hb variants have been identified, of these, 10 types of mutations account for half of Japanese hemoglobinopathies, and the incidence of the condition is around 1/3000 [Citation1]. Seventy percent of hemoglobinopathies are asymptomatic variants, so these disorders are often discovered accidentally by abnormal Hb A1c patterns using high performance liquid chromatography (HPLC) (ADAMS™ A1c HA-8181; Arkray Inc., Kyoto, Japan) [Citation1] of PaO2 and SpO2 by blood gas analysis [Citation2].
We detected a case of Hb Aalborg (HBB: c.223G>C). One of our proband’s siblings also presented with low SpO2 and further examination revealed the first familial case of Hb Aalborg in Japan. In addition to Hb Aalborg, our proband had an underlying chronic obstructive pulmonary disease (COPD). Herein we present this case as a rare addition to the hematological literature.
Case report
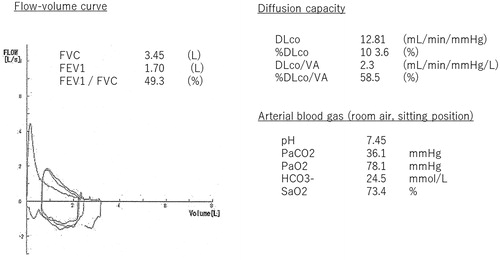
The proband was a male in his seventies, who was attending our facility because of shortness of breath. We followed him for a chronic aortic dissection; he also had a 58-pack/year smoking history. On a chest computed tomography (CT) scan, new aortic dissection was not detected. He was not anemic with a Hb value of 12.4 g/dL, same as the patient’s baseline value; however, his PaO2 of room air was 78.1 mmHg, but SpO2 was markedly decreased at 73.4% (). His forced vital capacity (FVC) was adequate at 3.45 L; however, his forced expiratory volume 1 secon (FEV1) was low at 1.7 L. (49.3% predicted), indicating a significant obstructive ventilatory pattern. The shape of his flow-volume curve was consistent with a diagnosis of COPD, as depicted in . His percent diffusion capacity for carbon monoxide (%DLCO) was 103.0% predicted, but his %DLCO divided by alveolar volume (%DLCO/VA) was low at 59.0%. Aortic expansion was observed on a CT scan, with a low attenuation area (LAA).
Figure 1. Pulmonary function test and arterial blood gas; FVC was 3.45 L and FEV1 and FEV1/FVC (1.7 L and 48.3%, respectively), was reduced. The %DLCO was kept at 103.0%, but %DLCO/VA was reduced (58.5%).
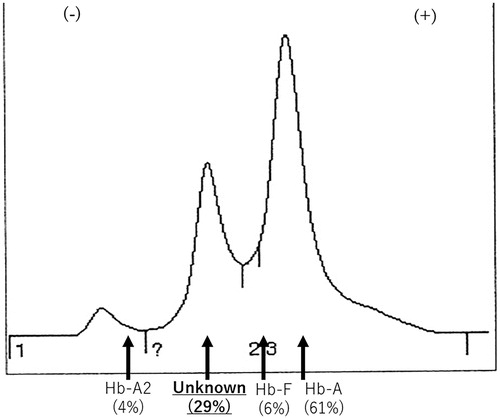
We subjected the Hb extracted from the hemolysate to reverse-phase HPLC. The optical density of the eluting solution showed an abnormal peak between the heme and normal β-globin peaks. The estimated Hb ratio was Hb A 61.0%, Hb F 1.0%, Hb A2 2.8%, and unknown Hb 27.0% (). We therefore concluded that the patient carried an unknown β chain variant. The sample was sent to Yamaguchi University, Yamaguchi, Japan for further analysis to determine the membrane properties of the erythrocytes. The median glycerol lysis time (GLT50) was within the normal range. Heinz bodies were not detected, however, the isopropanol test was weakly positive, suggestive of a hemoglobinopathy. The cellulose acetate membrane electrophoresis of Hb and isoelecric focusing yielded an abnormal band between Hb F and Hb S (HBB: c.20A>T), the same as our data. These findings strongly suggested an unstable hemoglobinemia.
Figure 2. The Hb A1c analysis by the HPLC method. The Hb A2 level was 4.0%, the estimated ratio of the unknown Hb was 29.0%, Hb F value was 6.0% and Hb A 61.0%.
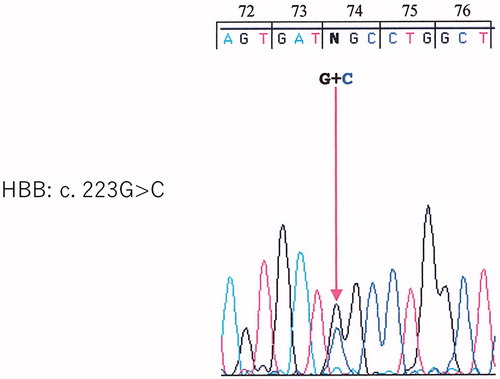
The proband’s β-globin gene was amplified by polymerase chain reaction (PCR) (Thermal cycler GeneAtlas Type G; Astec Co. Ltd., Fukuoka, Japan), and the nucleotide sequence was determined. Direct sequencing of exon 2 showed a missense mutation on the β-globin chain at codon 74 (GGC>CGC) (Gly→Arg), thus confirming the diagnosis of Hb Aalborg ().
Figure 3. Direct sequencing of exon 2 shows a missense mutation of the β-globin chain at codon 74 (GGC>CGC) (Gly→Arg).
We also evaluated the oxygen tension at 50% saturated hemoglobin (p50) from oxygen equilibrium curve (OEC) using Hemox™-analyzer (TCS Scientific Corporation, New Hope, PA, USA). As a result, the p50 values of our patients were 57.9 (normal reference range was 24.0-28.0 mmHg) ().
Table 1. Characteristics of the pulmonary function of the Hb Aalborg patients.
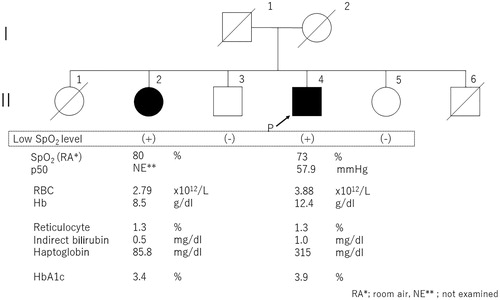
We confirmed that only II-2 was detected with a low SpO2 level, and oxygen therapy was given, so we decided to examine her. After conducting β-globin gene sequencing of this case, similar results were obtained. About II-2, the Heinz body was detected at a rate of 1/700 red blood cells (RBCs), and she had mild anemia (). This is the first Japanese familial report of Hb Aalborg. The symptoms of the proband (II-4) were shortness of breath and excessive sputum probably due to COPD. Notably, there was no anemia, so we treated the patient with expectorant and an anticholinergic agent.
Figure 4. The family pedigree. The sister (II-2) and proband (II-4) were diagnosed to carry Hb Aalborg.
Yamashiro and Hattori [Citation1] reported on abnormal Hbs in Japan, including the regions of where the variants were found. It is not rare for hemoglobinopathies detected abroad to be detected in Japan. However, it is all an isolated case, and there has only been one familial case of Hb Kansas, as reported by Ishiguro et al. in 1983 [Citation3]. At this time, we are reporting the first detection of familial Hb Aalborg in Japan.
Hb Aalborg was first reported in 1990 by Williamson et al. [Citation4]. Heinz bodies were readily detected in RBCs, but there was no specific evidence of hemolysis. The amino acid replacement was identified by fast atom bombardment mass spectrometry and consists of Gly→Arg at position β74(E18). Hb Aalborg is moderately unstable and is associated with mild anemia [Citation4]. In addition, Hb Aalborg has a reduced oxygen affinity due to the altered state of the Hb molecule in the tense and relaxed state [Citation5,Citation6]. This change shifts the oxygen dissociation curve to the right with an increase in the p50. The p50 is the partial pressure of oxygen when half of the Hb molecule is saturated [Citation7].
In our report, the proband (II-4) did not have anemia. On the other hand, II-2, had mild anemia and Heinz bodies were detected. In both cases no hemolytic involvement was observed, but the Hb A1c values were reduced. In addition, the SpO2 level of both patients was low and the value of p50 was elevated in our proband. This also suggests that Hb Aalborg is a low oxygen affinity variant.
The manner in which symptoms appeared varied among individual cases, consistent with the report by Williamson et al. [Citation4] describing the first case of Hb Aalborg. Given that two of six siblings of our patients had already died, it was not possible to evaluate all cases. However, Hb Aalborg would be transmitted in autosomal dominant fashion.
A few years ago, Panou et al. [Citation5] reported a Hb Aalborg patient who developed acute respiratory distress syndrome (ARDS), such as interstitial lung disease (ILD)-like clinical symptoms after cholecystectomy surgery. They reported that six out of seven cases caused 59.0-76.0% of %DLCO decrease and found no pulmonary disease. They speculated this phenomenon was due to the inability of heme bind to oxygen.
In our case, the proband’s %DLCO value of 104.0% was considered normal, however, %DLCO/VA, which is DLCO per unit displacement volume, decreased by 59.0%. The proband’s flow-volume curve was also suggestive of COPD, as were the LAA on the chest CT. In patients with pulmonary emphysema, the reduced diffusion capacity is sometimes masked by an increase in lung capacity arising from reduced elastic contractility due to alveolar wall destruction. For this reason, the diffusion capacity per area (calculated by DLCO/VA, %DLCO/VA) is a more accurate indicator. When DLCO reduction in patients with Hb Aalborg is evaluated, it is also necessary to check for the presence/absence of underlying pulmonary disease.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Yamashiro Y, Hattori Y. Hemoglobinopathies in Japan: characteristics and comparison with those of other ethnic groups. Rinsho Ketsueki. 2015;56(7):752–759.
- Yoko I, Yutaka T, Asako N, et al. Blood gas analysis suggested the abnormality of hemoglobin and diagnosis of the unstable hemoglobin disease (Hb Kansas) for a cyanosis patient. Hakodate Med J. 2007;31(1):21–23.
- Ishiguro K, Ohba Y, Hattori Y, et al. Hemoglobin Kansas in a Japanese family. Hemoglobin. 1983;7(6):573–579.
- Williamson D, Nutkins J, Rosthoj S, et al. Characterization of Hb Aalborg, a new unstable hemoglobin variant, by fast atom bombardment mass spectrometry. Hemoglobin. 1990;14(2):137–145.
- Panou V, Jensen P-DM, Pedersen JF, et al. Hemoglobin variant (hemoglobin Aalborg) mimicking interstitial pulmonary disease. Pulm Med. 2014;2014:701839.
- Verhovsek M, Henderson MP, Cox G, et al. Unexpectedly low pulse oximetry measurements associated with variant hemoglobins: a systematic review. Am J Hematol. 2010;85(11):882–885.
- Acheampong B, Chihak M, Rodriguez V. Hemoglobin Aalborg causing low oxygen saturation in a child. JCR. 2016;6(2):251–253.
