Abstract
On 11 March 2020, the World Health Organization (WHO) declared the novel SARS-CoV-2 virus responsible for causing COVID-19, a global pandemic. The virus primarily targets the respiratory system but can also affect other systems, notably causing hematological pathologies. Anemia, a common hematologic disorder, is characterized by the reduced oxygen-carrying capacity of red blood cells. The existing literature has a suspected link between anemia and severe COVID-19 cases. Researchers are currently investigating the long-term complications of COVID-19 in anemic patients, as these complications may play a crucial role in predicting patient prognosis. Anemic individuals are at a higher risk of experiencing severe COVID-19 infections due to several contributing pathophysiological mechanisms, including thrombotic, hemorrhagic, and autoimmune etiologies. The primary effect of these mechanisms is a decrease in circulating hemoglobin levels, reducing oxygen availability for cells. This exacerbates the hypoxia caused by COVID-19-induced acute respiratory distress syndrome (ARDS). This review offers a comprehensive overview of the evidence regarding the long-term complications of COVID-19 in anemic patients.
Introduction
The correlation between COVID-19 and anemia is of a complex nature. Both anemia and COVID-19 cause immune dysregulation, predisposing patients to autoimmune complications that are effectively interchangeable [Citation1,Citation2]. For example, COVID-19 patients might become anemic, and anemic patients are at risk of severe COVID-19 infection. Moreover, COVID-19 is clinically linked to thrombotic events caused by several mechanisms [Citation3,Citation4]. Inflammation leads to typical alterations of iron homeostasis hallmarked by increased iron acquisition and retention within macrophages along with reduced intestinal iron absorption [Citation5]. This results in a reduction of circulating iron levels and a reduced availability of the metal for erythropoiesis, where it is needed to produce hemoglobin (Hb). Together with cytokine-mediated inhibition of erythropoiesis, shortened erythrocyte half-life and reduced biological activity of the red cell hormone erythropoietin, this results in the development of anemia of inflammation (AI) [Citation6,Citation7].
Our review aims to investigate the relationship between COVID-19 infection and anemia, analyzing the resulting consequences and their effects on the well-being of patients.
COVID-19 and hemostasis
Viruses, bacteria, and other infectious pathogens are the major postulated environmental triggers of autoimmunity. In the scope of COVID-19, autoimmune complications have been reported early in the pandemic. For instance, cold agglutinin syndrome (CAD), autoimmune hemolytic anemia (AIHA), and Guillain-Barré are autoimmune complications that occur in the scope of COVID-19, in addition to thrombocytopenic purpura, which occurs 3–4 weeks post-infection [Citation8–10]. The effect of COVID-19, which results in thrombocytopenic purpura, can be attributed to different mechanisms summarized by Xu et al., who showed that the virus itself or the immunity against it can cause platelet’s decreased production, increased destruction, or both of them [Citation11].
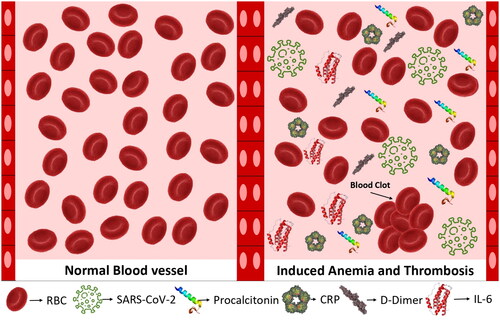
COVID-19 is a significant risk for thrombosis in patients [Citation12]. Inflammation and hemostasis engage in a bidirectional relationship, as inflammation induces activation of the hemostatic system, and this activation induces inflammatory reactions back () [Citation5]. In COVID-19, the excessive activation of the coagulation cascade is thought to be caused by the body’s elevated levels of inflammation () [Citation13]. Hospitals have been aggressively utilizing anticoagulants to reduce the occurrence of catastrophic thrombotic events, but even with this approach, a significant proportion of COVID-19 patients experience fatal thrombotic problems [Citation13].
Pathophysiology of anemia in COVID-19
A significant number of cases of hemolytic anemia have been documented in the context of COVID-19, mostly due to the development of auto-antibodies, despite the fact that AIHA has an estimated incidence of 13/100,000 in the normal population annually [Citation14]. It has been hypothesized that this might contribute to thrombosis and adverse outcomes in COVID-19 patients, considering the known risk of thrombosis in individuals with cold agglutinin hemolytic anemia [Citation15]. Hemolysis may also be brought on by viral infection-induced changes in their shape and functions [Citation16]. This is crucial when hemoglobinopathies or hereditary anemias are present [Citation17]. Severance et al. found that the SARS-CoV-2 infection caused oxidative stress, which is what caused hemolytic anemia in children with hereditary spherocytosis [Citation18]. However, oxidative stress contributes to hemolysis in other inherited hemolytic anemias, including hemoglobinopathies and not only in congenital spherocytosis. On the other hand, due to diminished immune response without cytokine storm and decreased T cell-mediated immunity, finally, the degree of anemia in AIHA is a factor in predicting the prognosis in COVID-19 patients [Citation19]. As a result, the National Haemoglobinopathy Panel (NHP) has produced recommendations on how to treat anemic patients with reference to planned blood transfusions and outpatient visits in order to lower the risk of exposure to SARS-CoV-2 and related COVID-19 severity in the patient population at risk [Citation17].
Conversely, the inflammatory state induced by COVID-19 leads to several interactions that contribute to a decrease in Hb levels. Among these interactions, the most significant is the hepcidin-related iron restriction of erythropoiesis. Researchers investigated the impact of COVID-19-induced hypoxia on erythropoiesis and iron metabolism [Citation6,Citation20]. A strong correlation between iron, IL-6, and hepcidin was observed in the context of COVID-19 infection. The release of IL-6 induced by the virus prompts hepcidin synthesis, leading to hyperferritinemia and iron-restricted erythropoiesis. This observation suggests a potential connection between COVID-19 inflammation and anemia, which extends beyond autoimmunity [Citation20]. Nevertheless, hypoferritinemia was observed in patients with severe COVID-19 infections, presenting a noteworthy finding in the context of the disease [Citation21]. Their analysis attributes this finding to retention of iron inside reticuloendothelial macrophages, and although a link between inflammation-induced hepcidin expression and serum iron was suspected, their correlation model failed to reveal it, implying involvement of other mechanisms [Citation21].
The interaction between AIHA and SARS-CoV-2 infection
In a study involving 94 documented cases, researchers investigated the phenomenon and reported their findings [Citation22]. AIHA is a common hematologic autoimmune complication in COVID-19 patients. A cross-sectional study found that COVID-19 patients with AIHA tend to have a worse prognosis and require longer hospital stays when their Hb levels fall below 12 g/L. The study reported that AIHA affected 14.7% of patients in intensive care units (ICUs) and 9% of non-ICU patients, with a mortality rate of 32% for those who tested positive for direct antiglobulin [Citation19]. Seven cases of AIHA, encompassing both warm and cold forms, were reported during the initial course of COVID-19. Four of these individuals were affected by lymphoid diseases, suggesting that SARS-CoV-2 infection may play a role in the development of autoimmunity [Citation23]. A number of cases of AIHA have been reported in a pediatric setting of COVID-19, which is significant given that SARS-CoV-2 infection can lead to hematologic autoimmune diseases in susceptible people, including both the elderly and children [Citation24]. A series of cases reported various instances of AIHA following COVID-19, as well as other complement-related anemic conditions. These conditions included paroxysmal nocturnal hemoglobinuria (PNH), cold agglutinin disease (CAD), aplastic anemia, and hemolytic uremic syndrome (HUS) [Citation25–27].
Autoimmune hemolytic anemia and associated anemia can lead to critical organ ischemia, decreased oxygen saturation, and hemodynamic disturbances [Citation19]. The occurrence of SARS-CoV-2-mediated immune hemolysis increases when it coincides with the cytokine storm associated with COVID-19. A number of cold and warm AIHA cases reported in the context of COVID-19 infection, which manifested during the SARS-CoV-2-induced cytokine storm, have been reported. The inflammatory reaction induced by SARS-CoV-2, which is abundant in cytokines, is thought to induce changes in antigen presentation, thereby creating cryptic antigens [Citation28]. The exact method by which AIHA contributes to COVID-19, however, remains unidentified. Moreover, it has been suggested that the complement system’s imbalance [Citation29], along with immune complexes and complement products found on RBC cells, may alter their rheology and thus promote the occurrence of intravascular thrombosis [Citation30]. This notion aligns with the heightened acute-phase response observed in COVID-19 patients. An illustrative example of this would be a COVID-19 patient who suffered from warm AIHA-induced disseminated intravascular coagulation leading to multiple organ failure [Citation31]. Patients with COVID-19 may experience thromboembolism due to the hypercoagulability and enhanced inflammatory response that may damage red blood cells (RBCs), making membranes brittle and less elastic [Citation32]. Finally, hemolysis-related iron and serum ferritin may fuel oxidative stress. It has been established that individuals with COVID-19 who have hyperferritinemia and impaired iron homeostasis have RBCs with altered ultrastructure, which lead to endothelial injury. Autoimmune hemolytic anemia may also cause pulmonary thrombosis [Citation33]. It is interesting to note that patients with COVID-19 may present with thrombosis linked to cold-agglutinin AIHA [Citation34]. A pulmonary embolism may also result with warm type AIHA. The probable mechanism of thrombosis in warm type AIHA may involve abnormal exposure to phosphatidylserine (PS), RBC-derived microparticles (MP), and nitric oxide scavenging. Red blood cell apoptosis increases PS exposure on the outer surface of the RBCs. Phosphatidylserine makes RBCs stickier and promotes the production of antiphospholipid antibodies. It serves as a docking site for enzyme complexes implicated in coagulation pathways. During hemolysis, RBC-derived MPs are released and function as tissue factors to cause thrombosis. D-dimer and thrombin–antithrombin complex production are also correlated with MPs. The released Hb from hemolyzed RBCs binds nitric oxide, which causes unchecked platelet aggregation and vasoconstriction, both of which result in thrombosis [Citation35]. These results point to a connection between these individuals’ erythrocyte abnormalities and thrombosis [Citation36].
Clinical course of COVID-19 in the presence of anemia
During the influenza seasons of 2003–2005, hospitalization records of four states in the USA showed that sickle cell disease (SCD) children were admitted 56 times more in comparison with non-SCD children [Citation37]. Likewise, anemia, coagulation disorders, and thrombocytopenia in COVID-19 patients predispose patients to hospitalization and more severe complications [Citation38]. Zhou et al. [Citation39] detected anemia in 15% of 191 patients who were hospitalized due to COVID-19; however, a higher prevalence of comorbidities including arterial cardiovascular disease, hypertension, or chronic kidney disease was detected in these anemic patients. These comorbidities are all known risk factors of mortality due to COVID-19 [Citation7]. In comparison to Zhou et al.’s findings [Citation39], other researchers confirmed anemia in 35.5% of 222 hospitalized patients [Citation40], while yet another study confirmed anemia in 61% of their 206 hospitalized patients of COVID-19 [Citation41], which are much higher rates.
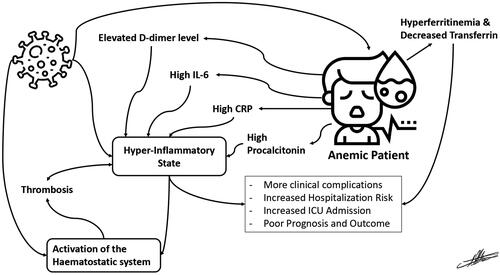
An association between anemia and poor clinical conditions, poor survival and longer hospital stays in COVID-19 patients was suggested [Citation7]. Anemia of inflammation is the commonest form of anemia amongst hospitalized patients, and the second commonest form of anemia in the world [Citation41]. Anemia, along with an inflammation-induced disruption of the iron homeostasis, is suggestive of more clinical risk and an increased rate of hospitalization, ICU admission, and need for mechanical ventilation. This risk can be confirmed by the increased ferritin and decreased transferrin typically found in COVID-19 patients with anemia () [Citation7,Citation42,Citation43]. In one study, researchers reported a link between anemia and increased mortality rates among hospitalized COVID-19 patients, although anemia was not associated with a higher rate of ICU admissions [Citation40]. The important finding indicates that people with anemia are more likely to have severe illness when compared to non-anemic patients. That is supported by the fact that COVID-19 patients with anemia are predisposed to more severe inflammatory reactions, coagulation abnormalities, and organ damage [Citation40].
Other factors of association
The relationship between COVID-19 and anemia remains unclear and hard to determine without close laboratory monitoring. Most studies did not consider age and sex differences when accounting for anemia in COVID-19 patients. Therefore, it is hard to confirm whether age or sex differences have an impact. Tao et al. argued that the sex and age of a patient have no significant effect on the outcome of COVID-19 across types of anemia [Citation40]. Telfer et al. support this claim since no significant correlation between SCD genotypes and age or sex was found in their survey of 195 patients with COVID-19 and rare inherited anemias [Citation44]. Despite not reaching statistical significance, mortality rates were higher among milder genotypes and female gender [Citation44]. On the contrary, a meta-analysis of 189 studies covering 57,563 COVID-19 patients noted a decline in Hb levels with age and higher pathological levels of ferritin in the elderly and in male patients [Citation45]. Also, Faghih Dinevari et al. suggest an indirect link between sex, age, and BMI to the COVID-19–anemia relationship, since their study concluded a significant relationship between these factors and anemia, thus influencing the hospitalization rates of anemic patients in cases of COVID-19 infection [Citation46]. Race and ethnicity might also have a role. A mini-review on sickle cell trait and exacerbated COVID-19 manifestations pointed out the potential connection between COVID-19 and the increased hypercoagulability state in sickle cell patients, despite the absence of a known direct link between the two thus far [Citation47]. This presents a clinical question regarding African Americans since sickle cell trait is higher among them [Citation47]. It is also important to underscore the relevance of genetic disorders. Paroxysmal nocturnal hemoglobinuria serves as a poignant example. This disorder manifests in patients due to the lack of complement inhibitory proteins, subsequently precipitating RBCs’ destruction, coagulation dysfunction, and intravascular hemolytic anemia. The core pathology lies in the unchecked synthesis of complement proteins – a phenomenon that intriguingly also underpins the pathogenesis of COVID-19 [Citation48]. However, it is worth noting that PNH is a relatively uncommon condition.
Inflammatory response COVID-19 in anemic patients
Upon investigating hematological laboratory findings of COVID-19 in anemic patients, an alteration of circulating biomarkers such as hyperferritinemia, C-reactive protein (CRP), interleukin-6 (IL-6), and procalcitonin that occurred in the post-acute phase of COVID-19 infection was noted (, ) [Citation50–52]. One study showed that hyperferritinemia was present in 38% of the study participants, predominantly in males, and that it was associated with severe disease [Citation50]. According to a study, individuals with higher ferritin levels are at a greater risk of contracting acute respiratory distress syndrome (ARDS). A prospective cohort study found that 16% of participants had elevated levels of CRP while 12% had elevated levels of IL-6 [Citation53]. Anemic patients of this study had higher levels of CRP and IL-6 and were associated with a more severe course of the infection [Citation50].
Table 1. A summery for laboratory findings related to anemia in COVID-19 patients across studies.
In , hematocrit levels were generally within the normal range, although at the lower end of it. Elevated ferritin and lowered iron and transferrin levels can be observed in COVID-19 patients. These readings correspond to the hyperferritinemic observations in COVID-19 patients. Also, CRP levels were rather high, as expected with the inflammatory state imposed by the virus and the high immune activation in patients. Elevated ferritin and lowered iron and transferrin levels can be observed in COVID patients. These readings correspond to the hyperferritinemic observations in COVID-19 patients. C-reactive protein levels were rather high, as expected with the inflammatory state imposed by the virus and the high immune activation of patients.
Long-term COVID-19 complications
The terms ‘long-haul COVID‘, ‘chronic COVID‘, and ‘post-COVID syndrome‘ are often used in medical research to describe persistent COVID-19 findings in those who are no longer positive for the virus [Citation56]. COVID-19 was linked to inflammatory syndromes and autoimmune diseases in various systems of the human body [Citation49]. These effects are mainly related to the thrombotic effect of the virus, resulting in irreversible damage in different organs. However, other observations that were made in long-COVID correspond to hematological issues other than thrombosis, including hyperferritinemia, iron deficiency, and anemia [Citation9]. In , three studies reported anemic or marginally anemic Hb levels in their patients. In another study, the levels of Hb were reduced to 7.1 and 7.6 upon admission and after release, respectively, which means that anemia remained in their case even after a negative PCR for SARS-CoV-2 [Citation57].
Ferritin is an indicator of iron storage in cells and is controlled by inflammation and iron availability [Citation58]. In COVID-19, hyperferritinemia is observed in ill patients, as is a hyperinflammatory state indicated by cytokine storms and excess IL-6 () [Citation59]. This state of inflammation and hyperferritinemia causes disruption of the iron homeostasis, sequentially resulting in iron deficiency and anemia, i.e. AI [Citation6]. These effects are of prognostic value, and may persist on the long term, causing late complications [Citation60].
In a study examining the late effects of COVID-19 in 60 children who had contracted the virus 1.5–2 months earlier, various complications were observed. Among these, iron deficiency anemia and coagulopathy were found to be relevant to the scope of our review [Citation61]. Anemia and iron deficiency were also observed when investigating mild to severe COVID-19 patients 60 days after the onset of the disease. Out of 109 patients, iron deficiency was found in 30% of patients, while anemia was present in 9%, even 2 months after the onset of the disease. Moreover, 38% of observed cases showed hyperferritinemia, more commonly associated with severe or critical condition [Citation50].
Finally, a persistent commitment to research and in-depth analysis is vital to surmount these challenges. It is through such rigorous scientific investigation that we can not only expand our understanding of SARS-CoV-2 but also unravel its complex interactions with human physiology. This involves comprehending the virus’s entry mechanisms, replication patterns, immune response evocation, and the consequent effects on different human organ systems (). Such knowledge will be invaluable in developing efficient therapeutics and prevention strategies against this formidable pathogen, ultimately contributing to global health security.
Conclusions
Anemia in the context of COVID-19 is critically important, as both conditions contribute to hypoxia and reduced oxygen availability, creating a high-risk combination. This interaction primarily occurs due to autoimmune mechanisms exacerbated by the hyperinflammatory and hyperferritinemic states associated with COVID-19 infection. The combined effects make patients more susceptible to severe complications, leading to increased hospitalization and mortality rates. A brief overview of laboratory findings in COVID-19 patients with anemia highlights the impact of SARS-CoV-2 infection on anemia, either by exacerbating its effects or by triggering its onset. Long-term complications are common among COVID-19 patients with anemia, as reports of lingering issues continue to emerge. The novel SARS-CoV-2 virus is not yet fully understood, and these aspects of the pandemic require further investigation.
Ethical approval
The article describes a review article. Therefore, no additional permission from our Ethics Committee was required.
Author’ contributions
LA-I: conceptualization; MA: figures design; LA-I, MA, MT, WA, KA, YA-B, AJN, and MAY: literature search and manuscript preparation; all authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors acknowledge Open Access funding provided by the Qatar National Library.
Disclosure statement
The authors report no conflicts of interest.
Data availability statement
All generated data are included in this published article.
Additional information
Funding
References
- Gracia-Ramos AE, Martin-Nares E, Hernández-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021;10(12):3592. doi: 10.3390/cells10123592.
- Kroll MH, Rojas-Hernandez C, Yee C. Hematologic complications of immune checkpoint inhibitors. Blood. 2022;139(25):3594–3604. doi: 10.1182/blood.2020009016.
- Fletcher-Sandersjöö A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027.
- Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127:104362. doi: 10.1016/j.jcv.2020.104362.
- Lanser L, Fuchs D, Kurz K, et al. Physiology and inflammation driven pathophysiology of iron homeostasis-mechanistic insights into anemia of inflammation and its treatment. Nutrients. 2021;13(11):3732. doi: 10.3390/nu13113732.
- Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50. doi: 10.1182/blood-2018-06-856500.
- Bellmann-Weiler R, Lanser L, Barket R, et al. Prevalence and predictive value of anemia and dysregulated iron homeostasis in patients with COVID-19 infection. J Clin Med. 2020;9(8):2429.
- Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol. 2021;33(2):155–162. doi: 10.1097/BOR.0000000000000776.
- Korompoki E, Gavriatopoulou M, Fotiou D, et al. Late-onset hematological complications post COVID-19: an emerging medical problem for the hematologist. Am J Hematol. 2022;97(1):119–128. doi: 10.1002/ajh.26384.
- Marchi G, Bozzini C, Bertolone L, et al. Red blood cell morphologic abnormalities in patients hospitalized for COVID-19. Front Physiol. 2022;13:932013. doi: 10.3389/fphys.2022.932013.
- Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0.
- Gorog DA, Storey RF, Gurbel PA, et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022;19(7):475–495. doi: 10.1038/s41569-021-00665-7.
- Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x.
- Gehrs BC, Friedberg RC. Autoimmune hemolytic anemia. Am J Hematol. 2002;69(4):258–271. doi: 10.1002/ajh.10062.
- Maslov DV, Simenson V, Jain S, et al. COVID-19 and cold agglutinin hemolytic anemia. TH Open. 2020;4(3):e175–e177. doi: 10.1055/s-0040-1715791.
- Russo A, et al. Implication of COVID-19 on erythrocytes functionality: red blood cell biochemical implications and morpho-functional aspects. Int J Mol Sci. 2022;23(4):2171. doi: 10.3390/ijms23042171.
- Roy NBA, Telfer P, Eleftheriou P, et al. Protecting vulnerable patients with inherited anaemias from unnecessary death during the COVID‐19 pandemic. Br J Haematol. 2020;189(4):635–639. doi: 10.1111/bjh.16687.
- Severance T, Rahim MQ, French J 2nd, et al. COVID-19 and hereditary spherocytosis: a recipe for hemolysis. Pediatr Blood Cancer. 2020;68(1):e28548.
- Algassim AA, Elghazaly AA, Alnahdi AS, et al. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2 infection. Ann Hematol. 2021;100(1):37–43. doi: 10.1007/s00277-020-04256-3.
- Maira D, Duca L, Busti F, et al. The role of hypoxia and inflammation in the regulation of iron metabolism and erythropoiesis in COVID-19: the IRONCOVID study. Am J Hematol. 2022;97(11):1404–1412. doi: 10.1002/ajh.26679.
- Hippchen T, Altamura S, Muckenthaler MU, et al. Hypoferremia is associated with increased hospitalization and oxygen demand in COVID-19 patients. Hemasphere. 2020;4(6):e492. doi: 10.1097/HS9.0000000000000492.
- Taherifard E, Taherifard E, Movahed H, et al. Hematologic autoimmune disorders in the course of COVID-19: a systematic review of reported cases. Hematology. 2021;26(1):225–239. doi: 10.1080/16078454.2021.1881225.
- Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune haemolytic anaemia associated with COVID‐19 infection. Br J Haematol. 2020;190(1):29–31. doi: 10.1111/bjh.16794.
- Hernández PV, Rivas YB, Sánchez EO, et al. Autoimmune hemolytic anemia in a pediatric patient with severe acute respiratory syndrome coronavirus 2 infection. Pediatr Infect Dis J. 2020;39(9):e288.
- Fattizzo B, Pasquale R, Bellani V, et al. Complement mediated hemolytic anemias in the COVID-19 era: case series and review of the literature. Front Immunol. 2021;12:791429. doi: 10.3389/fimmu.2021.791429.
- Cecchi N, Giannotta JA, Barcellini W, et al. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. 2022;196(6):1334–1336. doi: 10.1111/bjh.17947.
- Fattizzo B, Giannotta JA, Cecchi N, et al. SARS-CoV-2 vaccination in patients with autoimmune cytopenias: the experience of a reference center. Am J Hematol. 2021;96(11):E413–E416.
- AbouYabis AN, Bell GT. Hemolytic anemia complicating COVID-19 infection. J Hematol. 2021;10(5):221–227. doi: 10.14740/jh906.
- D’Alessandro A, Thomas T, Dzieciatkowska M, et al. Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level. J Proteome Res. 2020;19(11):4417–4427. doi: 10.1021/acs.jproteome.0c00365.
- Lam LKM, Reilly JP, Rux AH, et al. Erythrocytes identify complement activation in patients with COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;321(2):L485–L489. doi: 10.1152/ajplung.00231.2021.
- Damani J. Disseminated intravascular coagulopathy from warm autoimmune hemolytic anemia in a patient with COVID-19. Chest. 2020;158(4):A397. doi: 10.1016/j.chest.2020.08.388.
- Grobler C, Maphumulo SC, Grobbelaar LM, et al. Covid-19: the rollercoaster of fibrin(ogen), D-dimer, von Willebrand Factor, P-selectin and their interactions with endothelial cells, platelets and erythrocytes. Int J Mol Sci. 2020;21(14):5168. doi: 10.3390/ijms21145168.
- Solari D, Alberio L, Ribi C, et al. Autoimmune hemolytic anemia and pulmonary embolism: an association to consider. TH Open. 2021;5(1):e8–e13. doi: 10.1055/s-0040-1721733.
- Patil NR, Herc ES, Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection. Hematol Oncol Stem Cell Ther. 2022;15(4):213–216. doi: 10.1016/j.hemonc.2020.06.005.
- Elmassry M, Vutthikraivit W, Abdelmalek J, et al. Warm autoimmune hemolytic anemia as a rare cause of pulmonary embolism. Chest. 2020;158(4):A2095–A2096. doi: 10.1016/j.chest.2020.08.1810.
- Venter C, Bezuidenhout JA, Laubscher GJ, et al. Erythrocyte, platelet, serum ferritin, and P-selectin pathophysiology implicated in severe hypercoagulation and vascular complications in COVID-19. Int J Mol Sci. 2020;21(21):8234. doi: 10.3390/ijms21218234.
- Bundy DG, Strouse JJ, Casella JF, et al. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics. 2010;125(2):234–243. doi: 10.1542/peds.2009-1465.
- Mendy A, Apewokin S, Wells AA, et al. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv. 2020. doi: 10.1101/2020.06.25.20137323.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3.
- Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID-19: a retrospective cohort study. J Med Virol. 2021;93(3):1478–1488. doi: 10.1002/jmv.26444.
- Bergamaschi G, Borrelli de Andreis F, Aronico N, et al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med. 2021;21(2):247. doi: 10.1007/s10238-021-00699-8.
- Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x.
- McGonagle D, Sharif K, O’Regan A, et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev. 2020;19(6):102537. doi: 10.1016/j.autrev.2020.102537.
- Telfer P, De la Fuente J, Sohal M, et al. Real-time national survey of COVID-19 in hemoglobinopathy and rare inherited anemia patients. Haematologica. 2020;105(11):2651–2654. doi: 10.3324/haematol.2020.259440.
- Taneri PE, Gómez-Ochoa SA, Llanaj E, et al. Anemia and iron metabolism in COVID-19: a systematic review and meta-analysis. Eur J Epidemiol. 2020;35(8):763–773. doi: 10.1007/s10654-020-00678-5.
- Faghih Dinevari M, Somi MH, Sadeghi Majd E, et al. Anemia predicts poor outcomes of COVID-19 in hospitalized patients: a prospective study in Iran. BMC Infect Dis. 2021;21(1):170. doi: 10.1186/s12879-021-05868-4.
- Kehinde TA, Osundiji MA. Sickle cell trait and the potential risk of severe coronavirus disease 2019—a mini-review. Eur J Haematol. 2020;105(5):519–523. doi: 10.1111/ejh.13478.
- Chauhan AJ, Wiffen LJ, Brown TP. COVID-19: a collision of complement, coagulation and inflammatory pathways. J Thromb Haemost. 2020;18(9):2110–2117. doi: 10.1111/jth.14981.
- Hosseini P, Fallahi MS, Erabi G, et al. Multisystem inflammatory syndrome and autoimmune diseases following COVID-19: molecular mechanisms and therapeutic opportunities. Front Mol Biosci. 2022;9:804109. doi: 10.3389/fmolb.2022.804109.
- Sonnweber T, Boehm A, Sahanic S, et al. Persisting alterations of iron homeostasis in COVID-19 are associated with non-resolving lung pathologies and poor patients’ performance: a prospective observational cohort study. Respir Res. 2020;21(1):276. doi: 10.1186/s12931-020-01546-2.
- Terpos E, Ntanasis-Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID-19. Am J Hematol. 2020;95(7):834–847. doi: 10.1002/ajh.25829.
- Santosh Kumar Sidhwani TM, Khatoon A, Shaikh F, et al. Inflammatory markers and COVID-19 disease progression. J Infect Public Health. 2023;16(9):1386–1391. doi: 10.1016/j.jiph.2023.06.018.
- Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994.
- Oh SM, Skendelas JP, Macdonald E, et al. On-admission anemia predicts mortality in COVID-19 patients: A single center, retrospective cohort study. Am J Emerg Med. 2021;48:140–147. doi: 10.1016/j.ajem.2021.03.083.
- Lanser L, Burkert FR, Bellmann-Weiler R, et al. Dynamics in anemia development and dysregulation of iron homeostasis in hospitalized patients with COVID-19. Metabolites. 2021;11(10):653. doi: 10.3390/metabo11100653.
- Qian G-Q, Yang N-B, Ding F, et al. Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series. QJM. 2020;113(7):474–481. doi: 10.1093/qjmed/hcaa089.
- Wu T, Kang SC, Feng W, et al. A case report of aplastic anemia accompanied with COVID-19. Zhonghua Xue Ye Xue Za Zhi. 2020;41(4):340.
- Vogt A-CS, Arsiwala T, Mohsen M, et al. On iron metabolism and its regulation. Int J Mol Sci. 2021;22(9):4591.
- Gómez-Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID-19 patients – is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–251. doi: 10.1016/j.cca.2020.06.033.
- Edeas M, Saleh J, Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110.
- Zhvania M, Kvezereli-Kopadze M, Kutubidze T, et al. COVID-19 and children: complications and late outcomes. Georgian Med News. 2021;313:124–127.