Abstract
Interest in hot-melt extrusion techniques for pharmaceutical applications is growing rapidly with well over 100 papers published in the pharmaceutical scientific literature in the last 12 years. Hot-melt extrusion (HME) has been a widely applied technique in the plastics industry and has been demonstrated recently to be a viable method to prepare several types of dosage forms and drug delivery systems. Hot-melt extruded dosage forms are complex mixtures of active medicaments, functional excipients, and processing aids. HME also offers several advantages over traditional pharmaceutical processing techniques including the absence of solvents, few processing steps, continuous operation, and the possibility of the formation of solid dispersions and improved bioavailability. This article, Part I, reviews the pharmaceutical applications of hot-melt extrusion, including equipment, principles of operation, and process technology. The raw materials processed using this technique are also detailed and the physicochemical properties of the resultant dosage forms are described. Part II of this review will focus on various applications of HME in drug delivery such as granules, pellets, immediate and modified release tablets, transmucosal and transdermal systems, and implants.
INTRODUCTION
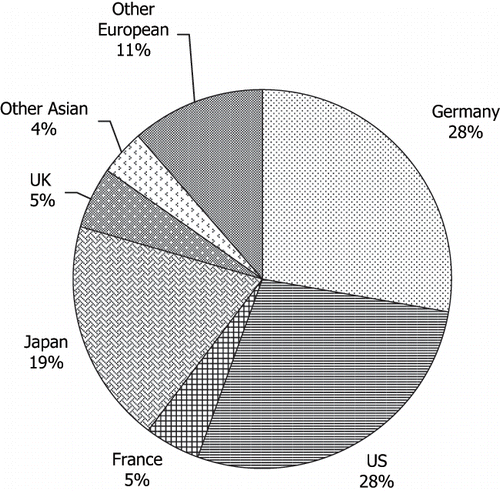
Hot-melt extrusion (HME) is one of the most widely used processing techniques within the plastics industry. Hot-melt extrusion is the process of pumping raw materials with a rotating screw under elevated temperature through a die into a product of uniform shape. Currently, more than half of all plastic products, including plastic bags, sheets, and pipes, are manufactured by this process (Kaufman et al., Citation1977). HME was first introduced in the plastics industry in the mid-nineteenth century to prepare polymeric insulation coatings to wires. Today, interest in HME techniques for pharmaceutical applications is growing rapidly with well over 100 papers published in the scientific literature in the last 12 years. The number of HME patents issued for pharmaceutical systems has steadily increased since the early 1980's () with international scope ().
FIGURE 1. The number of hot-melt extrusion patents issued for pharmaceutical applications from 1983 to 2006.
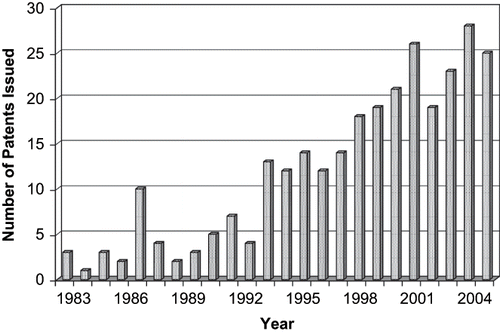
FIGURE 2. The number and percentage of hot-melt extrusion patents issued by country since 1983 for pharmaceutical applications.
Several research groups have demonstrated HME processes as a viable method to prepare pharmaceutical drug delivery systems, including granules (Follonier et al., Citation1995), pellets (Follonier et al., Citation1994; Young et al., Citation2002), sustained release tablets (Crowley et al., Citation2004b; Crowley et al., Citation2002; McGinity et al., Citation1997; Zhang, Citation1999; Zhang et al., Citation2000), transdermal and transmucosal drug delivery systems (Aitken-Nichol et al., Citation1996; Munjal et al., Citation2006; Prodduturi et al., Citation2005; Repka et al., Citation1999a, Citation2000b, Citation2001a, Citation2001b, Citation2002b, Citation2002d) and implants (Bhardwaj et al., Citation1997, Citation1998; Rothen-Weinhold et al., Citation2000; Sam, Citation1992). The HME technique is an attractive alternative to traditional processing methods.
HME offers many advantages to other pharmaceutical processing techniques. Molten polymers during the extrusion process can function as thermal binders and act as drug depots and/or drug release retardants upon cooling and solidification. Solvents and water are not necessary thereby reducing the number of processing steps and eliminating time-consuming drying steps. A matrix can be massed into a larger unit independent of compression properties. The intense mixing and agitation imposed by the rotating screw cause de-aggregation of suspended particles in the molten polymer resulting in a more uniform dispersion and the process is continuous and efficient.
It has been estimated that as many as 40% of all new molecular entities have poor bioavailability because of low aqueous solubility. This percentage is likely increasing due to the advent of combinatorial chemistry and the importance of lipophilic receptors (Kerns, Citation2001). Formulation of such compounds for oral delivery presents one of the most frequent and formidable challenges to formulation scientists. HME has been used to improve the bioavailability of drug substances especially those having low water solubility by formation of molecular dispersions (Breitenbach et al., Citation2003; Forster et al., Citation2001a; Kinoshita et al., Citation2002; Ndindayino et al., Citation2002c).
HME requires a pharmaceutical grade polymer that can be processed at relatively low temperatures due to the thermal sensitivity of many drugs. All components must be thermally stable at the processing temperature during the short duration of the heating process. Although this requirement may sometimes limit a pharmaceutical compound from HME processing, input of new techniques and equipment specifications over the last decade have expanded the list of actives not previously thought to be applicable for this emerging technology.
EQUIPMENT, PRINCIPLES OF EXTRUSION, AND PROCESS TECHNOLOGY
Hot-Melt Extrusion Equipment
Pharmaceutical-class extruders have evolved and adapted to mix drugs with carriers for various solid dosage forms as well as for the production of wet granulations. The major differences between a plastics extruder and a pharmaceutical-class extruder are the contact parts, which must meet regulatory requirements. Typically, the metallurgy of the contact parts must not to be reactive, additive or absorptive with the product. In addition, the equipment is configured for the cleaning and validation requirements associated with a pharmaceutical environment. Otherwise, the unit operations performed for a pharmaceutical product is virtually identical to a polymer extrusion process.
Extrusion processes can be categorized as either ram extrusion or screw extrusion. Screw extrusion consists of a rotating screw inside a heated barrel, while ram extrusion operates with a positive displacement ram capable of generating high pressures to push materials through the die. During ram extrusion, materials are introduced into a heated cylinder. After an induction period to soften the materials, a ram (or a piston) pressurizes the soft materials through the die and transforms them into the desired shape (Perdikoulias et al., Citation2003). High-pressure is the operating principle of ram extrusion. This technique is well suited for the precision extrusion of highly valuable materials. The ram exerts modest and repeatable pressure as well as a very consistent extrudate diameter. The major drawback of ram extrusion is limited melting capacity that causes poor temperature uniformity in the extrudate. Also, extrudates prepared by ram extrusion have lower homogeneity, in comparison with extrudates processed by screw extrusion.
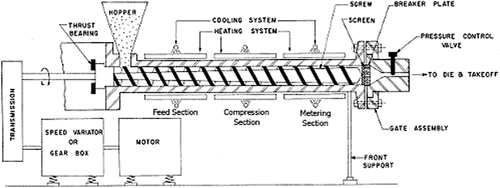
Unlike ram extrusion, a screw extruder provides more shear stress and intense mixing. At a minimum, a screw extruder consists of three distinct parts: a conveying system for material transport and mixing, a die system for forming, and downstream auxiliary equipment for cooling, cutting or collecting the finished goods. Individual components within the extruder are the feed hopper, a temperature controlled barrel, a rotating screw, die and heating and cooling systems (Griff, Citation1968). Additional systems include mass flow feeders to accurately meter materials into the feed hopper, process analytical technology to measure extrudate properties (near infra red systems and laser systems), liquid and solid side stuffers, vacuum pumps to devolitize extrudates, pelletizers, and calendaring equipment. Standard process control and monitoring devices include zone temperature and screw speed with optional monitoring of torque, drive amperage, and pressure and melt viscosity. Temperatures are normally controlled by electrical heating bands and monitored by thermocouples.
Single Screw Extruder
The single screw extruder is the most widely used extrusion system in the world. One screw rotates inside the barrel and is used for feeding, melting, devolatilizing, and pumping. Mixing is also accomplished for less demanding applications. Single screw extruders can be either flood or starve fed, depending upon the intended manufacturing process (Luker, Citation2003).
Single screw extruders are continuous, high-pressure pumps for viscous materials that can generate thousands of pounds of pressure while melting and mixing. Most extruder screws are driven from the hopper end. However, once screws are reduced to less than 18 mm, the screw becomes weak and solids transportation is far less reliable. To overcome these shortcomings, a vertical screw, driven from the discharge end, may be used. The discharge of such screws is two to four times stronger increasing solids transport (Luker, Citation2003).
Single screw extruders accept material into the feed section and convey materials along a flighted screw enclosed in a barrel. Single screws are typically flood fed, where the hopper sits over the feed throat and the screw RPM determines the output rate. Sometimes these devices are operated under starve fed conditions, where a feed system sets the mass flow rate and is independent of screw RPM. There are three basic functions of a single screw extruder: solids conveying, melting and pumping.
The forwarding of the solid particles in the early portion of the screw is a result of friction between the material and the feed section's bore. After solids conveying the flight depth begins to taper down and the heated barrel causes a melt to form. The energy from the heaters and shearing contribute to melting. Ideally, the melt pool will increase as the solid bed reduces in size until all is molten at the end of the compression zone. Finally, the molten materials are pumped against the die resistance to form the extrudate (Luker, Citation2003).
Twin-Screw Extruders
The first twin-screw extruders were developed in the late 1930's in Italy, with the concept of combining the machine actions of several available devices into a single unit. As the name implies, twin-screw extruders utilize two screws usually arranged side by side (). The use of two screws allows a number of different configurations to be obtained and imposes different conditions on all zones of the extruder, from the transfer of material from the hopper to the screw, all the way to the metered pumping zone (Mollan, Citation2003).
FIGURE 3. Twin screw prism USALAB digital 16 mm extruder (top), twin screw extruder (Courtesy of American Leistritz Co., Somerville, NJ) (bottom).
In a twin-screw extruder, the screws can either rotate in the same (co-rotating extruder) or the opposite (counter-rotating extruder) direction. The counter-rotating designs are utilized when very high shear regions are needed as they subject materials to very high shear forces as the material is squeezed through the gap between the two screws as they come together. Also, the extruder layout is good for dispersing particles in a blend. Generally, counter-rotating twin-screw extruders suffer from disadvantages of potential air entrapment, high-pressure generation, and low maximum screw speeds and output. Co-rotating twin-screw extruders on the other hand are generally of the intermeshing design, and are thus self-wiping (Breitenbach, Citation2002). They are industrially the most important type of extruders and can be operated at high screw speeds and achieve high outputs, while maintaining good mixing and conveying characteristics. Unlike counter-rotating extruders, they generally experience lower screw and barrel wear as they do not experience the outward “pushing” effect due to screw rotation.
These two primary types can be further classified as non-intermeshing and fully intermeshing. The fully intermeshing type of screw design is the most popular type used for twin-screw extruders () (Thiele, Citation2003). This design itself is self-wiping, where it minimizes the nonmotion and prevents localized overheating of materials within the extruder. The extruder operates by a first in/first out principle since the material does not rotate along with the screw. Non-intermeshing extruders, on the other hand, are often used for processing when large amounts of volatiles need to be removed and when processing highly viscous materials. Non-intermeshing extruders allow large volume de-volatization via a vent opening since the screws are positioned apart from one another. Non-intermeshing extruders are not susceptible to high torques generated while processing highly viscous materials for the same reasons (Mollan, Citation2003).
FIGURE 4. Twin screw design examples: intermeshing co-rotating twin-screw (top), and intermeshing counter-rotating twin-screw (Burns et al.). (Courtesy of American Leistritz Co., Somerville, NJ) (bottom).
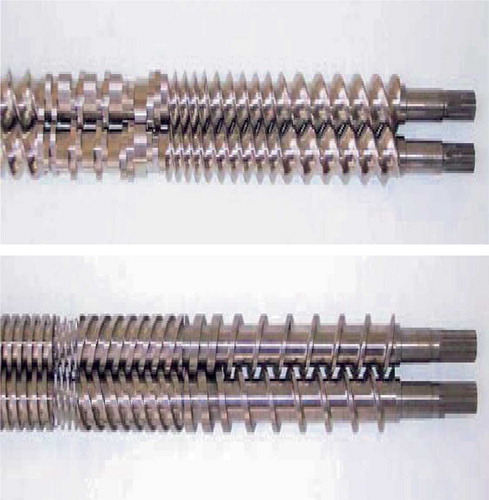
Twin-screw extruders have several advantages over single screw extruders, such as easier material feeding, high kneading, and dispersing capacities, less tendency to over-heat and shorter transit time. However, single-screw extruders do have the advantage over twin-screw extruders in terms of their mechanical simplicity and more reasonable cost (Repka et al., Citation2002a).
Most commercial extruders have a modular design to facilitate changing screws. The design of the screw has a significant impact on the process and can be selected to meet particular requirements such as high or low shear. Whelan and Dunning have reviewed the various screw designs available (Whelan et al., Citation1996). Specific screw features are displayed in . In an extrusion process, the dimensions of the screws are given in terms of L/D ratio, which is the length of the screw divided by the diameter (Steiner, Citation2003). For example, an extruder screw that is 1000 mm long and has a 25 mm diameter exhibits a 40:1 L/D. Typical extrusion process lengths are in the 20 to 40:1 L/D range, or longer. Extruder residence times are generally between 5 sec and 10 min, depending upon the L/D ratio, type of extruder, screw design, and how it is operated. The size of an extruder is generally described based on the diameter of the screw used in the system, i.e., 18–27 mm extruder (pilot scale) as compared with 60 mm extruder (production scale) (Steiner, Citation2003). Although the screw size difference appears small (∼2 fold) in the preceding example, the extruder output that results from doubling the screw size may be 10-fold, i.e., from 10 to 100 kg/h. This is due to the much larger volume available for processing as the screw size is increased.
FIGURE 5. Diagram of an extruder screw (repka et al., 2002a). 1) the channel depth is the distance from the screw roots to the inner barrel surface; 2) the flight clearance is the distance between the screw flight and the inner barrel surface; 3) the channel width is the distance between two neighboring flights; 4) the helix angle is the angle between the flight and the direction perpendicular to the screw axis.
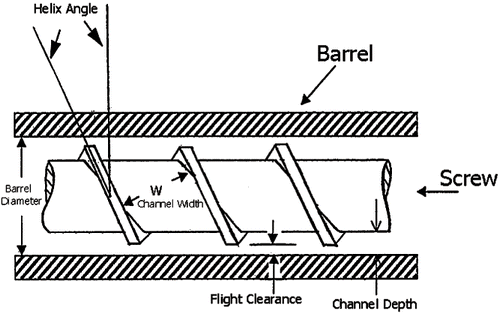
Screws are designed with several sections, with the function of each section ranging from feeding, mixing, compression, and metering. Most screws are made from surface coated stainless steel to reduce friction and the possibility of chemical reactions.
The screw is typically divided into three sections along the length of the barrel: feeding, melting or compression, and metering as shown in . The purpose of the feeding section is to transfer the materials from the hopper to the barrel. The channel depth is usually widest in this section to facilitate mass flow. A decrease in channel depth in the compression zone increases the pressure, which removes entrapped air (Chokshi et al., Citation2004). The polymer typically begins to soften and melt in the compression zone. The melt moves by circulation in a helical path by means of transverse flow, drag flow, pressure flow, and leakage. Thermoplastic polymers primarily exist in a molten state when entering the metering section. The function of the metering zone is to reduce the pulsating flow and ensure a uniform delivery rate through the die cavity. The mass flow rate of the extrudate is highly dependent upon the channel depth and the length of the metering section.
The die is attached at the end of the barrel. The shape of the die dictates the physical form or shape of the extrudate. Generally, the cross section of the extrudate will increase upon leaving the die, a phenomenon known as “die swell” depending on the viscoelastic properties of the polymers. This entropy driven event occurs when individual polymer chains recover from deformation imposed by the rotating screw by “relaxing” and increasing their radius of gyration.
Downstream Processing Equipment
Providing a usable melt to the die and downstream system is only part of an extruded pharmaceutical product. A wide variety of downstream systems are available following the extrusion process. Cooling the extrudate may be in the form of air, nitrogen, on stainless steel conveyors or rolls, or in water. Pellets or shapes may be extruded and wound or cut-to-length (). Co-extrusion allows the possibility of complex properties from a single structure, which can be beneficial for time-release products (Ghebre-Sellassie et al., Citation2003). Film and lamination systems are used to combine melt extrusion with substrates for transmucosal and transdermal applications. There have also been recent advances in cyclic molding, where a continuous melt process fills an accumulator that intermittently fills a complex mold. For film applications, chill rolls, and torque winders are used to rapidly cool and collect the extrudate (). Film thickness can be adjusted by changing the die opening, the mass flow rate introduced into the extruder, screw speed, the rotation speed of the chill rolls, or the torque winder. The chill roll temperature also influences the properties of the film and may be adjusted with appropriate downstream devices.
The Hot-Melt Extrusion Process
The different zones of the barrel are pre-set to specific temperatures prior to the extrusion process. The feedstock must have good flow properties. This requirement is usually met by insuring that the angle of the feed hopper exceeds the angle of repose of the feed materials. When this prerequisite is not met, the feedstock tends to form a solid bridge at the throat of the hopper resulting in erratic flow. In these situations, a force-feeding device such as a mass flow feeder or side stuffer can be used to direct the feedstock onto the rotating screw (Doetsch, Citation2003).
As the feedstock is moved along the length of the barrel, thermal energy is generated by shearing, imposed by the rotating screw and from conduction from the barrel via electrical heating bands. Pumping efficiency of the feeding section is dependent upon the friction coefficient between the feed materials and the surface of the barrel and screw. The friction on the inner surface of the barrel is the driving force for the material feed, whereas the friction at the surface of the screw restricts forward motion of the material (Luker, Citation2003). High friction along the barrel and low friction at the screw interface contribute to efficient mass flow in the feed section. Clearly, the bulk density, particle shape, and compression properties of the raw materials impact the feeding efficiency.
Material transfer should be efficient in order to maintain an increase in pressure in the compression zone and the metering zone. The pressure rise in these zones insures efficient output of the extrudate. It is also possible to fine-tune the barrel temperature at the feeding section in order to optimize the friction at the surface of the barrel. Inconsistent material feed may result in a “surge” phenomenon that will cause cyclical variations in the output rate, head pressure, and product quality. The temperature of the melting zone is normally set 15–60°C above the melting point of semi-crystalline polymers or the glass transition temperature of amorphous polymers (Griff, Citation1968; Mccrum et al., Citation1997; Rauwendaal, Citation1994; Whelan et al., Citation1996).
The efficiency of the melting process depends on the polymer properties and the extruder design. In general, polymers with low melt viscosities and high thermal conductivities exhibit a more efficient melting process. Changes in the screw design are sometimes warranted to improve the melting process and improve mass flow through the extruder. Solidified polymer components can block the channel if melting is incomplete and result in a surge of material around the blockage.
Processing conditions depend on the chemical stability and physical properties of the thermal polymer. Melt viscosity, molecular weight, glass transition temperature, and melting point (in the case of a semicrystalline polymer) should be considered to establish appropriate processing parameters. Polymers are subjected to a mechanical shear stress imposed by the rotating screw, and thermal stress due to the relatively high processing temperatures and pressures. Under these conditions, polymers may undergo chain scission, depolymerization or thermal degradation. Differential scanning calorimetry, thermogravimetric analysis, and gel permeation chromatography are often used to monitor polymer stability. Plasticizers, antioxidants, thermal lubricants, and other additives are often included in the formulation to address stability concerns (McGinity et al., Citation2003).
Wet Extrusion Versus Dry Extrusion
Based on the properties of the feedstock, extrusion processes can be classified as wet extrusion or dry extrusion. In wet extrusion, the feed stock is conditioned and softened with the addition of solvents prior to processing (Doetsch, Citation2003). The primary reason for wet extrusion is that extrudates have a superior finish due to the softening, plasticizing, and ripening action of the solvents. In some cases, such as cellulose nitrate, wet extrusion under low temperatures and pressures with minimum friction is required because the polymer is explosive when overheated using dry extrusion processes.
Compared to wet extrusion, dry extrusion is a solvent free process. The feedstock is generally in solid form and heat is required to soften or melt the materials. In the dry extrusion process, materials are softened by the heated barrel, the shearing effect of a rotating screw and friction during transit. The extrudate solidifies after exiting the extruder. For obvious reasons, most of the extrusion processes use the dry technique.
Mass Flow During Hot-Melt Extrusion
Many polymer melts behave as pseudoplastic fluids under typical processing conditions. The viscosity of a pseudoplastic fluid depends upon the shear rate and is described by the following power law equation (Eq. (1)) (Dreiblatt, Citation2003):(1)
where η is the viscosity of the polymer melt, γ is the shear rate, K is an exponential function of the temperature and depends on the properties of the polymer, and n is the power law constant (typically in the range of 0.25 to 0.9 for the polymer melt).
Minimum temperatures are required for extrusion; otherwise, the torque required to rotate the screw will overload the drive unit. Torque is directly proportional to melt viscosity. The dependence of polymer melt viscosity on the temperature at a given shear rate follows the Arrhenius equation (Eq. (2)) (Repka et al., Citation2002a):(2)
In Eq. (2), K′ is a constant depending on the structure and the molecular weight of the polymer; Ea is the activation energy of the polymer for the flow process, and it is a constant for the same type of polymer; R is the gas constant; and T is the temperature in degrees Kelvin.
Heat conduction from the electrical bands on the barrel contributes to the melting process. However, heat is also generated from shearing of the polymer melt. “Viscous heat generation” is the process of transforming mechanical energy from shearing into thermal energy. The rate of heat generation per unit volume due to viscous heat dissipation follows Eq. (3) (Repka et al., Citation2002a):(3)
in which m is a constant, γ is the shear rate and n is the power law constant.
MATERIALS USED IN HOT-MELT EXTRUSION
For a pharmaceutical material to be processed by HME, it must be able to deform easily inside the extruder and solidify upon its exit. The materials must meet the same levels of purity and safety as those prepared by traditional techniques. Most of the raw materials used in hot-melt extruded pharmaceuticals have been used in the production of other solid dosage forms such as tablets, pellets, granules, transdermal, and transmucosal systems (Aitken-Nichol et al., Citation1996; Munjal et al., Citation2006; Prodduturi et al., Citation2005; Repka et al., Citation1999a, Citation2000a, Citation2000b, Citation2001a, Citation2001b, Citation2002b, Citation2002c, Citation2005, Citation2006). Thermal stability of the individual compounds is a prerequisite for the process, although the short processing times encountered in this process may not limit all thermolabile compounds.
Hot-melt extruded dosage forms are complex mixtures of active medicaments and functional excipients. Functional excipients may be broadly classified as matrix carriers, release modifying agents, bulking agents, antioxidants, thermal lubricants, and miscellaneous additives. The selection and use of various excipients can impart specific properties to hot-melt extruded pharmaceuticals in a manner similar to those in traditional dosage forms.
The incorporation of plasticizers may lower the processing temperatures necessary for HME thus reducing drug and carrier degradation. Drug release from these systems can be modulated by the incorporation of various functional excipients. The dissolution rate of the active compound can be increased or decreased depending on the properties of the rate-modifying agent. For systems that display oxidative or free radical degradation during processing or storage, the addition of antioxidants, acid acceptors, and/or light absorbers may be warranted.
Carriers
In hot-melt extruded drug delivery systems, the active compound is embedded in a carrier formulation often comprised of one or more “meltable” substances and other functional excipients. The meltable substance is generally a polymer or low melting point wax. The selection of an appropriate carrier is important in the formulation and design of a hot-melt extruded dosage form. The properties of the carrier often dictate the processing conditions. The physical and chemical properties of the carrier can control the release of the active compound from the final dosage form. lists some of the carriers used to prepare hot-melt extruded dosage forms.
TABLE 1. Carriers Used to Prepare Hot-melt Extruded Dosage Forms
For systems employing nonpolymeric carrier materials, the compatibility between the drug substance and carrier should be addressed. The incorporation of a low melting compound into a low melting point wax may form a eutectic mixture or reduce the melting point of the mixture preventing the formation of a solid dosage form. The production of granules using carnauba wax has been reported (Miyagawa et al., Citation1996, Citation1999; Sato et al., Citation1997). The granules contained diclofenac sodium and could be produced at temperatures less than the reported melting point of the wax material. The use of waxes and other wax-based materials have the potential advantage of being relatively inert.
Drug release kinetics from hot-melt extruded dosage forms is highly dependent upon the choice of the carrier material. Carriers used in hot-melt extruded dosage forms have included water insoluble polymers and waxes such as ethyl cellulose or carnauba wax in which the rate of drug release are diffusion controlled. Water soluble polymers have included hydroxypropyl cellulose, polyethylene oxide, poly(vinyl pyrrolidone) in which the drug is released by a diffusion and erosion mechanism (Keleb et al., Citation2001; Repka et al., Citation2000a, Citation2001a). Functional excipients have also been used to modify drug release rates in these systems. Depending upon the physical and chemical properties of these additional excipients, various release profiles may be achieved. Functional excipients have been formulated into hot-melt extruded dosage forms to modify the drug release rate by altering the porosity or tortuosity of the dosage form. Viscosity increasing agents have been incorporated into polymeric matrices to limit and reduce the initial burst often observed with these systems.
The use of ionic and/or pH dependent polymers as the carrier matrix may achieve zero-order drug release or site specific drug delivery along the gastrointestinal tract. Swelling agents and super disintegrants such as AcDiSol® and Explotab® have been investigated as a method to modulate drug release. It has been reported that Explotab® could be used as a “super-absorbent” in hydroxypropylcellulose hot-melt extruded transdermal films to facilitate moisture uptake in wound care applications (Repka et al., Citation2000b). A similar approach of drug release modification was applied to wax-containing systems (Miyagawa et al., Citation1996, Citation1999; Sato et al., Citation1997). Hydroxypropylcellulose, Eudragit® L, and sodium chloride were incorporated into diclofenac sodium/carnauba wax matrices. Increasing the cellulose derivative or methacrylic acid copolymer concentration in the system resulted in a substantial increase in the release of diclofenac sodium. The release of diclofenac sodium from hydroxypropylcellulose/wax matrices was less pH dependent than the system containing wax/Eudragit® L since the methacrylic acid copolymer is insoluble in water or in solutions with pH < 6. The effect of sodium chloride was less pronounced and was attributed to the negligible swelling effect of this material.
Plasticizers
Plasticizers are typically low molecular weight compounds capable of softening polymers to make them more flexible. The use of polymeric carriers in HME often requires the incorporation of a plasticizer into the formulation to improve the processing conditions during the manufacturing of the extruded dosage form or to improve the physical and mechanical properties of the final product.
Plasticization of the polymer is generally attributed to the inter-molecular secondary valence forces between the plasticizer and the polymer. Plasticizers are able to decrease the glass transition temperature and the melt viscosity of a polymer by increasing the free volume between polymer chains (Aharoni, Citation1998). In doing so, the ease of movement of polymer chains with respect to each other is dramatically reduced. Plasticizers were also found to facilitate the fusion process of semi-crystalline polymers (Fried, Citation1995; Strobl, Citation1997). Less energy is usually required to melt semi-crystalline polymers following the addition of one or more plasticizers. With the addition of a plasticizer, a HME process can be conducted at lower temperatures and with less torque. Generally, both the active ingredient and the polymer will be more stable during the extrusion process due to these improved processing conditions. Materials commonly used as plasticizers that are approved by the Food and Drug Administration for use in pharmaceutical dosage forms are listed in according to their chemical structure.
TABLE 2. Common Plasticizers Used in Pharmaceutical Dosage Forms
Plasticizers used for the preparation of pharmaceutical dosage forms must have good efficiency, stability, polymer-plasticizer compatibility, and permanence. Triacetin, citrate esters, and low molecular weight polyethylene glycols have been investigated as plasticizers in hot-melt extruded systems. Recently, surfactants have also been shown to be promising plasticizers in producing solid dispersions by HME in addition to acting as solubilizers (Ghebremeskel et al., Citation2006). Additionally, several drug substances have been reported to function as plasticizers in hot-melt extruded dosage forms (Aitken-Nichol et al., Citation1996; Crowley et al., Citation2002, Citation2004a; Repka et al., Citation1999b; Zhang et al., Citation1999).
The physical and mechanical properties and drug release rate of pharmaceutical dosage forms is dependent on the permanence of the plasticizers. Permanence of a plasticizer during processing and storage is very important and the evaporation of highly volatile plasticizers from the dosage form during storage has been reported. Arwidsson et al. (Citation1991) reported a dramatic change in drug release properties of coated tablets due to volatilization of the plasticizer during curing and storage. Repka and McGinity (Citation1999a) demonstrated that the amount of plasticizer remaining in hot-melt extruded films over time was a function of the plasticizer type and storage conditions. Plasticizers may also improve the physical-mechanical properties of hot-melt extruded dosage forms. In transdermal films, the addition of a plasticizer to the polymer matrix can improve the film's flexibility (Aitken-Nichol et al., Citation1996; Repka et al., Citation1999a). Plasticizers often influence the product's tensile strength and elastic modulus.
Other Processing Aids
The excessive temperatures needed to process unplasticized or under plasticized polymers may lead to polymer degradation. The stability of polymers that are susceptible to degradation can be improved with the addition of antioxidants, acid receptors and or light absorbers during HME. One manufacturer of these materials recommends the incorporation of an antioxidant into formulations containing low molecular weight hydroxypropylcellulose (“Physical and chemical properties, klucel®, hydroxypropylcellulose,” Citation1997). Similarly, polyethylene oxide has been reported to be protected from free radical and oxidative degradation by the incorporation of an antioxidant (Crowley et al., Citation2002).
Antioxidants are classified as preventive antioxidants or chain-breaking antioxidants based upon their mechanism. Preventive antioxidants include materials that act to prevent initiation of free radical chain reactions. Reducing agents, such as ascorbic acid, are able to interfere with autoxidation in a preventive manner since they preferentially undergo oxidation. The preferential oxidation of reducing agents protects drugs, polymers, and other excipients from attack by oxygen molecules. These antioxidants are sometimes referred to as oxygen scavengers. They are most effective when used in a closed system where oxygen cannot be replaced once it is consumed. Chelating agents such as edetate disodium (EDTA) and citric acid are another type of preventive antioxidant that decrease the rate of free radical formation by forming a stable complex with metal ions that catalyze these reduction reactions.
Hindered phenols and aromatic amines are the two major groups of chain breaking antioxidants that inhibit free radical chain reactions. Commonly used antioxidants such as butylated hydroxyanisole, butylated hydroxytoluene and vitamin E are hindered phenols. Because the O-H bonds of phenols and the N-H bonds of aromatic amines are very weak, the rate of oxidation is generally higher with the antioxidant than with the polymer.
Other materials have been used to facilitate HME processing. Waxy materials like glyceryl monostearate have been reported to function as a thermal lubricant during hot-melt processing. Vitamin E TPGS has been reported to plasticize polymers and enhance drug absorption () (Crowley et al., Citation2002; Repka et al., Citation2000a).
TABLE 3. Common Processing Aids Used in Hot-melt Extruded Dosage Forms
Active Pharmaceutical Ingredients (APIs)
The properties of the active drug substance often limit the formulation and processing choices available to the pharmaceutical scientist in the development of dosage forms. HME is an anhydrous process, which avoids potential hydrolytic degradation pathways. In addition, poorly compactable materials can be prepared as tablets without a compression process by cutting an extruded rod to the desired dimensions.
As an initial assessment, the thermal, chemical, and physical properties of the drug substance must be characterized. Depending on the unique properties of the drug substance and the other materials in the formulation, the drug may be present as undissolved particles, a solid solution, or a combination in the final dosage form. The state of the drug in the dosage form may have a profound impact on the processability and stability of the product. The advantages and disadvantages of each form have been discussed in both injection molding (Cuff et al., Citation1998) and melt extrusion (Grunhagen et al., Citation1995) systems. Solid dispersion systems may be more stable and more easily processed than solid solution systems, but solid solution systems may be produced that are transparent and may have increased bioavailability of poorly soluble compounds. provides a partial listing of some of the drug substances that have been formulated and processed by HME.
TABLE 4. Drug Substances Processed by Hot-melt Extrusion Techniques
The active compound may help or hinder the functionality of the other components in the formulation. Oxprenolol hydrochloride was shown to melt under the HME processing conditions thus decreasing the viscosity of the extrudate to yield a material with poor handling properties (Follonier et al., Citation1994). In similar work preparing dosage forms by injection molding, Cuff and Raouf reported that fenoprofen calcium inhibited the hardening of a PEG-MCC matrix, resulting in an unusable product (Cuff et al., Citation1998). In contrast, Lidocaine was shown to lower the glass transition temperature of Eudragit? E/HDPE films (Aitken-Nichol et al., Citation1996), and hydrocortisone demonstrated a time dependent lowering of the glass transition temperature of HPC films (Repka et al., Citation1999b). Thus, the drug substance can be either beneficial or detrimental to properties of hot-melt extruded dosage forms.
PROPERTIES OF HOT-MELT EXTRUDED DOSAGE FORMS
Chemical Stability of Drug Substances During Hot-Melt Extrusion
The stability of the active ingredients during HME should be closely monitored since it is conducted at elevated temperatures. Hydrolysis, solvolysis, and oxidation are three primary mechanisms of drug degradation. Drugs containing carboxylic acids, phosphoric acids or carbonyl functional groups are vulnerable to hydrolysis (Conors et al., Citation1986). Water or a solvent must be present for hydrolysis or solvolysis to occur. Since HME is a solvent free process, hydrolysis and solvolysis are often not a concern. Oxidation is also a free-radical chain reaction with three distinctive stages: initiation, propagation and termination. Oxidative drug degradation during HME has been reported by Aitken-Nicol et al. (Citation1996) and Repka et al. (Citation1999b). Peroxides formed due to the oxidation of polymeric carrier were shown to induce the oxidation of the active ingredient. Since the drug and polymer share the same oxidation mechanisms, antioxidants for polymers discussed in the previous chapter are also applicable for active ingredients.
High-pressure liquid chromatography is the most commonly used technique to investigate the stability of drug substances. Stability indicating methods should be developed so that the active ingredient is separated from the degradants. The purity of drug peak can be studied using a photo diode-array detector to confirm the reliability of the assay.
Thermal and Crystalline Properties of Hot-Melt Extruded Dosage Forms
Drug and polymers are subjected to elevated temperatures, high pressure, and intense mixing during the HME process. At these elevated temperatures, the solubility of the drug in the polymer carrier is increased. Depending upon the processing conditions, some crystalline drugs either melt or become solubilized in the polymer matrix during the process. Recrystallization and nucleation of drug molecules from the polymer melt is retarded during the cooling of the extrudate due to reduced solute migration and the difficulty in nucleation in a highly viscous polymer medium. Furthermore, polymer viscosity increases dramatically with the decrease in the temperature.
Miscibility of the active forms with the excipients is an important factor to be considered for a successful hot-melt extrudate. Miscibility can experimentally be determined with differential scanning calorimetry, hot stage microscopy and theoretically utilizing solubility parameter calculations. Forster et al. demonstrated that melt extrusion of miscible components resulted in solid solution formation, whereas extrusion of an ‘immiscible’ component led to an amorphous drug dispersed in crystalline excipients (Forster et al., Citation2001c). Following HME, the active ingredient can be present in one of two forms: as a crystal embedded in the hardened polymer phase, or as individual molecules dissolved in the polymer matrix. Formation of a drug-polymer solid dispersion in hot-melt extruded dosage forms has been reported (Aitken-Nichol et al., Citation1996; Zhang et al., Citation2000). Complexation between drug and polymer may also contribute to the formation of a solid solution (Chang et al., Citation1975). Solid solutions containing the drug and an amorphous polymer are generally regarded as interstitial solid solutions where drug molecules occupy the interstitial space between the polymer chains. For a semi-crystalline polymer, some drugs were reported to be concentrated in the amorphous regions of the polymer (Delahaye et al., Citation1997), and some drugs were reported to be solubilized in the crystalline matrix of the polymer (Chiou et al., Citation1971). Because the drug is dispersed at a molecular level, a solid dispersion is a metastable form. It is susceptible to aging effects and the drug may recrystallize from the matrix during the storage. Crystallization of chloramphenicol palmitate from a solid dispersion in PVP was reported (Chiou et al., Citation1971).
The methods that have been used to characterize hot-melt extrudates are summarized in . In addition to characterizing the hot-melt extrudate, these methods can be used to differentiate between solid solutions (molecularly dispersed drug), solid dispersions in which drug is only partly molecularly dispersed and physical mixture of drug and carrier. It is challenging to precisely characterize systems, which are molecularly dispersed from those that are not due to the complexity of the systems, and different analytical methods may yield contrasting results. In general, dispersions in which no crystallinity can be detected are molecularly dispersed and the absence of crystallinity is used as a criterion to differentiate between solid solutions and solid dispersions.
TABLE 5. Common Methods Used for the Characterization of Hot-melt Extrudates
Thermoanalytical methods include those that examine the system as a function of temperature. Differential scanning calorimetry (DSC) has been widely used to study the thermal properties of materials used in HME. DSC can be used for the quantitative detection of transitions (melting point, glass transition) in which energy is required or liberated (i.e., endothermic and exothermic phase transformations). Generally, the hot-melt extrudate is scanned and compared to a physical mixture of the drug, polymeric carrier and other excipients. The lack of a melting transition in the DSC scan of the hot-melt extrudate indicates that the drug is present in an amorphous rather than crystalline form. Thermogravimetric analysis (TGA) is a measure of thermally induced weight loss of a material as a function of applied temperature. TGA is limited to studies involving either a weight gain or loss, and is commonly used to study desolvation and decomposition. TGA can be used as a screening tool for the thermal stability of materials used in HME. Microthermal analysis has been used to identify phase separation in hot-melt extrudates containing itraconazole and Eudragit E100 (Six et al., Citation2003). In this approach, differences in the thermal topography of hot-melt extrudates can be discerned.
Wide angle X-ray diffraction (XRD) is also used to characterize the crystalline properties of hot-melt extruded dosage forms. The principle of XRD is based on Bragg's law, in which parallel incident X-rays strike the crystal planes and are then diffracted at angles related to the spacing between the planes of molecules in the lattice. Crystallinity is reflected by a characteristic fingerprint region in the diffraction pattern. If the fingerprints of the drug and carrier do not overlay one another, the crystallinity of the drug and polymer following HME can be determined. Thus, X-ray diffraction can be used to differentiate between solid solutions, in which the drug is amorphous, and solid dispersions, in which it is at least partly present in the crystalline form, regardless of whether the carrier is amorphous or crystalline. However, the sensitivity of the XRD technique is limited and cannot generally detect crystallinity of less than 10% (Brittain, Citation1995).
Additional techniques to detect crystalinity include infrared spectroscopy, solid-state nuclear magnetic resonance, light microscopy, and scanning electron microscopy. Infrared spectroscopy can be used to detect changes in bonding between functional groups due to structural changes or a lack of crystal structure. IR can be used to differentiate between peaks that are sensitive to changes in crystallinity from those that are not (Taylor et al., Citation1997). Solid-state nuclear magnetic resonance (NMR) has been used to probe the crystallinity of materials. Although any NMR-active nucleus can be studied, most efforts have focused on 13C investigations. Microscopy is one of the best methods to study the crystalline properties of hot-melt extrudates. Both optical and electron methods are suitable to examine the surface morphology of samples to probe for the presence of crystalline particles or amorphous domains. It is also possible to obtain reliable particle size information using these techniques.
CONCLUSION
Although a relatively new technology in the pharmaceutical industry, HME has emerged as a viable approach for the development of drug delivery systems. Single and twin-screw extruders are replacing traditional batch processes because of the consistent and repeatable nature of continuous extrusion. The more recent introduction of twin-screw extruders with different screw designs allows them to be used to perform a large number of previously separate functions. Compared to solvent processes aiming at solid molecular dispersions melt extrusion technology offers a promising alternative. This technology is suitable for both high dose and potent compounds. The mixing that occurs in the barrel of the extruder during processing ensures good content uniformity of the active material in the finished product. Furthermore, the availability of a wide range of thermoanalytical and microscopic techniques allows for the characterization of physical and chemical stability of actives and/or excipients used in the melt extrudate with good predictability. The potential of automation and reduction of capital investment and labor costs have made HME worthy of consideration. High-energy input mainly related to shear forces and temperature pose problems in manufacturing of heat labile actives by HME. This can be overcome in most cases, however, by proper selection of screw assemblies, side stuffing techniques and extruder die designs to minimize the residence time in the extruder. The possible use of a broad selection of polymers, plasticizers and/or processing aids has opened an arena for continued research, processing and manufacture of various dosage forms in the field of formulation that will be discussed in Part II of this review.
ACKNOWLEDGMENT
Resources for this journal article were supported by NIH/NCRR Award #P20 RR021929.
REFERENCES
- Aharoni S. M. Increased glass transition temperature in motionally constrained semicrystalline polymers. Polymers For Advanced Technologies 1998; 9(3)169–201
- Aitken-Nichol C., Zhang F., McGinity J. W. Hot melt extrusion of acrylic films. Pharm. Res. 1996; 13(5)804–808
- Arwidsson H., Hjelstuen O., Ingason D., Graffner C. Properties of ethyl cellulose films for extended release. Part 2. Influence of plasticizer content and coalescence conditions when using aqueous dispersions. Acta Pharm. Nordica 1991; 3: 65–70
- Bhardwaj R., Blanchard J. In vitro evaluation of poly(D, L-lactide-co-glycolide) polymer-based implants containing the alpha-melanocyte stimulating hormone analog, melanotan-I. J. Control. Release 1997; 45(1)49–55
- Bhardwaj R., Blanchard J. In vitro characterization and in vivo release profile of a poly(D, L-lactide-co-glycolide)-based implant delivery system for the alpha-msh analog, melanotan-I. Int. J. Pharm. 1998; 170(1)109–117
- Breitenbach J. Melt Extrusion: From Process To Drug Delivery Technology. European Journal Of Pharmaceutics And Biopharmaceutics 2002; 54: 107–117
- Breitenbach J., Magerlein M. Melt extruded molecular dispersions. Pharmaceutical Extrusion Technology, I. Ghebre-Sellassie, C. Martin. Marcel Dekker Inc, New York 2003; 133
- Brittain H. G. Physical characterization of pharmaceutical solids. Marcel Dekker Inc, New York 1995; 70
- Bruce L. D. N. H. S., Malick A. W., Infeld M H., McGinity J. W. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. Eur. J. Pharm. Biopharm. 2005; 59(1)85–97
- Burns S. J., Higginbottom S., Corness D., Hay G., Barnwell S. G. Study of enteric-coated liquid-filled hard gelatin capsules with biphasic release characteristics. Int. J. Pharm. 1994; 110: 291–296
- Chang B. L., Nuessle N. O., Haney W. G. Characterization of hydrogen bonding between selected barbiturates and polyethylene glycol 4000 By Ir Spectral Analysis. J. Pharmaceut. Sci. 1975; 64: 1787–1797
- Chiou W. L., Reigelman S. Pharmaceutical applications of solid dispersion systems. J. Pharmaceut. Sci. 1971; 60: 1281–1302
- Chokshi R., Zia H. Hot-melt extrusion technique: A review. Iranian J. Pharm. Res. 2004; 3: 3–16
- Conors K. A., Amidon G. L., Stella V. J. Chemical stability of pharmaceuticals2nd. Wiley-Interscience, New York 1986
- Crowley M. M., Fredersdorf A., Schroeder B., Ucera S., Prodduturi S., Repka M. A., et al. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur. J. Pharm. Sci 2004a; 22(5)409–418
- Crowley M. M., Schroeder B., Fredersdorf A., Obara S., Talarico M., Kucera S., et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. Int. J. Pharm. 2004b; 271(1–2)77–84
- Crowley M. M., Zhang F., Koleng J. J., McGinity J. W. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002; 23(21)4241–4248
- Cuff G., Raouf F. A preliminary evaluation of injection molding as a technology to produce tablets. Pharmaceut. Tech. 1998; 22: 96–106
- De Brabander C., Van Den Mooter G., Vervaet C., Remon J. P. Characterization of ibuprofen as a nontraditional plasticizer of ethyl cellulose. J. Pharm. Sci. 2002; 91(7)1678–1685
- De Brabander C., Vervaet C., Fiermans L., Remon J. P. Matrix mini-tablets based on starch/microcrystalline wax mixtures. Int. J. Pharm. 2000; 199(2)195–203
- Delahaye N., Duclos R., Saiter J. M., Varnier S. Characterization of solid dispersion phase transition using a new optical thermal analyzer. Drug Dev. Ind. Pharm. 1997; 23: 293–303
- Doetsch W. Material handling and feeder technology. Pharmaceutical extrusion technology, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, New York 2003; 133, pp: 111–134
- Dreiblatt A. Process design. Pharmaceutical extrusion technology, I. Ghebre-Sellassie. Marcel Dekker, Inc, New York 2003; 133, pp: 153–169
- El-Egakey M. A., Soliva M., Speiser P. Hot extruded dosage forms Part I: Technology and dissolution kinetics of polymeric matrices. Pharm. Acta Helv. 1971; 46: 31–52
- Follonier N., Doelker E., Cole E. T. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained release capsules containing high loadings of freely soluble drugs. Drug Dev. Ind. Pharm. 1994; 20(8)1323–1339
- Follonier N., Doelker E., Cole E. T. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained release pellets prepared using polymeric materials. J. Contr. Release 1995; 36(3)243–250
- Forster A., Hempenstall J., Rades T. Characterization of glass solutions of poorly water-soluble drugs produced by melt extrusion with hydrophilic amorphous polymers. J. Pharm. Pharmacol. 2001a; 53(3)303–315
- Forster A., Hempenstall J., Tucker I., Rades T. The potential of small-scale fusion experiments and the gordon-taylor equation to predict the suitability of drug/polymer blends for melt extrusion. Drug Dev. Ind. Pharm. 2001b; 27(6)549–560
- Forster A., Hempenstall J., Tucker I., Rades T. Selection of excipients for melt extrusion with two poorly water-soluble drugs by solubility parameter calculation and thermal analysis. Int. J. Pharm. 2001c; 226: 147–161
- Fried J. R. Polymer science and technology. Ptr Prentice Hall: Englewood Cliffs, , N.J 1995
- Fukuda M., Peppas N. A., McGinity J. W. Floating hot-melt extruded tablets for gastroretentive controlled drug release system. J. Of Cont. Rel. 2006a; 115(2)121–129
- Fukuda M., Peppas N. A., McGinity J. W. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int. J. Pharm. 2006b; 31: 90–100
- Ghebre-Sellassie I., Martin C. Future trends. Pharmaceutical extrusion technology. Drugs and the pharmaceutical sciences, I. Ghebre-Sellasie, C. Martin. Marcel Dekker, New York 2003; 133: 383–392
- Ghebremeskel N. A., Vemavarapu C., Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble API: Stability testing of selected solid dispersions. Pharm. Res 2006; 23(8)1928–1936
- Griff A. L. Plastics extrusion technology2nd. Robert E. Krieger Publishing Company, Malabar 1968
- Grunhagen H. H., Muller O. Melt extrusion technology. Pharm. Manuf. Int. 1995; 166–170
- Hamaura T., Newton J. M. Interaction between water and poly(vinylpyrrolidone) containing polyethylene glycol. J. Pharm. Sci. 1999; 88(11)1228–1233
- Henrist D., Lefebvre R. A., Remon J. P. Bioavailability of starch based hot stage extrusion formulations. Int. J. Pharm. 1999; 187(2)185–191
- Henrist D., Remon J. P. Influence of the formulation composition on the in vitro characteristics of hot stage extrudates. Int. J. Pharm 1999a; 188(1)111–119
- Henrist D., Remon J. P. Influence of the process parameters on the characteristics of starch based hot stage extrudates. Int. J. Pharm. 1999b; 189(1)7–17
- Hulsmann, S., & Backensfeld, T. (2000). World 0057853.
- Hulsmann S., Backensfeld T., Bodmeier R. Stability of extruded 17 ss-estradiol solid dispersions. Pharm. Dev. Technol 2001; 6(2)223–229
- Hulsmann S., Backensfeld T., Keitel S., Bodmeier R. Melt extrusion—an alternative method for enhancing the dissolution rate of 17-estradiol hemihydrate. Eur. J. Pharm. Biopharm. 2000; 49: 237–242
- Kaufman H. S., Falcetta J. J. Introduction to polymer science and technology: an spe textbook1st. John Wiley & Son, New York 1977
- Keleb E. I., Vermeire A., Vervaet C., Remon J. P. Cold extrusion as a continuous single-step granulation and tabletting process. Eur. J. Pharm. Biopharm. 2001; 52(3)359–368
- Kerns E. H. High throughput physicochemical profiling for drug discovery. J. Pharm. Sci. 2001; 90(11)1838–1858
- Kidokoro M., Shah N. H., Malick A. W., Infeld M. H., McGinity J. W. Properties of tablets containing granulations of ibuprofen and an acrylic copolymer prepared by thermal processes. Pharmaceut. Dev. Tech. 2001; 6(2)263–275
- Kinoshita M., Baba K., Nagayasu A., Yamabe K., Shimooka T., Takeichi Y., et al. improvement of solubility and oral bioavailability of a poorly water-soluble drug, TAS-301, by its melt-adsorption on a porous calcium silicate. J. Pharm. Sci. 2002; 91(2)362–370
- Koleng J. J., McGinity J. W. Preparation and evaluation of rapid-release granules using a novel hot-melt extrusion technique. Paper Presented At The 16th Pharmaceutical Technology Conference, Athens, 1997
- Lindberg N. O., Tufvesson C., Holm P., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins Mpf 50 D. influence on intragranular porosity and liquid saturation. Drug Dev. Ind. Pharm. 1988; 14(13)1791–1798
- Lindberg N. O., Tufvesson C., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, Baker Perkins Mpf 50 D. Drug Dev. Ind. Pharm. 1987; 13: 1891–1913
- Liu J., Zhang F., McGinity J. W. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2001; 52: 181–190
- Luker K. Single-screw extrusion and screw design. Pharmaceutical extrusion technology. Drugs and the pharmaceutical sciences, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, Newyork 2003; 133: 39–68
- Lyons J. G. D. D. M., Kennedy J. E., Geever L. M., O'sullivan P., Higginbotham C. L. The use of agar as a novel filler for monolithic matrices produced using hot melt extrusion. Eur. J. Of Pharm. And Biopharm 2006; 64(1)75–81
- Mccrum N. G., Buckley C. P. Principles of polymer engineering. Oxford University Press, Oxford 1997
- McGinity J., Zhang F., World Patent No. 9749384, 1997
- McGinity J. W., Zhang F. Melt-extruded controlled-release dosage forms. Pharmaceutical extrusion technology, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, New York 2003; 133: 183–208
- Mididoddi P. K., Prodduturi S., Repka M. A. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Dev. Ind. Pharm. 2006; 32: 1059–1066
- Miller D. A, M. J. T., Yang W., Williams R. O., McGinity J. W. Hot-melt extrusion for enhanced delivery of drug particles. J. Pharm. Sci. 2006; 96: 361–376
- Miyagawa Y., Okabe T., Yamaguchi Y., Al E. Controlled release of diclofenac sodium from wax matrix granule. Int. J. Pharma. 1996; 138(2)215–224
- Miyagawa Y., Sato H., Okabe T., Nishiyama T., Miyajima M. A., Sunada H. In vivo performance of wax matrix granules prepared by a twin- screw compounding extruder. Drug Dev. Ind. Pharm. 1999; 25(4)429–435
- Mollan M. Historical overview. Pharmaceutical extrusion technology, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, Inc, New York, Ny 2003; 133: 1–18
- Munjal M., Stodghill S. P., Elsohly M. A., Repka M. A. Polymeric systems for amorphous D9-tetrahydrocannabinol produced by a hot-melt method. Part I: chemical and thermal stability during processing. J. Pharm. Sci. 2006; 95(8)1841–1853
- Nakamichi K., Nakano T., Yasuura H., Izumi S., Kawashima Y. The role of kneading paddle and the effects of screw revolution speed and water content on the preparation of solid dispersions using a twin-screw extruder. Int. J. Pharm. 2002; 241: 203–211
- Nakamichi K., Yasuura H., Fukui H., Oka M., Izumi S. Evaluation of a floating dosage form of nicardipine hydrochloride and hydroxypropylmethylcellulose acetate succinate prepared using a twin-screw extruder. Int. J. Pharm. 2001; 218(1–2)103–112
- Ndindayino F., Henrist D., Kiekens F., Van Den Mooter G., Vervaet C., Remon J. P. Direct compression properties of melt-extruded isomalt. Int. J. Pharm 2002a; 235(1–2)149–157
- Ndindayino F., Vervaet C., Van Den Mooter G., Remon J. Bioavailability of hydrochlorothiazide from isomalt-based moulded tablets. Int. J. Pharm. 2002b; 246(1–2)199–202
- Ndindayino F., Vervaet C., Van Den Mooter G., Remon J. P. Direct compression and moulding properties of co-extruded isomalt/drug mixtures. Int. J. Pharm. 2002c; 235(1–2)159–168
- Perdikoulias J., Dobbie T. Die Design. Pharmaceutical extrusion technology. Drugs and the pharmaceutical sciences, I. Ghebre-Sellassie, C. Martin. Marcel Dekkr, Inc, New York 2003; 133: 99–110
- Perissutti B., Newton J. M., Podczeck F., Rubessa F. Preparation of extruded carbamazepine and PEG 4000 as a potential rapid release dosage form. Eur. J. Pharm. Biopharm. 2002; 53(1)125–132
- Physical And Chemical Properties, Klucel®, Hydroxypropylcellulose. Hercules Incorporated, Aqualon Division, Wilmington, De 1997; 2–6
- Prodduturi S., Manek R. V., Kolling W. M., Stodghill S. P., Repka M. A. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J. Pharm. Sci 2005; 94(10)2232–2245
- Rauwendaal C. Polymer extrusion3rd. Hanser Publisher, Munich 1994
- Repka M. A., Gerding T. G., Repka S. L., McGinity J. W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot-melt extrusion. Drug Dev. Ind. Pharm. 1999a; 25(5)625–633
- Repka M. A., Gerding T. G., Repka S. L., McGinity J. W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Dev. Ind. Pharm. 1999b; 25(5)625–633
- Repka M. A., Gutta K., Prodduturi S., Munjal M., Stodghill S. P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005; 59(1)189–196
- Repka M. A., Gutta K., Prodduturi S., Munjal M., Stodghill S. P. Characterization of cellulosic hot-melt extruded films containing lidocaine. Eur. J. Pharm. Biopharm. 2005; 59(1)189–196
- Repka M. A., McGinity J. W. Influence of vitamin E TPGS on the properties of hydrophilic films produced by hot-melt extrusion. Int. J. Pharm. 2000a; 202(1–2)63–70
- Repka M. A., McGinity J. W. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials 2000b; 21(14)1509–1517
- Repka M. A., McGinity J. W. Bioadhesive properties of hydroxypropylcellulose topical films produced by hot-melt extrusion. J. Contr. Release 2001a; 70(3)341–351
- Repka M. A., McGinity J. W. Influence of chlorpheniramine maleate on topical films produced by hot-melt extrusion. Pharmaceut. Dev. Tech. 2001b; 6(3)295–302
- Repka M. A., McGinity J. W. Influence of chlorpheniramine maleate on topical hydroxypropylcellulose films produced by hot-melt extrusion. Pharmaceut. Dev. Tech. 2001c; 6(3)297–304
- Repka M. A., McGinity J. W., Zhang F., Koleng J. J. Hot-melt extrusion technology. Encyclopedia of pharmaceutical technology2nd, J. Swarbrick, J. Boylan. Marcel Dekker, New York 2002a; 203–266
- Repka M. A., Munjal M., Elsohly M. A., Ross S. Temperature stability and bioadhesive properties of d9-tetrahydrocannabinol incorporated hydroxypropyl cellulose polymer matrix systems. Drug Dev. Ind. Pharm. 2006; 32(1)21–32
- Repka M. A., O'haver J., See C. H., Gutta K., Munjal M. Nail morphology studies as assessments for onychomycosis treatment modalities. Int. J. Pharm. 2002b; 245: 25–36
- Repka M. A., Repka S. L., McGinity J. W., United States Patent No. 6375963 B1, 2002c
- Repka M. A., Repka S. L., McGinity J. W., United States Patent No. 6,375,963 B1, 2002d
- Ringrose B. J., Kronfli E. Feasibility study on the melt processing of radiation-grafted ethylene-vinyl acetate copolymer resins. Adv. Polym. Technol. 1999; 18(4)343–350
- Rippie E. G., Johnson J. R. Regulation of dissolution rate by pellet geometry. J. Pharmaceut. Sci. 1969; 58(4)428–431
- Rothen-Weinhold A., Oudry N., Schwach-Abdellaoui K., Frutiger-Hughes S., Hughes G. J., Jeannerat D., et al. Formation of peptide impurities in polyester matrices during implant manufacturing. Eur. J. Pharm. Biopharm. 2000; 49(3)253–257
- Sam A. P. Controlled release contraceptive devices—A status-report. J. Control. Release 1992; 22(1)35–46
- Sato H., Miyagawa Y. Dissolution mechanism of diclofenac sodium wax matrix granules. J. Pharmaceut. Sci. 1997; 86(8)929–934
- Six K., Murphy J., Weuts I., Craig D. Q. M., Verreck G., Peeters J., et al. Identification of phase separation in solid dispersions of itraconazole and eudragit (R) E100 using microthermal analysis. Pharm. Res. 2003; 20(1)135–138
- Sprockel O. L., Sen M., Shivanand P., Prapaitrakul W. A melt extrusion process for manufacturing matrix drug delivery systems. Int. J. Pharm. 1997; 155(2)191–199
- Steiner R. Extruder design. Pharmaceutical extrusion technology. Drugs and the pharmaceutical sciences, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, Inc, New York 2003; 133: 19–38
- Stepto R. F. T. Thermoplastic starch and drug delivery capsules. Polym. Int. 1997; 43(2)155–158
- Stepto R. F. T. Thermoplastic Starch. Paper Presented At The Macromol. Symp. 2000
- Strobl G. The physics of polymers: Concepts for understanding their structures and behavior2nd. Springer, Berlin 1997
- Taylor L. S., Zografi G. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 1997; 14(12)1691–1698
- Thiele W. Twin-screw extrusion and screw design. Pharmaceutical extrusion technology. Drugs and the pharmaceutical sciences, I. Ghebre-Sellassie, C. Martin. Marcel Dekker, New York 2003; 133: 69–98
- Van Laarhoven J. A. H., Kruft M. A. B., Vromans H. Effect of supersaturation and crystallization phenomena on the release properties of a controlled release device based on eva copolymer. J. Control. Release 2002; 82(2–3)309–317
- Van Laarhoven J. A. H., Kruft M. A. B., Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. Int. J. Pharm. 2002; 232(1–2)163–173
- Whelan T., Dunning D. The Dynisco Extrusion Processor Handbook1st. London School Of Polymer Technology, London 1996
- Wu C. M. J. Influence of methyl praben as a solid-state plasticizer on the physicochemical properties of eudragit RS PO hot-melt extrudates. J. Pharm. Biopharm. 2003; 56(1)95–100
- Young C. R. D. C., Cerea M., Farrell T., Fegely K. A., Rajabi Siahboomi A., McGinity J. W. Physicochemical characterization and mechanisms of release of theophylline from melt-extruded dosage forms based on a methacrylic acid copolymer. Int. J. Pharm. 2005; 301(1–2)112–120
- Young C. R., Koleng J. J., McGinity J. W. Production of spherical pellets by a hot-melt extrusion and spheronization process. Int. J. Pharm. 2002; 242: 87–92
- Zhang F. Hot-melt extrusion as a novel technology to prepare sustained-release dosage forms. Unpublished Dissertation, The University Of Texas At Austin, Austin 1999
- Zhang F., McGinity J. W. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharmaceut. Dev. Tech. 1999; 4(2)241–250
- Zhang F., McGinity J. W. Properties of hot-melt extruded theophylline tablets containing poly(vinyl acetate). Drug Dev. Ind. Pharm. 2000; 26(9)931–942
- Zhou F., Vervaet C., Remon J. P. Matrix pellets based on the combination of waxes, starches and maltodextrins. Int. J. Pharm. 1996; 133(1–2)155–160
- Zhu Y. S. N., Malick A. W., Infeld M. H., McGinity J. W. Controlled release of a poorly water-soluble drug from hot-melt extrudates containing acrylic polymers. Drug Dev. Ind. Pharm. 2006; 32(5)569–583
- Zhu Y. C., Shah N. H., Malick A. W., Infeld M. H., McGinity J. W. Solid-state plasticization of an acrylic polymer with chlorpheniramine maleate and triethyl citrate. Int. J. Pharm. 2002; 241(2)301–310