Abstract
The advent of high through-put screening in the drug discovery process has resulted in compounds with high lipophilicity and poor solubility. Increasing the solubility of such compounds poses a major challenge to formulation scientists. Various approaches have been adopted to address this including preparation of solid dispersions and solid solutions. Hot-melt extrusion is an efficient technology for producing solid molecular dispersions with considerable advantages over solvent-based processes such as spray drying and co-precipitation. Hot-melt extrusion has been demonstrated to provide sustained, modified, and targeted drug delivery. Improvements in bioavailability utilizing the hot-melt extrusion technique demonstrate the value of the technology as a potential drug delivery processing tool. The interest in hot-melt extrusion technology for pharmaceutical applications is evident from the increasing number of patents and publications in the scientific literature. Part II of this article reviews the myriad of hot-melt extrusion applications for pharmaceutical dosage forms including granules, pellets, tablets, implants, transmucosal, and transdermal systems.
INTRODUCTION
For decades, the value of “continuous processing” in the pharmaceutical industry has been recognized. Hot-melt extrusion (HME) is a manufacturing process widely used in plastics industry, and has significant potential as a continuous pharmaceutical process. During hot-melt extrusion of pharmaceutical dosage forms, a blend of active ingredient, thermoplastic polymeric carrier, and other processing aids, including plasticizers and antioxidants, is heated and softened inside the extruder and then pressurized through a die into granules, cylinders, or films (Crowley et al., Citation2004b; Crowley et al., Citation2002; Munjal et al., Citation2006a; Repka et al., Citation2005; Repka et al., Citation2003; Young Cr, Citation2005; Young, Citation2003; Zheng et al., Citation2004).
New chemical entities that demonstrate poor bioavailability due to solubility issues are prime candidates for hot melt extrusion. This technology may be applied to disperse such drugs in a given matrix at the molecular level via the formation of a solid solution. Coupled with the use of dispersed, amorphous, and molecularly dissolved systems, a number of other formulation techniques can be applied using the melt extrusion approach: in situ salt formation, particle size homogenization, and tailored release profiles for selected APIs. These applications as well as other demonstrate the potential of hot-melt extrusion as a drug delivery processing technology (Breitenbach, Citation2002a).
Hot-melt extruded drugs and pharmaceutical devices encompass both prescription products and over the counter medications. HME has also been shown to provide many different advantages in the production of thin films for both drug delivery and wound care applications (Repka et al., Citation2002a). Hot-melt extrusion technologies may offer numerous advantages over traditional methods. Shorter and more efficient times to the final product, environmental advantages due to elimination of solvents in processing (including the possibility of recycling), and increased efficiency of drug delivery to the patient make hot-melt extrusion an exciting challenge for the pharmaceutical scientist.
Part I of this review described hot-melt extrusion technology with respect to various types of equipment, principles of operation, process technology, raw materials utilized, and characterization of melt extruded dosage forms. The growing interest of the pharmaceutical industry for HME is evident from the increasing number of patents and publications available in the literature. Part II of this review focuses on various applications of HME in drug delivery including granules, pellets, immediate and modified release tablets, transmucosal and transdermal systems, and implants.
HOT-MELT EXTRUDED DOSAGE FORMS
Granules, Pellets and Spheres
Until recently, hot-melt extrusion had not received much attention in the pharmaceutical literature. Rippie and Johnson prepared pellets containing cellulose acetate phthalate using a rudimentary ram extruder in 1969 to study the dissolution rates based upon pellet geometry (Rippie et al., Citation1969). More recently, Mank et al. reported in 1989 and 1990 on the extrusion of a number of thermoplastic polymers to produce sustained release pellets (Mank et al., Citation1989; Mank et al., Citation1990).
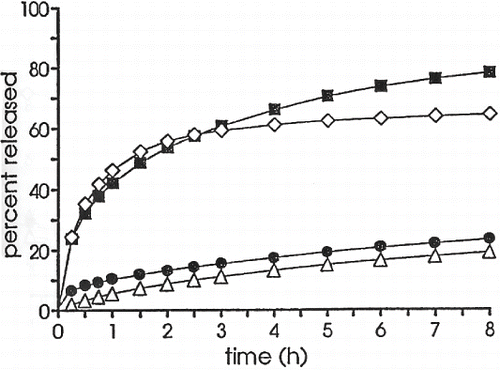
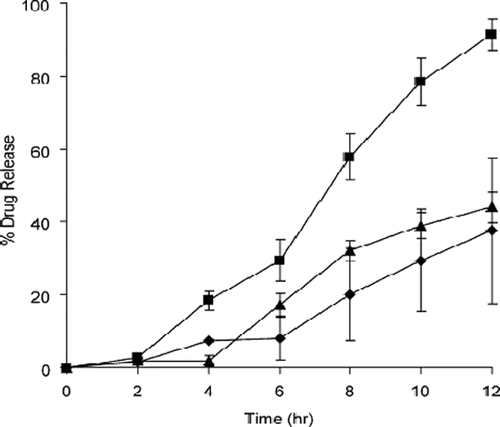
Follonier et al. in 1994 investigated the possibility of using hot-melt extrusion technology to produce sustained-release pellets (Follonier et al., Citation1994). It was the researchers' goal to prepare a dosage form in a simple and continuous manner. Thermal degradation was recognized as a limitation of this hot-melt process. Diltiazem hydrochloride, a relatively stable and freely soluble drug was incorporated into their polymer–based pellets for sustained release capsules. Polymers and plasticizers were selected prior to extrusion to maximize the possibility of a successful dosage form. In their study, the polymers investigated were ethyl cellulose (EC), cellulose acetate butyrate (CAB), poly(ethyl acrylate/methyl-methacrylate/trimethyl ammonio ethyl methacrylate chloride) (Eudragit® RSPM), and poly(ethylene-co-vinyl acetate) (EVAC). Triacetin and diethyl phthalate were investigated as plasticizers. The porosity of the pellets was determined by mercury porosimetry. The pellets exhibited a smooth surface and low porosity. The drug release characteristics of diltiazem were biphasic, with the CAB and EVAC pellets giving the slowest release rate (). These researchers also reported that the type and amount of plasticizer used, drying time of the polymers, extrusion temperatures, and plasticization times varied with each formulation. They observed that the stability of Eudragit® RSPM was adequate for extrusion at a temperature of 130°C.
FIGURE 1. Release profiles of diltiazem hydrochloride from extruded pellets based on various polymers (polymer/drug ratio 1:1, size 2 × 2 mm). (▪) EC; (Δ) CAB; (⋄) Eudragit®; (•) EVAC.
In a latter study, Follonier et al. examined different parameters influencing the release of diltiazem hydrochloride from hot-melt extruded pellets incorporated into hard gelatin capsules (Follonier et al., Citation1995). The drug release rate was found to depend upon polymer type, addition of pore-forming additives or hydrophilic polymers, and pellet size. The authors obtained in vitro release rates low enough to achieve therapeutic plasma levels for diltiazem hydrochloride with a once or twice daily administration. The addition of hydrophilic polymers helped avoid incomplete drug release due to encapsulated drug clusters in the insoluble matrix. The authors also incorporated various functional excipients, such as croscarmellose sodium (Ac-Di-Sol®) and sodium starch glycolate (Explotab®), into the pellet formulations to vary the drug release rate. The incorporation of swelling agents successfully reduced the initial burst release from the matrix.
In 1996 and 1997, Miyagawa, Sato et al. prepared controlled release matrices containing diclofenac as a model drug by hot-melt extrusion (Miyagawa et al., Citation1996; Miyagawa et al., Citation1999; Sato et al., Citation1997). The authors used a twin-screw compounding extruder to prepare wax matrix granules composed of carnauba wax, the model drug, and other rate controlling agents. Their study showed that a wax matrix with high mechanical strength could be obtained even when the composition was processed below the melting point of the wax. Dissolution of diclofenac from the wax matrix granules was strongly influenced by the formulation. Hydroxypropylcellulose, methacrylic acid copolymer (Eudragit L-100), and sodium chloride were investigated as dissolution rate controlling materials. The authors emphasized the advantages of using the twin-screw extruder for wax matrix tablets because of the low processing temperatures, high kneading and dispersing ability, and short residence time. The authors observed in their second study that the selection of rate controlling excipients based upon solubility and swelling characteristics had a significant impact on drug release properties from the wax matrix granules.
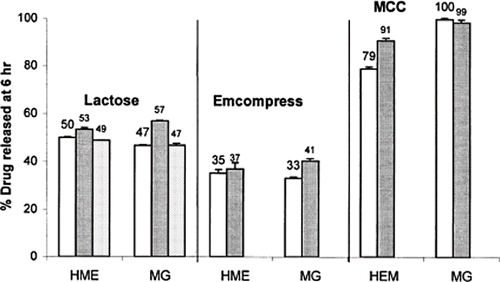
Liu et al. (Citation2001) compared the properties of wax based granules and tablets prepared by hot-melt extrusion to those prepared by high shear melt granulation. Powder blends containing phenylpropanolamine hydrochloride, Precirol®, Sterotex® K, and various excipients (microcrystalline cellulose, lactose, and Emcompress®) were extruded using a single screw extruder with open-end discharge. The extrudates were then passed through a 14-mesh screen to form granules. Hot-melt extruded granules were observed to be less spherical than high-shear melt granules and had lower bulk and tap densities. Content uniformity analysis of the hot-melt extruded granules exhibited less variability and decreased range relative to the granules produced using high shear, resulting in improved drug release reproducibility from the corresponding compressed tablets. At the same wax level, drug release from tablets decreased in the order of using microcrystalline cellulose, lactose, and Emcompress® as the filler excipients (). The observed differences in the dissolution properties of the tablets were due to the differences in the solubility, swellability, and density of the filler excipients.
FIGURE 2. The percentage phenylpropanolamine (PPA) released at 6 h from tablets containing 15% PPA, 30% Precirol, and 55% filler excipients prepared by hot-melt extrusion (HME) and high-shear melt granulation (MG). (□) Tablets compressed from granules within 20–40 mesh only; (▪) Tablets compressed from granules within 60–100 mesh only; (![]()
Hot-melt extrusion has also been used to prepare effervescent granules (Lindberg et al., Citation1988a; Lindberg et al., Citation1988b; Lindberg et al., Citation1987; McGinity et al., Citation2001; Robinson et al., Citation2000; Robinson et al., Citation2001; Tufvesson et al., Citation1987). Lindberg et al. prepared effervescent granules using a twin-screw extruder. During extrusion, sodium bicarbonate and anhydrous citric acid were added from separate inlet ports of the extruder, and ethanol was added as a liquid binder and pumped through a nozzle in the extruder barrel to facilitate the formation of the granules.
Koleng and McGinity utilized hot-melt extrusion technology for the preparation of rapid release granules (Koleng et al., Citation1997). A hot-melt extrusion process was used to granulate acetaminophen and filler excipients with low molecular weight poly(ethylene glycol)s. The resultant granules were then combined with additional excipients (disintegrants and lubricant) and compressed into tablet compacts. The granules exhibited improved drug release compared to the tablets. Tablets containing 15% poly(ethylene glycol) released greater than 80% of the incorporated acetaminophen after 30 min, as required for acetaminophen tablets in the USP 29.
In another study, Perissutti et al. utilized a ram extrusion technique to prepare rapid release dosage forms with uniform shape and density (Perissutti et al., Citation2002). This process was employed to form extrudates containing carbamazepine as a water-insoluble drug, polyethylene glycol 4000 as a low melting binder and lactose as hydrophilic filler. The extrudates disintegrated rapidly and an improved carbamazepine dissolution rate was observed which was attributed to enhanced surface contact between drug and dissolution medium. The extruded mixtures of an equivalent composition without lactose exhibited even more rapid release when compared to physical mixtures and extrudates containing lactose.
Zhang investigated the properties of poly (vinyl acetate) as a carrier for theophylline from matrix dosage forms prepared by hot-melt extrusion (Zhang et al., Citation2000). The influence of granule size and drug loading level on the drug release properties and the thermal stability of poly (vinyl acetate) (PVAc) were investigated. The rod shaped extrudates were ground and then compressed into tablets with various combinations of microcrystalline cellulose. As the size of the hot-melt extruded theophylline/PVAc granules was increased, a significant decrease in the release rate of the theophylline was observed. Since the drug was released from the matrix by a diffusion mechanism, the decrease in the drug release rate from the tablets containing larger granules was concluded to be a result of a longer diffusion pathway. PVAc was found to have a high solids carrying capacity when processed by hot-melt extrusion, with drug loads as high as 50%. The authors also studied the stability of PVAc to shear and stress using a Plasticorder® rheometer. The torque gradually decreased as the temperature of the polymer melt in the chamber was increased. No further change in the torque was observed after the initial heating step. This finding confirmed that PVAc was not susceptible to degradation by either thermal or shearing stress under the processing conditions.
Hot-melt extrusion has been used to prepare solid dispersions for immediate and sustained release applications. It has also been used to prepare solid dispersions in which the drug dissolution rate is enhanced. Huslman et al. demonstrated the hot-melt extrusion technique to increase the solubility rate of 17-Estradiol hemihydrate, a poorly water-soluble drug (Hulsmann et al., Citation2000; Hulsmann et al., Citation2001). The authors used PEG 6000, PVP or a vinylpyrrolidone—vinylacetate copolymer as polymers with Sucroester WE15 or Gelucire 44/14 as functional excipients. Rods were extruded, cut into granules and then compressed into tablets. The solid dispersions exhibited a significant increase in dissolution rate compared to the pure drug or to the physical mixtures. A 30-fold increase in dissolution rate was obtained from a formulation containing 10% 17-Estradiol, 50% PVP, and 40% Gelucire 44/14.
Ghebremeskel and co-workers investigated the overall performance of solid dispersions processed using surfactants as plasticizers (Ghebremeskel et al., Citation2006). Solid dispersions of a poorly soluble drug were prepared using PVP-K30, Plasdone-S630, and HPMC-E5 as the polymeric carriers and Tween-80 and Docusate Sodium as surfactants. The solid dispersions were produced by hot melt extrusion at temperatures 10°C above and below the glass transition temperature (Tg) of the carrier polymers using a 16 mm-Haake Extruder. The extruded solid dispersions containing polymeric carriers such as Plasdone S-603 and PVP-K30 (in addition to surfactants) were more stable than those containing surfactants only. The enhanced stability of the dispersions was attributed to the surfactants ability to reduce melt viscosity and increase API solubility and homogeneity in the carrier polymer. The authors convincingly demonstrated that surfactants are promising plasticizers to produce solid dispersions by hot melt extrusion, in doing so improving dissolution rates without compromising the physical stability of the systems.
Young et al. successfully prepared spherical controlled release theophylline pellets by a hot-melt extrusion and spheronization process (Young et al., Citation2002). A powder blend of anhydrous theophylline, Eudragit® Preparation 4135 F, microcrystalline cellulose, and polyethylene glycol 8000 powder was sieved, blended, and then melt-extruded in a Randcastle Microtruder®. The hot-melt extruded pellets were prepared by first cutting a thin, extruded composite rod into symmetrical pellets. The pellets were then spheronized in a traditional spheronizer at elevated temperatures. The melt-extruded matrix pellets exhibited diffusion-controlled drug release. Drug release from the acrylic matrix system was influenced by the pH of the dissolution medium since the solubility of the matrix polymer, Eudragit® Preparation 4135 F, is pH dependent. The surface morphology of the pellets was found to depend upon the spheronization parameters.
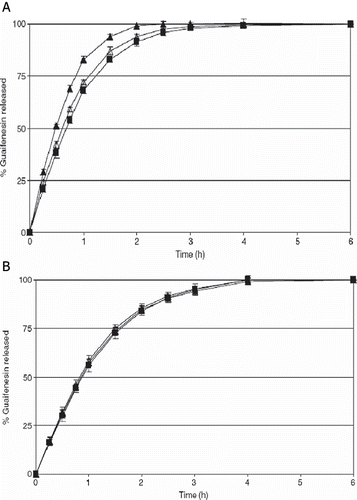
In a recent study, Young et al. studied the film coating of melt-extruded beads of guaifenesin with polymer Eudragit® L30 D-55 and designed a melt extruded pellet system with pH-dependent drug release properties (Young et al., Citation2007). The powder blends of guaifenesin, PEO, and functional excipients were processed using a melt-extrusion and spheronization technique and then film-coated in a fluidized bed apparatus. The authors report that despite initial miscibility of the drug/polymer blend, the pellets were morphologically unstable since PEO and guaifenesin recrystallized during storage. The addition of ethylcellulose to the extruded powder blend and film coating with Eudragit® L30 D-55 stabilized the drug release properties of the thermally processed pellets (). Film coating was demonstrated to be an effective process for providing melt-extruded beads with stable, pH-dependent drug release properties despite the low melting point of the thermoplastic carrier.
FIGURE 3. Stability of guaifenesin release rate from melt-extruded pellets (A) With out ethyl cellulose (B) With ethyl cellulose upon storage at 40C/75%RH in sealed HDPE containers with silica desiccants. Key: ▴ Initial; Δ 1 Month; ▪ 3 Months.
Brabender et al. investigated the bioavailability of ibuprofen from hot-melt extruded mini-matrices based on ethyl cellulose and a hydrophilic excipient, xanthum gum (De Brabander et al., Citation2004). During in vivo evaluation an oral dose of 300 mg ibuprofen was administered in hard gelatin capsules to healthy volunteers (n = 9) in a randomized cross-over study and compared with a commercially available sustained release product (Ibu-slow®). One mini-matrix formulation (F-1) consisted of 30% ibuprofen, 35% ethyl cellulose, and 35% hydroxypropyl methylcellulose, while the second formulation (F-2) contained 60% ibuprofen, 20% ethyl cellulose, and 20% xanthum gum. Both formulations behaved in vivo as sustained release formulations with an HVDt50% Cmax value (time span during which the plasma concentration is at least 50% of the Cmax value) of 7.6 and 12.0 hr for formulations F-1 and F-2, respectively, whereas a value of 5.2 h was obtained for Ibu-slow® (). Although the experimental formulations exhibited significantly lower Cmax, Tmax, and AUC0—24 h values than the corresponding values of commercial formulation, the relative bioavailability of both experimental formulations was about 80%. The authors concluded that the mini-matrices formulated with ethyl cellulose in combination with xanthum gum or hydroxypropyl methylcellulose can be used to prepare sustained release dosage forms.
TABLE 1. Mean Pharmacokinetic Parameters (± SD) After Oral Administration of 300 mg Ibuprofen to Healthy Volunteers (n = 9): Ibu-slow® 600 (1/2 tablet), Mini-matrix F-1 Containing HPMC and Mini-matrix F-2 Containing Xanthan Gum
Recently hot melt extrusion technology has been demonstrated as a viable approach for nanoparticle engineering by Miller et al. (Citation2006). Micronized particles of amorphous Itraconazole (ITZ) stabilized with PVP or HPMC were produced and subsequently melt extruded with poloxamer 407 and PEO 200 M to deaggregate and disperse the particles into the hydrophilic polymer matrix. The HME process did not alter the properties of the micronized particles. Dissolution testing conducted revealed that the drug release rate of the micronized particles was improved by HME due to particle deaggregation and enhanced wetting. From oral dosing of rats, it was determined that the two extrudate formulations performed similarly in vivo as confirmed by their statistically equivalent AUC values (). On the basis of results from supersaturation dissolution testing, the authors concluded that rapid precipitation of ITZ occurred upon entrance into the more neutral pH environment of the small intestine resulting in a brief opportunity for absorption. This study suggested that perhaps the optimum formulation approach for ITZ was to control drug release so as to retard precipitation as pH is increased and extend the absorption window in the small intestine.
TABLE 2. Pharmacokinetic Parameters Calculated Using Noncompartmental Analysis in Win-nonlin of Rats Dosed with Micronized Particle Extrudate (MPE) Compositions and Crystalline ITZ
Tablets and Capsules
Crowley et al. investigated the physicochemical properties and drug release mechanism from ethyl cellulose (EC) matrix tablets prepared by direct compression or hot-melt extrusion of binary mixtures of a water soluble drug (Guaifenesin) and a polymer (Crowley et al., Citation2004b). Ethyl cellulose was separated into “fine” or “coarse” particle size fractions corresponding to 325-80 and 80-30 mesh particles, respectively. Direct compression tablets containing 30% guaifenesin were prepared at 10, 30 or 50 KN compaction forces and the extruded tablets were processed at temperatures of 80–90°C and 90–110°C. The results of this study demonstrated that the guaifenesin release rate was dependent upon the particle size of ethyl cellulose and the processing conditions employed to prepare the tablets. The guaifenesin release rate was slower in tablets prepared with the “fine” ethyl cellulose particle size as compared to “coarse” ethyl cellulose particle size. Tablets prepared by hot-melt extrusion exhibited considerably slower drug release relative to those prepared by direct compression. The surface morphology of the hot-melt extruded tablets was found to depend upon processing temperature. The release profiles of hot-melt extruded tablets were found to be in good agreement with Higuchi diffusion model while those prepared by direct compression using “coarse” ethyl cellulose were found to release guaifenesin by both diffusion and erosion mechanisms.
Zhang and McGinity describe a novel method to prepare sustained release matrix tablets directly from a single screw hot-melt extruder (McGinity et al., Citation1997; Zhang et al., Citation1999). These researchers studied the properties of polyethylene oxide (PEO) as a drug carrier and studied the release mechanism of chlorpheniramine maleate (CPM) from matrix tablets. Large diameter rods (4.5 mm) were extruded and cut into tablets. PEG 3350 was included as a plasticizer to facilitate processing. The stability of the primary polymer, PEO, as a function of processing temperature was determined using gel permeation chromatography. The authors report that polymer type, temperature, and residence time in the extruder impacted the PEO stability. In addition, the researchers showed that additional mixing of the components occurred in the barrel of the extruder, since the content uniformity of the extruded tablets was within 99.0 to 101.0% of the theoretical content. As the PEG 3350 concentration increased, the release of CPM from the extruded matrix tablets was found to increase. The rate of hydration and dissolution rate of the entire matrix system were thus accelerated due to the presence of the plasticizer. The rate of drug release was only slightly affected by changes in drug content until the drug loading reached 20%.
Zhu and co-workers investigated the influence of a lipophilic thermal lubricant, glyceryl monostearate (GMS), on the processing conditions and properties of chlorpheniramine maleate (CPM) tablets prepared by hot-melt extrusion (Zhu, Citation2004). CPM tablets containing Eudragit® RS PO, triethyl citrate (TEC), and GMS were prepared by hot-melt extrusion. CPM and the excipient components of the extruded tablets were found to be stable at the thermal processing temperatures as determined by thermogravimetric analysis. The incorporation of either TEC or GMS into the powder blend decreased the drive amps and the torque values during the hot-melt extrusion process. The glass transition temperature of Eudragit® RS PO and the melting point of GMS were determined using differential scanning calorimetry from a physical mixture, and the results demonstrated that these two materials were not miscible in the molten state. An increase in the thermal lubricant (GMS) level in the Eudragit® RS PO system resulted in an increase the rate of drug release from the tablets. Both TEC and GMS facilitated thermal processing. While TEC lowered both the glass transition temperature and the melt viscosity of the acrylic polymer, GMS only decreased the melt viscosity of the acrylic polymer and had no effect on the glass transition temperature. The authors concluded that the evaluation of GMS as a thermal lubricant would potentially allow more drugs and polymers to be processed by the hot-melt extrusion process.
Crowley et al. studied the thermal stability of polyethylene oxide (PEO) in sustained release tablets prepared by hot-melt extrusion (Crowley et al., Citation2002). The weight average molecular weight of the polymer was determined using gel permeation chromatography. The chemical stability of PEO was found to be dependent on both the storage and processing temperature, and the molecular weight of the polymer (). Storage of the polymer above its melting point significantly increased polymer degradation, and the degradation process was accelerated as the molecular weight of the polymer was reduced. The thermal stability of high molecular weight PEO (1,000,000 or PEO 1M) in sustained release chlorpheniramine maleate (CPM) tablets prepared by hot-melt extrusion was found to depend on the processing temperature and screw speed. Lower molecular weight PEO (100,000 or PEO 100K) was demonstrated to be a suitable processing aid for PEO 1M. Incorporation of PEO 100K reduced the melt viscosity, friction and chain entanglements between the PEO 1M molecules thereby lowering its degradation. As the percentage of PEO 100K in the powder blend increased, the drive amperage decreased and the stability of PEO 1M increased (). Also, incorporation of PEO 100K did not alter the release rate of CPM. Vitamin E, Vitamin E Succinate, and Vitamin E TPGS were found to be suitable stabilizers for PEO, however, ascorbic acid was shown to degrade the polymer in solution. Thermal analysis demonstrated that Vitamin E Succinate and Vitamin E TPGS were dispersed at the molecular level in hot-melt extruded tablets. Solubilized Vitamin E Succinate and Vitamin E TPGS suppressed the melting point of the polyethylene oxide.
FIGURE 4. Relationship of polymer molecular weight in the thermal stability of PEO when stored at 60°C, 75% relative humidity, (♦) 1,000,000, (▪) 600,000 and (▴) 200,000.
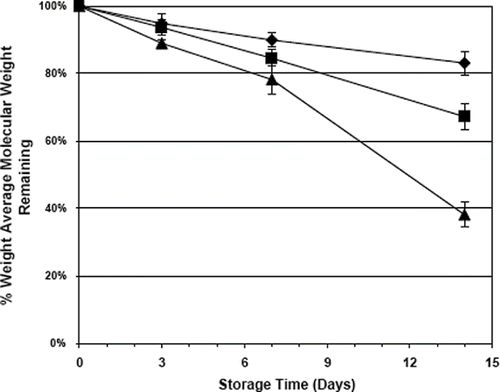
TABLE 3. Extrusion Stability of PEO 1M and the Influence of PEO 100K on PEO Weight Average Molecular Weight as Measured by Gel Permeation Chromatography, ± SD, n = 3
Young et al. described a novel carrier system based on Acryl-EZE® to prepare extended release tablets and pellets using a Randcastle ¾” single screw extruder (Young et al., Citation2005). These researchers studied the physicochemical properties of melt-extruded cylindrical rods, tablets, and pellets containing Acryl-EZE® and the influence of hydroxypropyl methylcellulose and carbomer as gelling agents on the mechanisms and kinetics of drug release from thermally processed matrices. The excipient blends were physically and chemically stable during processing, and the resulting dosage forms exhibited pH-dependent dissolution properties. Extrusion of blends containing HPMC or carbomer changed the mechanism and kinetics of drug release from the thermally processed dosage forms. Thus, hot-melt extrusion was shown to be an effective process for the preparation of controlled release matrix systems based on Acryl-EZE®.
Bruce et al. demonstrated the feasibility of hot-melt extruded tablets using Eudragit® S100 as the polymeric carrier to target delivery of 5-aminosalicylic acid (5-ASA) to the colon (Bruce et al., Citation2005). A preplasticization step was necessary when incorporating triethyl citrate (TEC) into the formulation in order to achieve uniform mixing of the polymer and plasticizer, effectively reduce the polymer glass transition temperature (Tg), and to lower the processing temperatures. The concentration of TEC in the extrudates not only influenced the processing temperature, but also influenced the drug release rates from the extruded tablets due to leaching of the TEC during dissolution testing (). A heat induced interaction between citric acid and 5-ASA was observed and attributed to an amide bond formation between 5-ASA and citric acid during hot melt processing. There was no evidence of a binding interaction between 5-ASA and the polymeric carrier.
FIGURE 5. Influence of TEC concentration and pre-plasticization on the drug release rate of hot-melt extrudate tablets containing 25% w/w 5-ASA. (▴) Formulation pre-plasticized with 12% w/w TEC; (♦) Formulation, not pre-plasticized with TEC; (▪) Formulation, pre-plasticized with 23% TEC.
Zheng et al. investigated the physicochemical and dissolution properties of theophylline tablets coated using a novel solvent-free powder coating process (Zheng et al., Citation2004). The ammonio methacrylate copolymers, Eudragit® RS PO, and Eudragit® RL PO (95:5), were pre-plasticized by a hot-melt extrusion process using a plasticizer and thermal lubricant, and then cryogenically ground into a fine powder. The powder coating process involved three steps including priming, powder layering and curing. The use of a solid primer at the initial stage of powder coating increased the adhesion of the pre-plasticized acrylic polymers to the substrates. At 8% w/w total weight gain, immediate release theophylline tablets that were powder coated with Eudragit® RS PO/RL PO demonstrated a 12 h sustained release profile. Theophylline release from powder-coated tablets was significantly influenced by curing temperature, plasticizer concentration, coating level, and particle size of the coating powder. The release rate of theophylline tablets decreased significantly with increasing curing temperature, increasing plasticizer concentration and increasing coating level. Tablets powder-coated with larger particles exhibited a faster drug release rate than tablets powder-coated with smaller particles. Eudragit® RS PO/RL PO powder-coated tablets demonstrated a stable drug release profile after 3-month storage at 25°C / 60% RH and 40°C / 75% RH. This novel powder coating process was demonstrated to be an efficient coating method to produce stable sustained release dosage forms.
Another unique application of the hot-melt extrusion technique is reported by Nakamichi et al. at Nippon Shinyaku Company (Nakamichi et al., Citation2001). The authors prepared a floating sustained release dosage form composed of nicardipine hydrochloride and hydroxypropyl methylcellulose acetate succinate using a twin screw extruder. Rods were extruded and then cut into tablets of different sizes. A dosage form with very small and uniform pores was obtained by selecting a screw that generated high pressure near the die, and by adjusting the processing temperatures. The authors demonstrated that the extruded, floating enteric polymer system was retained in the stomach for up to 6 h.
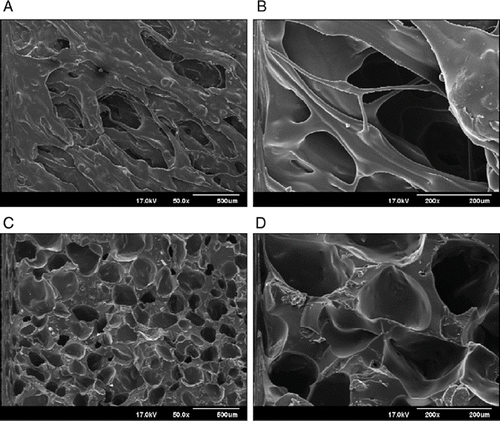
Fukuda et al. investigated the influence of sodium bicarbonate on the physicochemical properties of controlled release hot-melt extruded (HME) tablets containing Eudragit® RS PO and/or Eudragit® E PO (Fukuda et al., Citation2006a). Acetohydroxamic acid and chlorpheniramine maleate were used as model drugs. Sodium bicarbonate was incorporated into the tablet formulations and the drug release properties and buoyancy in media for HME tablets and directly compressed (DC) tablets were investigated. The HME tablets prepared from the powder blend containing both Eudragit® RS PO and sodium bicarbonate exhibited sustained release properties and the tablets floated on the surface of the media for 24 h. The cross-sectional morphology of the HME tablets () showed a porous structure due to carbon dioxide gas generation as a result of thermal decomposition of sodium bicarbonate in the softened acrylic polymers at elevated temperature during the extrusion process. In contrast, the DC tablets prepared in this study were not buoyant and exhibited rapid drug release in the dissolution media. The drug release rate from floating HME tablets was controlled by both the incorporation of Eudragit® E PO into the matrix tablet and the diameter of the die used in the extrusion equipment.
FIGURE 6. Morphologies of surface and cross-sectional floating HME tablets prepared from formulation containing 10% sodium bicarbonate: (A) Surface 50x, (B) Surface 200x, (C) Cross-sectional 50x, (D) Cross-sectional 200x.
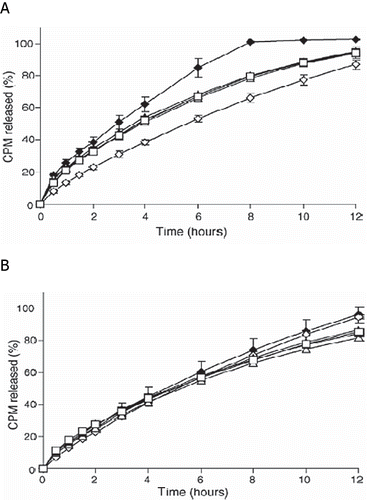
In another study, these researchers also investigated the influence of pH, buffer species, and ionic strength on the release mechanism of chlorpheniramine maleate (CPM) from matrix tablets containing chitosan and xanthan gum prepared by a hot-melt extrusion process (Fukuda et al., Citation2006b). Drug release from hot-melt extruded tablets containing either chitosan or xanthan gum was pH and buffer species dependent. In contrast, the release from the HME tablets containing both chitosan and xanthan gum was found to be independent of pH and buffer species as compared to directly compressed tablets containing both the polymers (). The authors proposed that the pH- and buffer species-independent sustained release HME tablet may exhibit the same release profile in the GI tract due to formation of a hydrogel in acidic media similar to stomach fluid. The resulting hydrogel retards drug release in neutral and slightly alkaline media, irrespective of ionic strength.
FIGURE 7. CPM release profiles from (A) DC tablets and (B) HME tablets in 900 mL of either (♦) 0.1N HCl, (⋄) pH 4.0 acetate buffer, (▴) pH 4.0 citrate buffer, (Δ) pH 4.0 phosphate buffer, (▪) pH 6.8 phosphate buffer, or (□) pH 7.4 phosphate buffer at 37 ± 0.5°C (USP 27 apparatus 2, 100 rpm). Each point represents the mean ± S.D., n = 3.
Prapaitrakul et al. prepared disks using a hot-melt extrusion technique in which the molten materials were forced into a mold (Prapaitrakul et al., Citation1991). The disks contained glyceryl fatty acid esters (Gelucire), polyethylene glycol fatty acid esters, or a combination of the two with chlorpheniramine maleate as a model drug. The release of the drug into distilled water, pH 1.2 buffer, and pH 7.5 buffer exhibited square root of time dependence. An increase in the fatty acid ester hydrophilic-lipophilic balance (HLB) from 1 to 14 resulted in a 10-fold increase in the drug release rate. The maximum release rate was seen from the fatty acid ester with a melting point of 44°C. The pH of the dissolution medium had a minimal impact on the rate of drug release. The release rate was modified by blending Gelucires of different melting points and HLB values.
In a later study, these researchers prepared disks containing polyethylene, polycaprolactone, polyvinyl acetate, and cellulose acetate butyrate with theophylline as a model drug at a 50% loading (Sprockel et al., Citation1997). The authors reported an 8-fold difference in the effective diffusion coefficient between the various polymers. The effective diffusion coefficient increased 10-fold when the theophylline load was increased from 50 to 70% in the polycaprolactone and polyethylene disks. Disks with theophylline content greater than 70% by weight could not be made. The release rate was modified using soluble additives (sucrose, sodium chloride, Pluronic®, and PEG).
Vervaet et al. developed an alternative technique for enteric delivery using hot-melt extrusion (Mehuys, Citation2005). The enteric polymers polyvinyl acetate phthalate (PVAP) and hydroxypropylmethylcellulose acetate succinate (HPMC AS) were premixed with triacetin and extruded into hollow cylinders using a co-rotating twin-screw extruder. The hollow pipes were filled with a model drug (hydralazine) and both open ends of the cylinders were closed, yielding hot-melt extruded enteric capsules. The resulting enteric capsules had excellent gastro-resistance.
Transdermal and Transmucosal Films
Currently, films for transdermal/transmucosal drug delivery and wound care applications are produced more frequently by casting from organic or aqueous solvents (Aitken-Nichol et al., Citation1996). However, there are several problems with the casting technique. Gutierrez-Rocca and McGinity showed that physical aging of both aqueous and solvent cast acrylic films resulted in a decrease in elongation or elasticity and an increase film tensile strength (Gutierrez-Rocca et al., Citation1993). This mechanical instability was related to the relaxation of the polymer chains as they moved toward a state of equilibrium. Also, it has been demonstrated that the type and level of plasticizers, curing time, and temperature have a significant effect on the dissolution rate of drugs from films formed from aqueous dispersions (Schmidt et al., Citation1993; Steuernagel, Citation1997).
Aitken-Nichol et al. (Citation1996) investigated the viability of hot-melt extrusion technology in 1996 for the production of thin, flexible acrylic films for topical drug delivery The authors noted that the manufacturing process was not restricted by solvent concerns. The authors compared cast films with hot-melt extruded films. Eudragit® E100 was the primary thermoplastic polymer extruded. The authors reported that hot-melt extrusion was a viable technology for the production of free films of this acrylic resin. Although triethyl citrate was an acceptable plasticizer for this polymer, these researchers found that lidocaine hydrochloride also plasticized for the acrylic films. The authors concluded that the differences in the dissolution rate and ductile properties between cast films and extruded films were due to the amount of drug dissolved in the polymer.
Repka et al. discussed the numerous disadvantages of solvent casting methods for preparing transdermal films (Repka et al., Citation1999). These researchers produced hydroxypropylcellulose films using a Killion hot-melt extruder. Several plasticizers and two model drugs were incorporated into the HPC films. The influence of the plasticizers and drugs on the physical-mechanical properties of the films was investigated. The authors found that HPC films could not be produced without a plasticizer due to high torque levels on the extruder. All plasticizers examined in this study were found to be stable under the study processing conditions except PEG 400. The influence of processing temperature and storage time on the two model drugs (chlorpheniramine maleate and hydrocortisone) was also investigated. Chlorpheniramine maleate proved to be an excellent plasticizer for hydroxypropyl cellulose. The hot-melt extruded films were found to be mechanically and chemically stable for up to 12 months. The authors also reported that chlorpheniramine maleate was fully dissolved in the hydroxypropyl cellulose film up to the 10% level. Hydrocortisone was also found to be a good plasticizer, however, its chemical stability was found to be a function of processing temperature and residence time in the extruder.
Repka et al. also reported that the mechanical properties of the films depended on whether the testing was performed perpendicular to flow from the extruder or in the direction of flow from the extruder. All extruded films exhibited a decrease in tensile strength and a large increase in percent elongation when testing was performed perpendicular to flow versus in the direction of flow. These results were in contrast to those reported by Aitken-Nichol et al. (Citation1996). The discrepancy between the two studies is likely due to polymer compatibility between polyethylene and Eudragit E100 in the Aitken-Nichol study. However, these studies illustrate how “flow orientation” can impact the mechanical properties of hot-melt extruded delivery systems.
Crowley et al. (Citation2004a) investigated the physicochemical and mechanical properties of hot-melt extruded polyethylene oxide (PEO) films containing either guaifenesin (GFN) or ketoprofen (KTP) as model drugs. Drug loaded films containing up to 30% GFN and 15% KTP were successfully prepared by a hot-melt extrusion process. The results of this study demonstrated that GFN and KTP were stable during the hot-melt extrusion process. Crystallization of GFN on the surface of the extruded films was observed at all concentrations investigated using scanning electron microscopy (SEM) and X-ray diffraction (XRD). However, SEM studies did not reveal KTP crystallization until reaching the 15% loading level. Melting points corresponding to the crystalline drugs were not observed in the films, suggesting miscibility in the molten polymer. GFN and KTP were found to decrease drive load, increase PEO stability, and plasticize the polymer during extrusion. The Hansen solubility parameters predicted miscibility between PEO and KTP and poor miscibility between PEO and GFN. The percentage elongation decreased with increasing GFN concentrations and significantly increased with increasing levels of KTP. Increasing concentrations of both GFN and KTP decreased the tensile strength of extruded films.
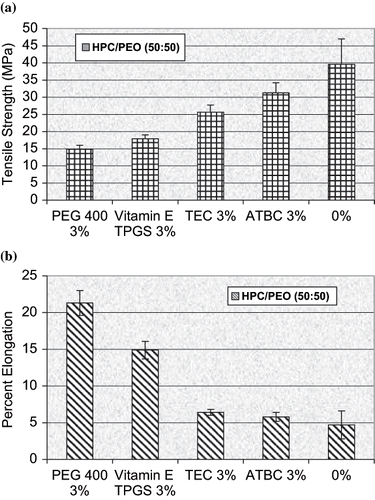
Repka and McGinity prepared films containing hydroxypropyl cellulose and polyethylene oxide by hot-melt extrusion with and without Vitamin E TPGS as a formulation additive (Repka et al., Citation2000a). Addition of 1, 3, and 5% Vitamin E TPGS decreased the glass transition temperature of the extruded films containing either a 50:50 or 80:20 ratio of HPC to PEO in an almost linear fashion. The glass transition temperature of the film containing 3% Vitamin E TPGS was lowered by over 11°C compared to the film without Vitamin E TPGS (). The films containing 3% Vitamin E TPGS had similar mechanical properties to films containing 3% PEG 400, but a 3 fold increase in percent elongation was observed compared to films containing 3% triethyl citrate and 3% acetyltributyl citrate (). Vitamin E TPGS also facilitated the processing of the HPC/PEO films by decreasing the barrel pressure, drive amps, and torque of the extruder.
FIGURE 8. Glass transition temperatures of HPC/PEO (50:50) hot-melt extruded films containing vitamin E TPGS and three conventional plasticizers (n = 4).
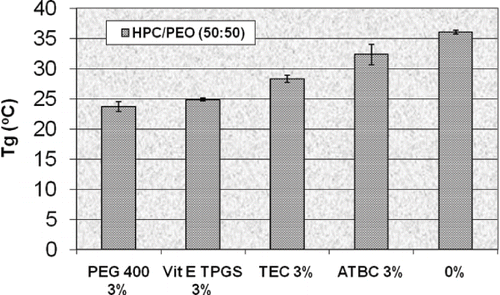
FIGURE 9. (a) Tensile strength and (b) Percent elongation of HPC/PEO 50:50 rRatio hot-melt extruded films containing vitamin E TPGS and three conventional plasticizers (n = 6).
Currently, a marketed denture adhesive is prepared by hot-melt extrusion using thermoplastic polymers that adhere to the mucous membrane when wetted (Repka et al., Citation2002b). A bioadhesive film has the benefit of simplifying dosage form design and reducing preparation costs, due to the elimination of the adhesive layer in the system. The film has the desirable adhesion strength so that retention of the film at the application site is achieved.
Repka and McGinity conducted bioadhesion testing of hot-melt extruded hydroxypropyl cellulose films containing various additives on human subjects using a Chatillon testing apparatus (Repka et al., Citation2000b). These researchers found that force of adhesion, elongation at adhesive failure, and modulus of adhesion were a function of the type of additive in the extruded film. The force of adhesion was highest for the films containing carbomer (Carbopol® 971p) and polycarbophil (Noveon AA-1®). This study demonstrated that a single layer HPC film could be produced with the bioadhesive incorporated into the matrix, thus eliminating a separate “adhesive layer” and simplifying the process of transdermal and transmucosal delivery systems.
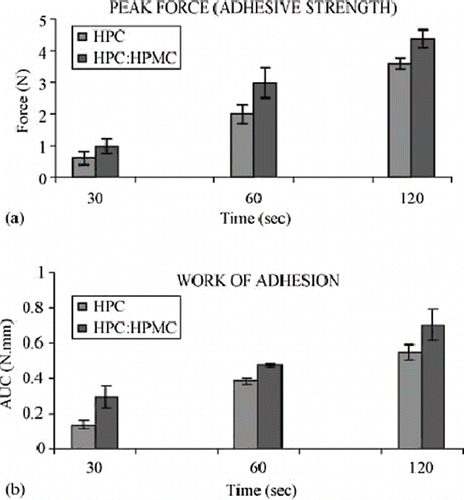
Repka et al. extensively investigated hot-melt extrusion techniques as effective tools for preparing transmucosal drug delivery systems. These researchers characterized hot-melt extruded hydroxypropyl cellulose or polyethylene oxide, which contained clotrimazole, a drug used for oral candidiasis therapy (Prodduturi et al., Citation2004, Citation2005; Repka et al., Citation2003). The film was processed at a temperature range of 125–130°C utilizing a Killion extruder (ModelKLB-100) equipped with a 6-inch flex-lip die. The extruded films demonstrated excellent content uniformity and post-processing drug content was 93.3%. The films were determined to exhibit desirable and consistent release properties and bioadhesive strength. In a similar study using hydroxypropyl cellulose with the same drug, the sustained release properties varied with molecular weight of the polymer carrier (). This behavior was attributed to greater and stronger polymer entanglements in films containing higher molecular weight grades of the polymer, which lead to slower polymer erosion. The films were found to be stable at 25°C / 60% RH for up to 3 months with no significant degradation or recrystallization of the drug. The authors also demonstrated similar sustained release properties with the carrier, polyethylene oxide, and Clotrimazole in which drug release was dependent on the molecular weight of PEO. The solid-state characterization of the drug and the polyethylene oxide polymer were performed utilizing differential scanning calorimetry and X-ray diffractometry. The authors also investigated hot melt extruded films containing hydroxypropyl cellulose and hydroxypropyl methyl cellulose with lidocaine (Repka et al., Citation2005). Two film formulations were extruded and compared, one containing only hydroxypropyl cellulose and the other containing Hydroxypropyl cellulose: Hydroxypropyl methyl cellulose in a 80:20 ratio. Thermal analysis of the films using differential scanning calorimetry suggested that the drug was amorphous, which was confirmed by wide angle X-ray diffractometry. Sustained release properties were observed from both matrices. Dissolution profiles suggested that hydroxypropyl methylcellulose retarded the drug release from the films prepared from both polymers. However, the mechanism of drug release from both of the films was predominantly diffusion of the drug through the polymer matrices. Incorporation of hydroxypropyl methylcellulose also increased both adhesive strength and work of adhesion as compared to the hydroxypropyl cellulose-only films (). These results indicate that hot-melt extrusion is a viable technique for preparation of muco-adhesive films containing locally acting therapeutic agents.
FIGURE 10. Release profiles of clotrimazole from hot-melt extruded Klucel® films as a function of polymer molecular weight.
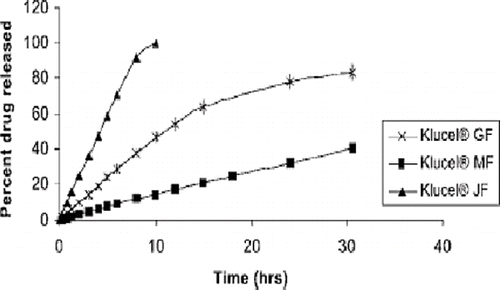
FIGURE 11. (a) Peak force (adhesive strength) and (b) Work of adhesion of HPC and HPC: HPMC films measured using texture analyzer and rabbit intestinal mucosa as a substrate (n = 5).
Repka et al. (Citation2004) report formulations and processes for topically delivery of ketoconazole from polyethylene oxide films prepared by hot melt extrusion technology for the treatment of onychomycosis in fingernails or toenails. The extruded films demonstrated excellent content uniformity and post processing drug content. The in vitro permeability profiles demonstrated that nail samples treated with an “etchant” had a significant increase in drug permeability compared to control. Differential scanning calorimetry thermograms indicated that a ketoconazole solid solution was formed within hot melt extruded films resulting in the increase in permeability.
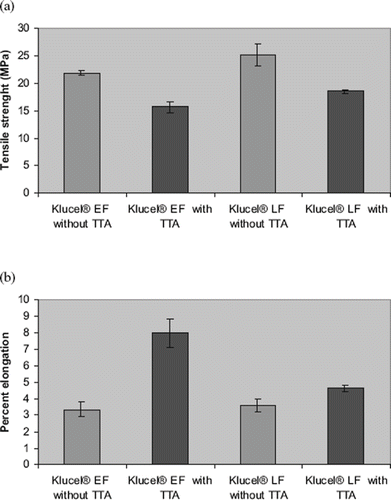
Repka et al. demonstrated the use of tartaric acid (TTA) as a plasticizer in hot-melt extruded hydroxypropyl cellulose (HPC) films containing polymeric additives (Mididoddi et al., Citation2006). Hot-melt extruded films were prepared using two different molecular weights of Klucel® (EF, MW: 80,000 and LF MW: 95,000) with ketoconazole (one batch of each MW with and without TTA 4%) using a Killion single-screw extruder. TTA functioned as an effective plasticizer, increasing percent elongation and decreasing tensile strength of the HPC films (). In this study, TTA was shown to be a potential candidate for transnail applications in film devices prepared by hot-melt extrusion technology.
FIGURE 12. Influence of tartaric acid on (a) Tensile strength and (b) Percent elongation of hot-melt extruded hydroxypropyl cellulose films.
Repka et al. report the preparation of transmucosal film formulations of Δ9-tetrahydrocannabinol (THC), the active ingredient of Marinol®/Dronabinol capsules. Due to the poor solubility and significant first-pass metabolism of THC, oral absorption is low and erratic, resulting in low bioavailability. In view of these limitations, several other routes of administration have been investigated to improve bioavailability of THC, namely pulmonary, ophthalmic, sublingual, rectal, and transdermal routes. The authors prepared THC transmucosal matrix systems by hot-melt extrusion, utilizing various processing aids (Munjal et al., Citation2006a, Citation2006b; Repka et al., Citation2006). The chemical stability of the drug in the polymeric matrices was investigated with respect to processing temperature, processing time, formulation additives, and storage conditions. Their data suggested that at processing temperatures of 160 and 200°C, the degradation of the cannabinoid was linear as a function of time. Weight loss could be controlled by blending the polymers, polyethylene oxide, and hydroxypropyl cellulose, of which polyethylene oxide was determined to be more effective. Although higher temperatures reduced the polymer melt viscosity, THC, and other materials were chemically unstable. However, matrix stability was found to be improved at relatively lower processing temperatures. The THC oxidative degradation rate was investigated by drug-excipient compatibility, use of antioxidants, cross-linking the polymeric matrices, adjusting microenvironment pH, and the effect of moisture content. THC instability in polyethylene oxide—vitamin E succinate films was determined to be due to a chemical interaction between the drug and the vitamin as well as with the atmospheric oxygen. Oxygen quenching of oxygen by reducing agents such as ascorbic acid was effective, with 5.8% THC degraded in the ascorbic acid-containing films relative to the control (31.6%) after 2 months of storage at 40°C. The authors also report that incorporation of THC resulted in an increase in the bioadhesive strength of polymer matrices.
Implants
Rothen-Weinhold et al. prepared long-acting poly(lactic acid) implants containing vapreotide, a somatostatin analogue, by hot-melt extrusion (Rothen-Weinhold et al., Citation2000). The authors reported that the peptide degraded during processing with the formation of a lactoyl lactyl-vapreotide conjugate. The authors determined that residual lactide in the polylactic acid significantly influenced the formation of the peptide impurity, illustrating that carrier purity impacts the quality of the dosage form.
Melanotan-I (MT-I) release rates from biodegradable implants of poly(D,L lactide-co-glycolide) (PLGA) copolymer prepared by melt extrusion have been reported (Bhardwaj et al., Citation1997, Citation1998). The in-vitro release of MT-I exhibited a triphasic profile with an initial rapid release followed by a secondary phase of slow release, then a tertiary phase of rapid release due to erosion of the polymer. The initial rapid release observed with PLGA (50:50 molar ratio of lactic/glycolic acid) polymers were less than 5% of the drug load and the tertiary phase commenced after about 3 weeks. The factors controlling the drug release were polymer degradation and erosion, which were controlled by the physical properties of the polymer such as molecular weight and viscosity.
A contraceptive vaginal ring containing polyethylene vinylacetate copolymers, etonogestrel and ethinyl estradiol was prepared by melt extrusion (Van Laarhoven et al., Citation2002). The powders were compounded in a twin-screw extruder, granulated and then spun into fibers. After leaving the extruder, the strands were cooled to room temperature and granulated using a strand granulator. The steroids were completely dissolved in the polymer melt. A co-extrusion procedure was used to prepare the coaxial fibers in which two single screw extruders were connected to a spinning block. The molten polymers were delivered to two gear pumps to provide precise flow control of both polymers to the spinneret. Finally, the membrane and core polymers were combined in the spinneret to form the coaxial fiber.
Implants composed of block copolymers have also been studied. Witt et al. investigated the degradation of ABA triblock copolymers, consisting of poly(lactide-coglycolide) A-blocks and poly(oxyethylene) B-blocks, and PLG, poly(lactide-co-glycolide), with respect to swelling behavior, molecular weight loss and polymer erosion (Witt et al., Citation2000). Implants were prepared by either compression molding or extrusion, using a laboratory ram extruder. Insertion of an elastic B block did not lower the processing temperature, although the entanglement of the polymer chains was significantly reduced. In this report, the influence of implant geometry was found to be insignificant.
QUALITY CONTROL AND REGULATORY CONSIDERATIONS
Pharmaceutical products prepared by hot-melt extrusion have been approved in the United States, Europe, and Asia (Breitenbach, Citation2002b). Comprehensive documentation of the process monitoring and control devices must be accomplished. These parameters include feed rate, temperature, screw speed, pressure, melt viscosity, drive amperage, and torque. Cleaning is accomplished by disassembly and removal of any excess material from the screw, barrel, and die. These surfaces can then be swabbed and analyzed to satisfy cleaning validation requirements.
A VIEW TO THE FUTURE
Hot-melt extrusion technology is an increasingly attractive process for the manufacture of drug delivery systems. A wide range of dosage forms and applications from oral to topical and parenteral can be prepared. Solid dispersions and/or solid solutions of the drug embedded in the carrier matrices may allow for sustained release application and dissolution rate improvement. Solvents are not required and a broad selection of carriers and functional excipients are available.
Drug and matrix degradation may result from high process temperatures and shear forces. However, these challenges can be overcome by formulation, equipment design, and engineering approaches. Selection of low melting carriers or the use of compatible plasticizers can reduce process temperatures. Extruder and screw design can also reduce shear forces and residence time. Given these considerations, even drugs known to be thermally labile have been processed.
Interest in hot-melt extrusion as a pharmaceutical process continues to grow. The published literature discloses innovative and promising new approaches for drug delivery systems, such as effervescent granules, fast dissolving systems, complex formation, and solid dispersions/solutions.
ACKNOWLEDGMENT
Resources for this journal article were partially supported by NIH/NCRR Award set #P20RR021929.
REFERENCES
- Aitken-Nichol C., Zhang F., McGinity J. W. Hot melt extrusion of acrylic films. Pharmaceutical Research 1996; 13(5)804–808
- Bhardwaj R., Blanchard J. In vitro evaluation of poly(d, l-lactide-co-glycolide) polymer- based implants containing the alpha-melanocyte stimulating hormone analog, melanotan-i. Journal of Controlled Release 1997; 45(1)49–55
- Bhardwaj R., Blanchard J. In vitro characterization and in vivo release profile of a poly(d, l-lactide-co-glycolide)-based implant delivery system for the alpha-msh analog, melanotan-i. International Journal of Pharmaceutics 1998; 170(1)109–117
- Breitenbach J. Melt extrusion: From process to drug delivery technology. European Journal of Pharmaceutics and Biopharmaceutics 2002a; 54: 107–117
- Breitenbach J. Melt extrusion: From process to drug delivery technology. European Journal of Pharmaceutics and Biopharmaceutics 2002b; 54(2)107–117
- Bruce L. D., Shah N. H., Waseem Malick A., Infeld M. H., McGinity J. W. Properties of hot-melt extruded tablet formulations for the colonic delivery of 5-aminosalicylic acid. European Journal of Pharmaceutics and Biopharmaceutics 2005; 59(1)85–97
- Crowley M. M., Fredersdorf A., Schroeder B., Ucera S., Prodduturi S., Repka M. A., et al. The influence of guaifenesin and ketoprofen on the properties of hot-melt extruded polyethylene oxide films. Eur. J. Pharm. Sci. 2004a; 22(5)409–418
- Crowley M. M., Schroeder B., Fredersdorf A., Obara S., Talarico M., Kucera S., et al. Physicochemical properties and mechanism of drug release from ethyl cellulose matrix tablets prepared by direct compression and hot-melt extrusion. International Journal of Pharmaceutics 2004b; 271(1–2)77–84
- Crowley M. M., Zhang F., Koleng J. J., McGinity J. W. Stability of polyethylene oxide in matrix tablets prepared by hot-melt extrusion. Biomaterials 2002; 23(21)4241–4248
- De Brabander C., Vervaet C., Van Bortel L., Remon J. P. Bioavailability of ibuprofen from hot-melt extruded mini-matrices. Int. J. Pharm 2004; 271(1–2)77–84
- Follonier N., Doelker E., Cole E. T. Evaluation of hot-melt extrusion as a new technique for the production of polymer-based pellets for sustained-release capsules containing high loadings of freely soluble drugs. Drug Development and Industrial Pharmacy 1994; 20(8)1323–1339
- Follonier N., Doelker E., Cole E. T. Various ways of modulating the release of diltiazem hydrochloride from hot-melt extruded sustained-release pellets prepared using polymeric materials. Journal of Controlled Release 1995; 36(3)243–250
- Fukuda M., Peppas N. A., McGinity J. W. Floating hot-melt extruded tablets for gastro-retentive controlled drug release system. Journal of Controlled Release 2006a; 115(2)121–129
- Fukuda M., Peppas N. A., McGinity J. W. Properties of sustained release hot-melt extruded tablets containing chitosan and xanthan gum. Int. J. Pharm 2006b; 31: 90–100
- Ghebremeskel N. A., Vemavarapu C., Lodaya M. Use of surfactants as plasticizers in preparing solid dispersions of poorly soluble api: Stability testing of selected solid dispersions. Pharm. Res 2006; 23(8)1928–1936
- Gutierrez-Rocca J. C., McGinity J. W. Influence of aging on the physical-mechanical properties of acrylic resin films cast from aqueous dispersions and organic solutions. Drug Development and Industrial Pharmacy 1993; 19(3)315–332
- Hulsmann S., Backensfeld T. PCT Int. Appl. World Patent No. 0057853, 2000
- Hulsmann S., Backensfeld T., Bodmeier R. Stability of extruded 17 ss-estradiol solid dispersions. Pharmaceutical Development and Technology 2001; 6(2)223–229
- Hulsmann S., Backensfeld T., Keitel S., Bodmeier R. Melt extrusion—an alternative method for enhancing the dissolution rate of 17 beta-estradiol hemihydrate. European Journal of Pharmaceutics and Biopharmaceutics 2000; 49(3)237–242
- Koleng J. J., McGinity J. W. Preparation and evaluation of rapid-release granules using a novel hot-melt extrusion technique. Paper presented at the Abstracts of the 16th Pharmaceutical Technology Conference, AthensGreece, 1997
- Lindberg N. O., Myrenas M., Tufvesson C., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, baker perkins mpf 50d—determination of mean residence time. Drug Development and Industrial Pharmacy 1988a; 14(5)649–655
- Lindberg N. O., Tufvesson C., Holm P., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, baker perkins mpf 50-d—influence on intragranular porosity and liquid saturation. Drug Development and Industrial Pharmacy 1988b; 14(13)1791–1798
- Lindberg N. O., Tufvesson C., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, baker perkins mpf 50-d. Drug Development and Industrial Pharmacy 1987; 13(9–11)1891–1913
- Liu J. P., Zhang F., McGinity J. W. Properties of lipophilic matrix tablets containing phenylpropanolamine hydrochloride prepared by hot-melt extrusion. European Journal of Pharmaceutics and Biopharmaceutics 2001; 52(2)181–190
- Mank R., Kala H., Richter M. Preparation of extrusion pellets containing drugs on the base of thermoplastics.1. Investigation of drug release. Pharmazie 1989; 44(11)773–776
- Mank R., Kala H., Richter M. Preparation of extrusion pellets containing drugs on the base of thermoplastics.2. Investigations on the improvement of the drug release on the base of thermoplastics. Pharmazie 1990; 45(8)592–593
- McGinity J., Zhang F. PCT Int. Appl. World Patent No. 9749384, 1997
- McGinity J. W., Robinson J. R. U.S. Pat. Appl. Publ. US Application Patent No. 20010006677, 2001
- Mehuys E. R. J., Vervaet C. Production of enteric capsules by means of hot-melt extrusion. Eur J Pharm Sci. 2005; 24(2–3), Eur J Pharm Sci.
- Mididoddi P. K., Prodduturi S., Repka M. A. Influence of tartaric acid on the bioadhesion and mechanical properties of hot-melt extruded hydroxypropyl cellulose films for the human nail. Drug Development and Industrial Pharmacy 2006; 32: 1059–1066
- Miller D. A. M. J. T., Yang W., Williams R. O., McGinity J. W. Hot-melt extrusion for enhanced delivery of drug particles. J.Pharm Sci 2006; 96: 361–376
- Miyagawa Y., Okabe T., Yamaguchi Y., Miyajima M., Sato H., Sunada H. Controlled-release of diclofenac sodium from wax matrix granule. International Journal of Pharmaceutics 1996; 138(2)215–224
- Miyagawa Y., Sato H., Okabe T., Nishiyama T., Miyajima M., Sunada H. In vivo performance of wax matrix granules prepared by a twin-screw compounding extruder. Drug Development and Industrial Pharmacy 1999; 25(4)429–435
- Munjal M., ElSohly M. A., Repka M. A. Polymeric systems for amorphous d9-tetrahydrocannabinol produced by a hot-melt method. Part ii: Effect of oxidation mechanisms and chemical interactions on stability. J Pharm Sci 2006a; 95(11)2473–2485
- Munjal M., Stodghill S. P., ElSohly M. A., Repka M. A. Polymeric systems for amorphous d9-tetrahydrocannabinol produced by a hot-melt method. Part i: Chemical and thermal stability during processing. J Pharm Sci 2006b; 95(8)1841–1853
- Nakamichi K., Yasuura H., Fukui H., Oka M., Izumi S. Evaluation of a floating dosage form of nicardipine hydrochloride and hydroxypropylmethylcellulose acetate succinate prepared using a twin-screw extruder. International Journal of Pharmaceutics 2001; 218(1–2)103–112
- Perissutti B., Newton J. M., Podczeck F., Rubessa F. Preparation of extruded carbamazepine and peg 4000 as a potential rapid release dosage form. European Journal of Pharmaceutics and Biopharmaceutics 2002; 53(1)125–132
- Prapaitrakul W., Sprockel O. L., Shivanand P. Release of chlorpheniramine maleate from fatty-acid ester matrix disks prepared by melt-extrusion. Journal of Pharmacy and Pharmacology 1991; 43(6)377–381
- Prodduturi S., Manek R. V., Kolling B., Stodghill S. P., Repka M. A. Water vapor sorption isotherms of hot-melt extruded hydroxypropyl cellulose films: Effect on physico-mechanical properties, release characteristics and stability. J. Pharm. Sci 2004; 93(12)3047–3056
- Prodduturi S., Manek R. V., Kolling W. M., Stodghill S. P., Repka M. A. Solid-state stability and characterization of hot-melt extruded poly(ethylene oxide) films. J Pharm Sci 2005; 94(10)2232–2245
- Repka M. A., Gerding T. G., Repka S. L., McGinity J. W. Influence of plasticizers and drugs on the physical-mechanical properties of hydroxypropylcellulose films prepared by hot melt extrusion. Drug Development and Industrial Pharmacy 1999; 25(5)625–633
- Repka M. A., Gutta K., Prodduturi S., Munjal M., Stodghill S. P. Characterization of cellulosic hot-melt extruded films containing lidocaine. European Journal of Pharmaceutics and Biopharmaceutics 2005; 59(1)189–196
- Repka M. A., McGinity J. W. Influence of vitamin e TPGS on the properties of hydrophilic films produced by hot-melt extrusion. International Journal of Pharmaceutics 2000a; 202(1–2)63–70
- Repka M. A., McGinity J. W. Physical-mechanical, moisture absorption and bioadhesive properties of hydroxypropylcellulose hot-melt extruded films. Biomaterials 2000b; 21(14)1509–1517
- Repka M. A., McGinity J. W., Zhang F., Koleng J. J. Hot-melt extrusion technology. Encyclopedia of pharmaceutical technology2nd, J. Swarbrick, J. Boylan. Marcel Dekker, New York 2002a; 203–266
- Repka M. A., Mididoddi P. K., Stodghill S. P. Influence of ‘etching’ on human nails for the assessment of treatment modalities for onychomycosis. Int. J. Pharm 2004; 283(1–2)95–106
- Repka M. A., Munjal M., ElSohly M. A., Ross S. Temperature stability and bioadhesive properties of d9-tetrahydrocannabinol incorporated hydroxypropyl cellulose polymer matrix systems. Drug Development and Industrial Pharmacy 2006; 32(1)21–32
- Repka M. A., Prodduturi S., Stodghill S. P. Production and characterization of hot-melt extruded films containing clotrimazole. Drug Development and Industrial Pharmacy 2003; 29(7)757–765
- Repka M. A., Repka S. L., McGinity J. W., US Patent No. 6375963, 2002b
- Rippie E. G., Johnson J. R. Regulation of dissolution rate by pellet geometry. Journal of Pharmaceutical Sciences 1969; 58: 428–431
- Robinson J. R., McGinity J. W., US Patent No. 6071539. U.S, 2000
- Robinson J. R., McGinity J. W., Delmas P. PCT Int. Appl. World Patent No. 0180822, 2001
- Rothen-Weinhold A., Oudry N., Schwach-Abdellaoui K., Frutiger-Hughes S., Hughes G. J., Jeannerat D., et al. Formation of peptide impurities in polyester matrices during implant manufacturing. European Journal of Pharmaceutics and Biopharmaceutics 2000; 49(3)253–257
- Sato H., Miyagawa Y., Okabe T., Miyajima M., Sunada H. Dissolution mechanism of diclofenac sodium from wax matrix granules. Journal of Pharmaceutical Sciences 1997; 86(8)929–934
- Schmidt P. C., Niemann F. The miniwid-coater.3. Effect of application temperature on the dissolution profile of sustained-release theophylline pellets coated with Eudragit RS 30-D. Drug Development and Industrial Pharmacy 1993; 19(13)1603–1612
- Sprockel O. L., Sen M. H., Shivanand P., Prapaitrakul W. A melt-extrusion process for manufacturing matrix drug delivery systems. International Journal of Pharmaceutics 1997; 155(2)191–199
- Steuernagel C. R. Latex emulsions for controlled drug delivery. Aqueous polymeric coatings for pharmaceutical dosage forms, J. W. McGinity. Marcel Dekker Inc, New York 1997; 79: 582
- Tufvesson C., Lindberg N. O., Olbjer L. Extrusion of an effervescent granulation with a twin screw extruder, baker perkins mpf 50-d. Acta Pharmaceutica Suecica 1987; 24(2)84–84
- Van Laarhoven J. A. H., Kruft M. A. B., Vromans H. In vitro release properties of etonogestrel and ethinyl estradiol from a contraceptive vaginal ring. International Journal of Pharmaceutics 2002; 232(1–2)163–173
- Witt C., Mader K., Kissel T. The degradation, swelling and erosion properties of biodegradable implants prepared by extrusion or compression moulding of poly(lactide-co-glycolide) and aba triblock copolymers. Biomaterials 2000; 21(9)931–938
- Young C. R., Crowley M., Dietzsch C., McGinity J. W. Physicochemical properties of film-coated melt-extruded pellets. Journal of Microencapsulation 2007; 24(1)57–71
- Young C. R. D. C., McGinity J.W. Compression of controlled-release pellets produced by a hot-melt extrusion and spheronization process. Pharm Dev Technol. 2005; 10(1)133–139
- Young C. R., Dietzsch C., Cerea M., Farrell T., Fegely K. A., Rajabi S. A., et al. Physicochemical characterization and mechanisms of release of theophylline from melt-extruded dosage forms based on a methacrylic acid copolymer. Int J Pharm 2005; 301(1–2)112–120
- Young C. R., Koleng J. J., McGinity J. W. Production of spherical pellets by a hot-melt extrusion and spheronization process. International Journal of Pharmaceutics 2002; 242(1–2)87–92
- Young C. R. K. J. J., McGinity J.W. Properties of drug-containing spherical pellets produced by hot-melt extrusion and spheronization process. Journal of Microencapsulation 2003; 20(5)613–625
- Zhang F., McGinity J. W. Properties of sustained-release tablets prepared by hot-melt extrusion. Pharmaceutical Development and Technology 1999; 4(2)241–250
- Zhang F., McGinity J. W. Properties of hot-melt extruded theophylline tablets containing poly(vinyl acetate). Drug Development and Industrial Pharmacy 2000; 26(9)931–942
- Zheng W., Cerea M., Sauer D., McGinity J. W. Properties of theophylline tablets powder-coated with methacrylate ester copolymers. J. Drug Deliv. Sci. Tech. 2004; 14(4)319–332
- Zhu Y. S., Malick A. W., Infeld M.H., McGinity J.W. Influence of a lipophilic thermal lubricant on the processing conditions and drug release properties of chlorpheniramine maleate tablets prepared by hot-melt extrusion. Journal of Drug Delivery Science and Technology 2004; 14(4)313–318