Abstract
RNA interference (RNAi) is emerging as a powerful approach in cancer treatment. siRNA is an important RNAi tool that can be designed to specifically silence the expression of genes involved in drug resistance and chemotherapeutic inactivity. Combining siRNA and other therapeutic agents can overcome the multidrug resistance (MDR) phenomenon by simultaneously silencing genes and enhancing chemotherapeutic activity. Moreover, the therapeutic efficiency of anticancer drugs can be significantly improved by additive or synergistic effects induced by siRNA and combined therapies. Co-delivery of these diverse anticancer agents, however, requires specially designed nanocarriers. This review highlights the recent trends in siRNA/anticancer drug co-delivery systems under the major categories of liposomes/lipid, polymeric and inorganic nanoplatforms. The objective is to discuss the strategies for nanocarrier-based co-delivery systems using siRNA/anticancer drug combinations, emphasizing various siRNA targets that help overcome MDR and enhance therapeutic efficiency.
Introduction
Worldwide, there is a continuing interest in identifying new strategies for cancer treatmentCitation1,Citation2. Finding and modulating the genes responsible for cancer pathology is one such approach. A growing number of studies have suggested that RNA interference (RNAi) as a promising gene manipulation technique with therapeutic potential when used alone or in conjunction with other treatmentsCitation3–5. However, siRNA or shRNA-based therapy relies on successful delivery to the targetCitation6,Citation7. Hence, these approaches require a safe and efficient carrier system for in vivo use.
The use of viral vectors for therapeutic gene delivery has been controversial because of possible immunogenic and undesirable gene mutation effectsCitation8. The availability of various non-viral, nanoparticle-based delivery systems has contributed to tremendous advancements in siRNA-based therapeutics for cancerCitation9. It has been shown that nanoparticle delivery systems improve the systemic stability of siRNA, prevent premature degradation and rapid in vivo clearance of siRNAs, and enhance selectivity toward the targetCitation10–12.
In addition, siRNA has been widely explored for use in combination therapyCitation13–16. Combination therapy relies on the simultaneous action of multiple therapeutic entities to exploit additive or synergistic effects and enhance therapeutic efficiency. In clinical settings, combination chemotherapy refers to the grouping of multiple chemotherapeutic agents that use different mechanisms to treat cancer. The combination strategy not only enhances therapeutic efficiency but also reduces the risk of severe side effects caused by cytotoxicity of individual drugsCitation17. The use of siRNA in combination with other anticancer therapeutics has been shown to improve outcomes by either increasing the sensitivity of cancer toward a therapeutic modality or by working in an additive or synergistic fashionCitation18.
Advancements in nano-drug delivery systems improved the co-delivery of siRNA and other therapeutic agentsCitation19. Nanoparticle carriers supporting the combination of anticancer therapeutics, such as chemotherapy agents, photodynamic sensitizers or small molecule inhibitors, with siRNA have been developed. This review primarily focuses on the nanodelivery system advancements for siRNA-chemotherapeutic combination(s) in cancer treatment.
Significance of siRNA in combination with other therapeutics
Cancers are highly heterogenic in nature and often become resistant to therapiesCitation20. Resistance may develop toward different treatment modalities, including chemotherapy, radiation therapy and photodynamic therapy (PDT). The mechanisms of treatment resistance are complex, although several molecular mechanisms have been elucidatedCitation21.
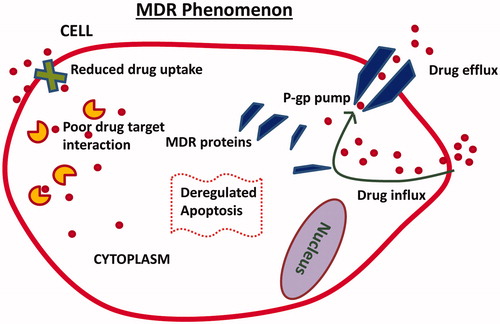
The development of multidrug resistance (MDR) poses a significant challenge. Many researchers have comprehensively reviewed the molecular mechanisms of MDR in cancerCitation21–23. Increased drug efflux, altered levels of intracellular target and overexpression of resistance-related, anti-apoptotic genes leading to the expression of MDR proteins are prominent mechanisms of MDR in cancer cells (). MDR ultimately results in a lower cellular concentration of the drug, which limits apoptosis and prevents other cytotoxic events. siRNA-based gene therapy has been shown to effectively overcome MDR in cancer, when combined with chemotherapeuticsCitation5,Citation24–26. The suppression of genes related to MDR may increase the chemosensitivity of cancer cells and improve treatment efficacy.
Figure 1. Illustration depicts multidrug resistance (MDR) mechanisms in cancer cells. Enhanced drug efflux, expression of MDR proteins, reduced drug uptake, poor drug target interaction and deregulated apoptosis are some of the important mechanisms.
PDT involves the treatment of cancer with multiple components, including light, photosensitizers and oxygenCitation27. The localized excitation of photosensitizer molecules by light results in the conversion of molecular oxygen to reactive oxygen species, which interact with biomolecules in cancer cells and kill them by triggering apoptosis. The combination of siRNA with PDT enhances therapeutic responses in cancerCitation28,Citation29. Many studies demonstrated that autophagy-related genes are major targets for siRNAs to improve cancer cells’ response to PDTCitation30,Citation31. Co-delivery of a photosensitizer and siRNA via nanoparticles might be an important treatment strategy. PDT combined with siRNA has also been utilized in cancer immunotherapyCitation32,Citation33. Activating human immune cells (T-cells) to attack cancer cells is a strategic way of utilizing the body’s own immune system against cancer. By suppressing certain genes in immune inhibitory pathways with siRNA, it is possible to safely and effectively render T-cells immunogenic against cancer.
siRNA therapy complements radiation therapy by targeted suppression of specific genes that cause radiation resistance, resulting in an enhanced tumor response to radiationCitation34–36. The 5′ adenosine monophosphate-activated protein kinase (AMPK) pathway has been shown to be upregulated in tumors that exhibit radiation therapy resistanceCitation37. Recent reports suggest that the overexpression of proteins like PD-L1, HuR and Ape-1 causes radiation resistance in some cancersCitation34,Citation36,Citation38. Increased DNA damage repair machinery is also a prominent mechanism of radiation resistance. Although new developments in nanomedicine have explored the use of nanoparticles for radio-sensitizationCitation39, there are not extensive investigations on radionuclide/siRNA co-delivery using nanoparticle drug delivery systems, until now.
To achieve the best therapeutic efficiency out of the combination of siRNA and other therapeutics precise and efficient nanoparticle delivery systems are required. The following section discusses various nanoparticle systems that have been particularly investigated for siRNA-based combination therapy in cancer.
Nanoparticle-assisted co-delivery of siRNA and other chemotherapy agents
Chemotherapeutic drugs have been used as a front-line treatment for many cancers. However, tumor heterogeneity and the development of drug resistance have resulted in the reduced effectiveness of numerous cancer drugs that are commonly used in the clinicCitation40. Nucleic acid therapeutics in combination with drugs compliment chemotherapy in various ways such as silencing specific genes involved in drug resistance and anti-apoptosis mechanism, by restoring tumor suppressor genes and by introducing apoptotic genes. The combination of chemotherapy with RNAi can be achieved by either co-treatment with individual therapeutic agentsCitation41 or by co-delivery of chemotherapeutics and siRNA via a single carrierCitation42; the latter known to be more efficientCitation43,Citation44.
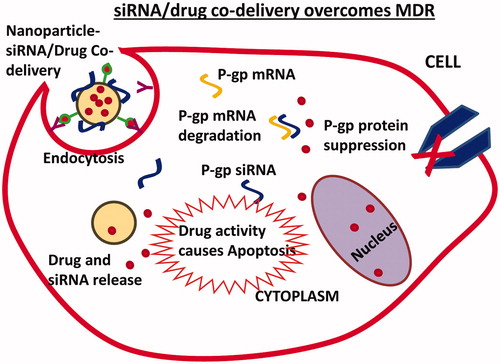
Co-delivery of multiple agents can be achieved with nanoparticle carriers that have the capability of packaging and releasing individual therapeutic agents in a controlled fashion at the desired siteCitation44. Various nanoparticle carriers, such as liposomes, polymer-based nanoparticles and inorganic nanoparticles, have been introduced for co-delivery of siRNA and chemotherapeutics. The co-delivery of siRNA and chemotherapeutics by nanoparticles results in a combined therapeutic response due to siRNA-based target gene silencing and cytotoxic drug activity ().
Figure 2. Nanoparticle-based co-delivery of siRNA and chemotherapeutic drug overcomes MDR by siRNA-mediated p-glycoprotein (p-gp) suppression. Increased drug accumulation in cells triggers apoptosis mechanisms.
The next section discusses some successful strategies in nanoparticle-based nucleic-chemotherapeutic co-delivery systems. Relevant examples of siRNA in combination with PDT, immunotherapy and radiotherapy are also mentioned.
Liposomes or lipid-based nanoparticles
Liposomes are well known for their ability to carry nucleic acid therapeutics and/or chemotherapeutic agentsCitation45. The easy manipulation of the lipid components to suit nucleic acid interaction and co-encapsulation of one or multiple drugs make liposomes advantageous for combination drug delivery. Co-delivery efficiency may depend on how the anticancer agents are packaged and their interaction with the lipid components of the carrierCitation46. Cationic liposomes usually utilize ionic interactions between the positively charged liposome surface and the negatively charged siRNA to form a siRNA-liposome complexCitation47. However, if the carrier liposome is anionic or neutrally charged, siRNAs may be encapsulated in the aqueous coreCitation48. Proper packaging of nucleic acid in liposomes can also occur through condensation using cationic polymers like protamineCitation48 or polyethylenimine (PEI)Citation49, which enhances siRNA stability. Depending on the solubility, chemotherapy drugs are either encapsulated in the aqueous core or embedded in the lipid bilayer during liposome preparation. Therefore, the solubility and stability of nucleic acid and chemotherapeutics are important factors.
Lipid-based nanoparticles typically harness the cationic property of lipids by modifying the physicochemical properties of nanoparticles made of different materials. For example, calcium phosphate nanoparticles use lipid to support the delivery of nucleic acid therapeuticsCitation50. Such nanoparticles that utilize the properties of multiple materials during fabrication are generally categorized as hybrid nanoparticles for specific drug delivery applications.
Liposomal nanocarriers have benefitted from more advancement in their application as co-delivery systems. Simple PEGylated liposomesCitation51, advanced stimuli-responsive liposomesCitation52,Citation53 and targeting liposomesCitation54,Citation55 have all been constructed for siRNA/chemotherapeutic co-delivery in cancer. Using a PEGylated liposome to co-deliver, BCL2 siRNA with docetaxel (DTX) successfully inhibited lung cancer in vitro and in vivoCitation51. The PEGylation of the liposomal carrier prolonged the circulation time of liposomes in vivo and facilitated passive co-delivery of BCL2 siRNA and DTX to the tumor. Synergism was reported between these therapeutic molecules in an A549 xenograft model upon successful co-delivery using liposomesCitation51. Another study by Yao et al.Citation53 used a pH-sensitive liposome to co-deliver sorafenib and a therapeutic siRNA in cell culture and a mouse model of liver cancer. The pH-sensitive liposome was constructed by modifying pH-sensitive carboxymethyl chitosan onto a liposome preloaded with siRNA and sorafenib. Drug release was pH-sensitive, and the liposome successfully co-delivered siRNA and sorafenib. Peng et al.Citation52 developed a thermosensitive magnetic liposome for co-delivery of shRNA and doxorubicin in gastric cancer in vitro and in vivo. This advantageous system combined magnetic targeting, thermosensitive controlled release and synergistic antitumor efficacyCitation52.
The functionalization of lipids with small molecule ligands for active targeting of specific receptors overexpressed in cancer cells offers significant advantages for cancer treatment. Recently, ligand conjugated liposomal systems have been optimized for targeted delivery of a combination of siRNA and chemotherapeutic drug(s). Yang et al.Citation55 reported the use of folic acid (FA)-modified liposomes for the targeted co-delivery of Bmi1 siRNA and doxorubicin in KB, HeLa and Hep3B cancer cells, and a KB xenograft tumor model. They observed folic acid receptor-expression-dependent cell uptake of FA-modified liposomes (N-[1-(2,3-Dioleoyloxy)propyl]-N,N,N-trimethylammonium methyl-sulfate (DOTAP)/Chol/mPEG-DSPE/FA-PEG-Chol) in cancer cells. In vivo administration resulted in effective Bmi1 gene silencing, and enhanced doxorubicin’s cytotoxicity. The combination of doxorubicin and Bmi1 siRNA delivered via folate-liposome had an enhanced therapeutic effect against cancer, compared with individual agents. Since this liposomal carrier enhanced hepatic accumulation, the co-delivery strategy may be useful against liver cancer; however, this approach remains to be investigated.
Another study demonstrated the targeted co-delivery of siRNA and a chemotherapeutic agent to liver tumorsCitation54. A liposomal carrier for the co-delivery of vimentin siRNA and doxorubicin was prepared from a mixture of lipids: DMKE (O,O'-dimyristyl-N-lysyl glutamate), cholesterol, galactosylated ceramide, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and PEG2000-DSPE (distearoyl phosphatidylethanolamine). Targeting was conducted by modifying the nanoparticle system with galactosyl ligand-targeted to asialoglycoprotein receptors expressed in hepatic cancer cells. This Gal-DOX-siRNA-L co-delivery system demonstrated great potential for synergistic antitumor therapy.
Modifying liposomal carriers using multiple targeting moieties might be a feasible option in co-delivering siRNA and chemotherapeutic. This strategy was successfully used by Yang et al.Citation56 to treat brain tumors in vitro and in vivo. The cationic liposome was modified using dual-peptides that targeted the low-density lipoprotein (LDL)-protein receptor-related protein receptor (Angiopep-2) and the neuropilin-1 receptor. The liposome loaded with vascular endothelial growth factor (VEGF) siRNA and DTX was targeted against glioma, and the peptide-mediated cell penetration enhanced the co-delivery of VEGF siRNA and DTX to the tumor cells. Anti-angiogenesis and cellular apoptosis effects were combined with this approach. The synergism between siRNA and the chemotherapeutic agent effectively inhibited tumor growth.
Lipid-based nanoparticles have also been used in a combination of siRNA and PDT to treat some cancers. In a typical study, Chen et al.Citation28 demonstrated that a combination of hypoxia-inducible factor (HIF)-1α siRNA-loaded lipid-calcium phosphate nanoparticle (LCP) and photosan-mediated PDT significantly inhibited head and neck tumor growth in vitro and in vivo. The anisamide-modified LCP nanoparticles/HIF-1α siRNA, which significantly inhibited cell growth and enhanced cell death and proliferation when combined with PDT, were targeted to cancer cells overexpressing sigma receptors. When systemically administered, LCP nanoparticles/HIF-1α siRNA reduced HIF-1α expression in mouse xenograft tumors. When PDT was combined with this siRNA-based gene silencing, tumor regression was significantly enhanced. The combination of LCP-based HIF-1α delivery and photosan-mediated PDT was more effective than HIF1α siRNA or PDT alone. A recent study demonstrated that LCP nanoparticle delivery of VEGF-siRNA combined with photosan-mediated PDT significantly reduced tumor growth in xenograft models of human head and neck squamous cell carcinomaCitation57. This LCP nanoparticle was also modified with anisamide to target sigma receptors in cancer cells.
The antitumor efficacy of small molecule inhibitors is enhanced upon co-delivery of therapeutic siRNA using a single liposomal vehicle. Inhibition of the Ras-group of oncoproteins is considered difficult. However, scientists have developed MAPK (mitogen-activated protein kinases)/ERK (extracellular signal-regulated kinases) kinase inhibitors that act downstream of Ras in the mitogen-activated protein (MAP) kinase pathway, indirectly targeting RasCitation4. The effect of these MEK inhibitors was further enhanced by co-delivery of Mcl1-specific siRNA via a cationic liposomeCitation58. The hydrophobic MEK inhibitor molecules were encapsulated in the lipid bilayer and were then complexed with the Mcl1 siRNA and co-delivered to achieve enhanced antitumor effects in cell culture and a mouse model. Cationic liposomes have also been used to combine multiple siRNAs with a chemotherapeutic agent. Saad et al.Citation59 used this strategy to co-deliver MRP1 and BCL2 siRNAs with doxorubicin to overcome MDR and to target different pathways or cell growth inhibition mechanisms.
Hence, the application of liposomes in co-delivery of siRNA and other therapeutics have a major impact in experimental and translational cancer therapeutics. Some of the well-known challenges with liposomes are their unsatisfactory in vivo stability and burst release of the loaded drug. Many of these challenges have been addressed to a great extent by modification of liposome surface or bilayer with additional componentsCitation60–63. The limitations still existing in liposomal platforms may be improved by newer technological advances that may allow continued translational success in cancer therapy.
As an alternative to liposomes, niosomes are widely-used drug delivery carriers because of their similar propertiesCitation64–66. Niosomes are known for more than two decades in drug delivery applicationsCitation66–68. Niosomes are formed by the self-association of non-ionic surfactants and cholesterol in an aqueous phase and have shown good physicochemical stability and excellent loading efficiency of drugs of both hydrophilic and hydrophobic nature and also oligonucleotidesCitation64,Citation65,Citation69. The biodegradable, biocompatible and non-toxic nature of niosomes and their easy manipulation with targeting ligands makes them attractive drug delivery systems for cancer therapyCitation70. In an earlier study, the hydrophilic anticancer agent doxorubicin was encapsulated in niosomes for studying its metabolism, tissue distribution and antitumor activity in a mouse modelCitation67. Niosomes were prepared from sorbitan monostearate (Span 60), cholesterol and choleth-24 (a 24 oxyethylene cholesteryl ether) in typical molar ratios and used as a carrier for doxorubicin for treating MAC 15A subcutaneously implanted tumor in mice. This niosomal formulation enhanced the tumor accumulation of doxorubicin compared to free doxorubicin, leading to doubling of tumoricidal activity. Later, the same group studied the doxorubicin-containing niosomal formulation in ovarian tumor models in miceCitation68. The C16G2 (a hexadecyl diglycerol ether)-based niosomes were equally potent when compared with free doxorubicin. Further, the study also demonstrated that span 60 niosomes were able to reduce the IC50 value of doxorubicin, though modestly, as compared to C16G2 niosomes and free doxorubicin. Niosomes were also efficiently delivered oligonucleotides to mammalian cells. In a typical study, niosomes were prepared by synthetic aminolipid, 2,3-di(tetradecyloxy)propan-1-amine and polysorbate-80 for delivery of antisense oligonucleotides against Renilla luciferase mRNA in HeLa cellsCitation71. The niosomes were not cytotoxic and efficiently transfected HeLa cells using oligonucleotides when used at a charge ratio of 1:14, in comparison with a cytotoxic commercial transfection reagent, lipofectamine.
Recently, niosomes have explored for combinatorial drug delivery for cancer therapy. For instance, Rajput et al.Citation72 demonstrated that an acidic milieu-sensitive multi-lamellar gold niosomes were able to co-deliver Akt-siRNA and thymoquinone (TQ) in tamoxifen-resistant and Akt-overexpressing MCF7 breast cancer cells. TQ was encapsulated in the hydrophobic core of the niosomes whereas siRNA was electrostatically bound with cationic niosomes. This combination delivery strategy enhanced the sensitivity of breast cancer cells to tamoxifen by efficient knockdown of Akt expression. Studies in xenograft tumor models in mice revealed that niosome formulation of Akt siRNA and TQ combination enhanced the apoptosis and MDM2 inhibition in therapy-resistant cancerCitation72. These studies suggest that niosomes are promising an alternative to liposomes which is capable of delivering drug or gene or a combination of both, in cancer therapy.
Polymer nanoparticles
Polymers offer a promising nanoparticle platform for siRNA/anticancer drug co-delivery. These materials have been extensively studied as a delivery system for siRNA and chemotherapyCitation73,Citation74. Polymer nanoparticles can be easily fine-tuned for any size and allow good packaging of the payload by physical or ionic interactions. They are ideal platforms with which to construct core-shell nanoparticles, allowing them to carry multiple agents, such as siRNA, plasmid DNA (pDNA) and chemotherapeutic drugs, simultaneouslyCitation24. Cationic polymers, including polyethyleneimine, chitosan and poly-l-lysine (PLL), are commonly employed for gene delivery applications because of their strong ionic interaction with nucleic acids to form polyplexes. Nanoparticles fabricated from amphiphilic polymers, like polylactic acid-co-glycolic acid (PLGA) and atellocollagen, have also shown promise as drug and gene delivery vehicles. Due to the distinct physicochemical properties of different polymers, most co-delivery strategies use multiple polymers in the nanoformulation processCitation75,Citation76.
In contrast to the soft nature of liposomes, polymer nanoparticles are solid particles that carry drugs in their network or matrix, and are more stable. Due to these properties, the entrapped or encapsulated drugs show more sustained and controlled release patterns over time. However, drug release also depends on the type of interaction between the drug and the polymer backbone of the nanoparticleCitation77. The physically conjugated drug may result in slower and more controlled release, usually over many days, in contrast to the fast, but time-dependent, release of the weakly bound drug. Nevertheless, the desired controlled release properties can be achieved for each individual payload based on how the nanoparticle co-delivery system was formulated.
PLGA or polylactic acid (PLA) nanoparticles are sought-after platforms for co-delivery of siRNA and chemotherapeutics. PLGA nanoparticles have a well-defined spherical and nano-capsular structure and are biocompatible. These nanoparticles offer high drug loading capacity and are easy to manipulate to carry siRNA therapeutics. PEI-modified PLGA nanoparticles are attractive nanoparticle platform for carrying siRNA and combination drugs as demonstrated by Su et al.Citation78. PLGA nanoparticles carrying paclitaxel with PEI as a copolymer was synthesized, and the siRNA was electrostatically adsorbed onto its surface. Since STAT3 overexpression is implicated in paclitaxel resistance in lung cancer, they combined STAT3 siRNA with paclitaxel in the same nanoparticle carrier. The co-delivery efficiency of this nanoparticle system was monitored in A549 lung cancer cells with Oregon green paclitaxel and cy5-labeled STAT3 siRNA. The researchers concluded that STAT3 siRNA co-delivered PLGA-PEI nanoparticles exerted higher cytotoxicity than their STAT3 siRNA-free counterpart. Moreover, sensitivity to treatment increased in paclitaxel-resistant A549 lung cancer cells after the co-delivery of STAT3 siRNA and paclitaxel by PLGA-PEI nanoparticlesCitation78.
In a similar approach, Patil et al.Citation79 designed PLGA-PEI nanoparticles for siRNA/drug co-delivery for lung cancer therapy in vitro and in vivo. They in-situ-coated PEI to PLGA in the nanoparticle preparation itself, instead of coating PEI into the preformed PLGA nanoparticle with loaded drugCitation79. Biotin, the target ligand, was introduced into the PLGA-PEI nanoparticle system as a PLA-PEG-Biotin conjugate. The resulting nanoparticle system loaded with paclitaxel and p-gp siRNA was used to treat biotin-receptor-overexpressing, drug-resistant JC (mammary adenocarcinoma) tumors in BALB/C mice. They observed that paclitaxel, either in its free form or as encapsulated in PLGA-PEI nanoparticles, did not suppress tumor growth, while the co-delivery of paclitaxel with p-gp siRNA effectively silenced the specific gene and enhanced the tumor’s response to paclitaxel.
Recently, we demonstrated the efficacy of chitosan-coated PLA nanoparticles as co-delivery vehicles for P62 siRNA, Beta-5 pDNA and cisplatin (CDDP) in cisplatin-resistant ovarian cancer cellsCitation24. The CDDP was encapsulated in the PLA core, and the siRNA/pDNA was adsorbed onto the cationic chitosan coating of the nanoparticle through electrostatic interaction. By suppressing the autophagy regulatory protein P62 using siRNA and restoring the proteasome Beta-5 function using pDNA via chitosan-coated, CDDP-loaded PLA nanoparticles, the sensitivity of ovarian cells to CDDP significantly improved.
Chitosan is a biodegradable and biocompatible polymer with attractive properties for the delivery of nucleic acids in combination with chemotherapeutics. This polymer is a well-known gene delivery vehicle that forms rapid, self-assembled complexes with siRNA or DNA to form nano-sized particles. The presence of many free amino groups allows chitosan to undergo a crosslinking reaction with counter ions, such as thiamin pyrophosphate or tripolyphosphate, resulting in ionic gelation of chitosan and production of nanoparticles under optimal cation-to-anion ratios. siRNA or drug may be entrapped in the crosslinked chitosan network, increasing particle stability, but decreasing the rate of drug release. Since most chemotherapeutic drugs are hydrophobic, the co-delivery of chemotherapeutics would be difficult without chemically modified chitosan or supporting chitosan with other hydrophobic polymers.
Recently, Wei et al.Citation80 introduced a modified chitosan-based nanoparticle system for co-delivery of mTERT siRNA and paclitaxel. The nanoparticle platform was fabricated using N-((2-hydroxy-3-trimethylammonium) propyl) chitosan chloride (HTCC) with a positive charge for interaction with siRNA. This system efficiently delivered siRNA in vivo. This chitosan-based nanoparticle was then developed into a two-in-one co-delivery system encapsulating paclitaxel and mTERT siRNA. This co-delivery strategy using an HTCC nanoparticle enhanced the efficacy of paclitaxel due to simultaneous delivery with mTERT siRNA, resulting in significant tumor suppression in vivo. Importantly, this chitosan-based nanoparticle system assisted the synergistic activity of paclitaxel and mTERT siRNA and thereby shows promise for the delivery of a siRNA/hydrophobic drug combination in cancer therapyCitation80.
Chitosan is also useful in the co-delivery of hydrophilic drug and siRNA. In a recent study, a carboxymethyl chitosan nanoparticle was designed to co-deliver siRNA toward IL17RB (associated with cell growth, proliferation migration and poor prognosis in patients with breast cancer) and doxorubicin in breast cancer cellsCitation81. Simultaneous delivery of IL17RB siRNA and doxorubicin was possible with this carboxymethyl chitosan nanoparticle, and resulted in efficient suppression of IL17RB-related genes, like NF-kB and Bcl-2, apoptosis and inhibited migration of MDA-MB361 breast cancer cells.
In addition, chitosan is used as a prominent component of co-delivery vehicles due to its high biocompatibility. In a typical study, a chitosan-based triblock micellar structure was prepared for the co-delivery of p-glycoprotein siRNA and doxorubicin to overcome MDR in a liver tumor model in vivoCitation75. The N-succinyl chitosan–PLL–palmitic acid (NSC–PLL–PA) nanoparticles, in which n-succinyl chitosan served as a hydrophilic shell, improved the half-life of micelles and reduced the toxicity of PLL. Doxorubicin was encapsulated in the palmitic acid core, and siRNA was adsorbed onto the micelles using a PLL skeleton. The co-delivery of p-gp siRNA and doxorubicin using NSC–PLL–PA nanoparticles bypassed drug efflux transporters by downregulating p-gp expression and increased doxorubicin accumulation in the cancer cells. This chitosan-micelle-based co-delivery strategy ultimately improved the antitumor activity through the synergistic activity of p-gp siRNA and doxorubicin.
Various polymers with distinct physicochemical properties are being used together to fabricate nanoparticles with interesting features for co-delivery of nucleic acid therapeutics and anticancer drugs. Among the cationic polymers, polyethyleneimine (PEI) has been extensively used, alone or in combination with other polymers, in siRNA co-delivery systems. Shen et al.Citation42 designed a poly(D,L-lactide-co-glycolide) (PLGA) nanoparticle bearing disulfide-linked reducible PEIs covered by hyaluronic acid (HA) for co-delivery of COX-2 siRNA and DTX. The hydrophobic drug DTX was incorporated into the PLGA core, and the siRNA was electrostatically linked to the cationic PEI layer of the nanoparticle. The presence of HA was used to target the therapeutic agents toward CD44-overexpressing gastric cancer cells. This co-delivery strategy suppressed COX-2 gene expression in the target cells. This PEI-based nanoparticle system has promise as an excellent co-delivery system targeting CD44-overexpressing tumors, as supported by in vivo biodistribution data showing that the HA-modified nanoparticle system allowed enhanced DTX accumulation in tumors, compared with reticulo-endothelial system (RES) organs.
The presence of numerous free amino groups allows PEI to be conjugated with various moieties to improve its physicochemical properties for co-delivery formulation. Recently, Xu et al.Citation82 prepared a pH-sensitive nano-formulation using PEI for co-delivery of siRNA and doxorubicin in models of metastatic lung cancer. Doxorubicin was conjugated to PEI using a pH-sensitive hydrazone bond; the system was complexed with BCL2 siRNA. The pH-sensitive linkage in nanoparticles allowed controlled, the pH-responsive release of doxorubicin in vivo. The combination with BCL2 siRNA enhanced the therapeutic efficiency of doxorubicin in a B16F10 lung tumor model. This PEI-based nanoparticle system is a promising strategy for pulmonary co-delivery of siRNA and the chemotherapeutic agent(s) to treat lung cancer.
Triblock copolymer systems are also attractive delivery systems for siRNA/chemotherapeutic combinationsCitation83. It was shown that triblock copolymer methoxy-poly(ethylene glycol)-block-poly(L-glutamate-hydrazide)-block-poly(N,N-dimethylaminopropylmethacrylamide) (PDMAPMA) was functionalized with folate (FA/m-PEG-b-P(LG-Hyd)-b-PDMAPMA) for co-delivery of p-gp siRNA and doxorubicinCitation84. Doxorubicin was conjugated to the poly(L-glutamate-hydrazide) blocks using a pH-sensitive linkage. The nanoparticles were formed by self-assembly of these polymers and doxorubicin. The p-gp siRNA was then electrostatically bound to cationic PDMAPMA blocks to form nanocomplexes. The drug and siRNA release in vitro was pH-dependent. The nanoparticles were targeted by FA toward MCF-7 cells and showed enhanced cell uptake and cytotoxicity.
Biswas et al.Citation85 demonstrated a different triblock copolymer nanoparticle system based on poly(amidoamine) dendrimer (generation 4)-poly(ethylene glycol)-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (G(4)-D-PEG-2 K-DOPE) for co-delivery of siRNA and anticancer drug. The lipid modification of the nanoparticle system enhanced the binding, protection and transfection efficiency of model siRNA (siGFP), promoting a high level of target gene suppression by siRNA. The co-delivery of doxorubicin with siRNA was efficiently achieved by dendrimer-based triblock copolymer complex (G(4)-D-PEG-2 K-DOPE) or dendrimer-micelle formulation, as measured by the cell uptake experiment. Thus, novel triblock copolymers are consistently developed and tested. Although many studies are in the preliminary stages, triblock copolymers appear to be promising for co-delivery of siRNA and chemotherapy agents.
Inorganic nanoparticles
Inorganic nanoparticles are reliable systems for co-delivery of siRNA and other therapeutics. They have unique physicochemical properties, including small size, large surface-area-to-volume ratio, stability in a biological environment, a surface that is easily functionalized for the ligand or therapeutic molecules and optical or magnetic propertiesCitation86. Common inorganic nanoplatforms include gold, iron oxide, mesoporous silica, calcium phosphate nanoparticles, carbon nanotubes (CNTs) and quantum dots.
Recent studies suggest that gold nanoparticles or nanorods (AuNP) are excellent gene delivery systems, due to their stability, biocompatibility and ability to undergo surface modifications and conjugationsCitation86–88. siRNA can be directly conjugated to gold nanoparticles via electrostatic interactions or thiol bonds or can be adsorbed to a layer of cationic polymer coated onto gold nanoparticle surfaces. However, for co-delivery purposes, the polymer coating strategy has been usefulCitation89. A recent example demonstrated the co-delivery of doxorubicin, ASCL1 siRNA and octreotide (targeting ligand) using a gold nanorod targeted at neuroendocrine (NE) cancer cellsCitation90. The doxorubicin was conjugated to the gold nanoparticle via a pH-sensitive hydrazone linker; siRNA was complexed by poly-l-arginine coating. The targeting ligand octreotide was also attached to the poly-l-arginine polymer. Treatment of NE cells with this multi-component co-delivery system resulted in efficiently targeted cell uptake, enhanced ASCL1 gene silencing and effective cell proliferation inhibition. Hence, targeted delivery of ASLC1 siRNA and doxorubicin using Au nanorods could be a potential strategy for effective NE tumor treatment. Recently, Esteban-Fernández et al.Citation91 developed a gold nanowire-based system, optimized for ultrasound micromotor-propelled gene silencing, to deliver siRNA in HEK-293 and MCF-7 cell lines. The novel technology uses a rolling circle amplification DNA strand to wrap the gold nanowire as an anchor to siRNA for delivery. This system shows promise for efficient siRNA delivery.
Mesoporous silica nanoparticle (MSN) is a widely investigated platform for drug and gene delivery applications. MSNs are chemically stable, biocompatible and non-toxic. The physicochemical properties of MSNs favor the development of co-delivery platforms for siRNA and other cancer therapeuticsCitation15,Citation92. A recent study reported the development of functionalized MSN for co-delivery of siRNA and a chemotherapeutic agentCitation93. MSNs were modified with PEI-PEG and loaded with epirubicin and BCl2 siRNA for simultaneous delivery. The release of BCL2 siRNA from MSN was pH-dependent. Compared with free epirubicin, MSN carrying epirubicin, or siRNA alone, the MSN BCL2-siRNA/epirubicin co-delivery system exhibited cytotoxicity in vitro and enhanced therapeutic efficiency in vivo. The therapeutic effect was synergistic.
Another strategy for co-delivering siRNA and chemotherapeutics utilized hollow silica nanoparticlesCitation94. The hollow MSN achieved high drug loading and controlled release of doxorubicin. BCL2 siRNA was electrostatically adsorbed to the FA-PEI modified MSN containing doxorubicin for targeted co-delivery into HeLa cells. The pH-responsive cellular release of siRNA/doxorubicin may help prevent their premature release and possible side effects caused by non-specific activity. Upon targeting FR-overexpressing Hela cells, the simultaneous delivery of BCL2 siRNA and doxorubicin resulted in effective BCL2 gene silencing and enhanced cytotoxicity. Using hollow MSNs may enhance the potential of anticancer therapy.
Recent studies show that calcium phosphate nanoparticles may be an excellent alternative for siRNA and chemotherapeutic delivery systemCitation95–98. These nanoparticles are non-toxic and biocompatible and are in the generally regarded as safe for human use as per Food and Drug Administration (FDA). These advantages may facilitate rapid clinical translation for drug and gene delivery applications. Recent studies show the possibility of incorporating siRNA (or any nucleic acid therapeutic) and other agents into calcium phosphate nanoparticlesCitation95,Citation96. For siRNA co-delivery purpose, calcium phosphate nanoparticles may be modified to improve the physicochemical characteristics. For instance, Zhang et al.Citation99 encapsulated VEGF siRNA and gemcitabine into calcium phosphate nanoparticles. The calcium phosphate nanoparticles were prepared using a microemulsion technique, encapsulating VEGF siRNA and gemcitabine together. Then, the siRNA/drug-loaded calcium phosphate nanoparticle was coated with high-density lipid-PEG (DSPE-PEG). Systemic administration of this LCP nanoparticle resulted in targeted co-delivery of VEGF siRNA and doxorubicin to subcutaneous and orthotopic NSCLC tumor xenograft models. The strategy significantly enhanced apoptosis and reduced cell proliferation, leading to a dramatic reduction in tumor growth. Importantly, the in vivo toxicity of the LCP co-delivery system was minimal, while the VEGF silencing and apoptotic induction demonstrated the feasibility of this system as a successful siRNA/small molecule drug co-delivery vehicle. The same group extended the use of LCP to co-delivering a combination of c-myc-siRNA targeting the myc oncogene and gemcitabine to H460 and A549 NSCLC tumor cellsCitation50. These studies revealed that nucleic acid therapeutics can be efficiently combined with small molecule drugs in LCP nanoparticles for cancer therapy.
Functionalized CNTs have also emerged as a unique gene delivery systemCitation100,Citation101. CNTs have high mechanical strength and the ability to transport across the cell membrane without undergoing endocytosis. Due to their intrinsic hydrophobic nature, CNTs must be functionalized for drug delivery applications. Pereira et al.Citation102 investigated the use of ammonium-functionalized, multi-walled nanotubes (NTs)/cationic liposome hybrid to co-deliver Polo-1 kinase siRNA and doxorubicin. They tested a series of functionalized (f) MWNT-DOTAP:Chol liposome hybrid formulations for optimal in vitro transfection efficiency, gene silencing and doxorubicin delivery. The F-MWNT-liposome nanoparticles loaded with doxorubicin and complexed with PLK1 siRNA efficiently co-delivered drug and siRNA to A549 lung cancer cells. The two therapeutic agents evoked cytotoxicity synergistically. Although recent advancements that identify newer CNT-based drug and gene delivery systems have been made, the research data are limited, perhaps indicating that challenges to therapeutic applications remain. Additional studies are required to improve the pharmacokinetics and toxicity profile of CNT-based drug delivery vectors.
Although most of these inorganic nanoparticle platforms have shown potential as co-delivery vehicles for siRNA and other anticancer agents, most have not been evaluated for material-related toxicity. Most of these inorganic nanoparticles were only tested in vitro. The lack of information regarding the in vivo toxicity and efficiency is considered a major issue that hinders the path forward for inorganic nanoparticles as drug delivery systems for cancer therapy. Systemic studies regarding the safety, pharmacokinetics and mechanism of toxicities for inorganic nanoparticles are warranted.
Apart from siRNAs, these nanoparticle systems such as liposomes, polymers or inorganic systems have been utilized in exploring combinations of miRNA or pDNA therapeutics with chemotherapeutics or other therapeutic agents, including siRNA. provides examples of miRNA, pDNA and combinations, some using siRNA as a co-delivery agent, have also been co-delivered with other cancer therapeutics via a variety of nanoparticles.
Table 1. Co-delivery of miRNA, miRNA inhibitor or plasmid DNA with other cancer therapeutic agents via nanoparticles.
Conclusion
Nanoparticles are versatile platforms used to deliver nucleic acid therapeutics and other anticancer drugs. The advantages of nanoparticles include improved drug stability, protection from rapid bio-clearance or bio-degradation, altered pharmacokinetics, enhanced bioavailability, promotion of the preferential accumulation of drugs in tumor sites via enhanced permeation and retention effects, and increased therapeutic index. Over the last decade, nanoparticle systems have been successfully harnessed for the co-delivery of multiple anticancer agents. Co-delivery improves the therapeutic efficiency by achieving additive or synergistic effects and/or by overcoming MDR. The use of siRNA in combination with other anticancer agents is advantageous since multiple pathways or regulatory proteins involved in cancer cell growth, progression, metastasis, or drug resistance can be simultaneously manipulated to achieve an enhanced therapeutic response. Many liposomal, polymer-based and inorganic nanoparticles have been proposed for use in co-delivery of siRNA with chemotherapeutics, small molecule inhibitors or photodynamic agents. In addition to siRNA, other nucleic acid therapeutics, such as miRNA and pDNA, has been under investigation for co-delivery with anticancer agents. Many of the nanoparticle co-delivery systems using siRNA and anticancer drugs show great promise and high efficiency in treating cancers.
Although recent advancements using nanoparticle-based co-delivery have been made, challenges remain. One important issue may be the increased complexity of these systems, especially when multiple components are used in carrier fabrication. The material safety of each component should be validated before their application in drug delivery systems. Once a multicomponent system is created, multiple levels of safety evaluation must be carried out to monitor and prevent off-target effects or undesired toxicity. To quickly translate these co-delivery systems into clinical settings, the use of FDA-approved materials in their fabrication is recommended. By modifying nanoparticles with targeting ligands and molecules against specific receptors that are overexpressed in cancer cells, the efficiency of siRNA/cancer therapeutic co-delivery can be significantly enhanced. These strategies may also increase the potential of siRNA/drug combination delivery systems in overcoming drug resistance.
Acknowledgements
The authors thank Ms. Kathy Kyler at the office of Vice President for Research, OUHSC, for editorial assistance. Rajagopal Ramesh is an Oklahoma TSET Research Scholar and holds the Jim and Christy Everest Endowed Chair in Cancer Developmental Therapeutics.
Disclosure statement
The authors declare no competing financial interests.
Additional information
Funding
References
- Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol 2013;31:1592–605.
- Tong R, Kohane DS. New strategies in cancer nanomedicine. Annu Rev Pharmacol Toxicol 2016;56:41–57.
- Mansoori B, Shotorbani SS, Baradaran B. RNA interference and its role in cancer therapy. Adv Pharm Bull 2014;4:313–21.
- Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget 2015;6:24560–70.
- Ganesh S, Iyer AK, Weiler J, et al. Combination of siRNA-directed gene silencing with cisplatin reverses drug resistance in human non-small cell lung cancer. Mol Ther Nucleic Acids 2013;2:e110.
- Kanasty R, Dorkin JR, Vegas A, et al. Delivery materials for siRNA therapeutics. Nat Mater 2013;12:967–77.
- Vorhies JS, Nemunaitis J. Nonviral delivery vehicles for use in short hairpin RNA-based cancer therapies. Expert Rev Anticancer Ther 2007;7:373–82.
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet 2003;4:346–58.
- Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet 2014;15:541–55.
- Young SW, Stenzel M, Yang JL. Nanoparticle-siRNA: a potential cancer therapy? Crit Rev Oncol Hematol 2016; 98:159–69.
- Williford JM, Wu J, Ren Y, et al. Recent advances in nanoparticle-mediated siRNA delivery. Annu Rev Biomed Eng 2014;16:347–70.
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov 2009;8:129–38.
- Esmaeili MA. Combination of siRNA-directed gene silencing with epigallocatechin-3-gallate (EGCG) reverses drug resistance in human breast cancer cells. J Chem Biol 2015;9:41–52.
- Dong H, Yao L, Bi W, et al. Combination of survivin siRNA with neoadjuvant chemotherapy enhances apoptosis and reverses drug resistance in breast cancer MCF-7 cells. J Cancer Res Ther 2015;11:717–22.
- Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano 2013;7:994–1005.
- Wu Y, Zhang Y, Zhang W, et al. Reversing of multidrug resistance breast cancer by co-delivery of P-gp siRNA and doxorubicin via folic acid-modified core-shell nanomicelles. Colloids Surf B Biointerfaces 2016;138:60–9.
- Komarova NL, Boland CR. Cancer: calculated treatment. Nature 2013;499:291–2.
- Xiao B, Ma L, Merlin D. Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy. Expert Opin Drug Deliv 2017;14:65–73.
- Saraswathy M, Gong S. Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment. Mater Today 2014;17:298–306.
- Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 2010;1805:105.
- Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol 2010;596:47–76.
- Krishna R, Mayer LD. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur J Pharm Sci 2000;11:265–83.
- Pérez-Tomás R. Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem 2006;13:1859–76.
- Babu A, Wang Q, Muralidharan R, et al. Chitosan coated polylactic acid nanoparticle-mediated combinatorial delivery of cisplatin and siRNA/plasmid DNA chemosensitizes cisplatin-resistant human ovarian cancer cells. Mol Pharm 2014;11:2720–33.
- Yang H, Ding R, Tong Z, et al. siRNA targeting of MDR1 reverses multidrug resistance in a nude mouse model of doxorubicin-resistant human hepatocellular carcinoma. Anticancer Res 2016;36:2675–82.
- Nascimento AV, Singh A, Bousbaa H, et al. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater 2017;47:71–80.
- Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011;61:250–81.
- Chen WH, Lecaros RL, Tseng YC, et al. Nanoparticle delivery of HIF1α siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett 2015;359:65–74.
- Wang X, Liu K, Yang G, et al. Near-infrared light triggered photodynamic therapy in combination with gene therapy using upconversion nanoparticles for effective cancer cell killing. Nanoscale 2014;6:9198–205.
- Xue LY, Chiu SM, Oleinick NL. Atg7 deficiency increases resistance of MCF-7 human breast cancer cells to photodynamic therapy. Autophagy 2010;6:248–55.
- Kim J, Lim H, Kim S, et al. Effects of HSP27 downregulation on PDT resistance through PDT-induced autophagy in head and neck cancer cells. Oncol Rep 2016;35:2237–45.
- Mao CP, Hung CF, Wu TC, et al. Immunotherapeutic strategies employing RNA interference technology for the control of cancers. J Biomed Sci 2007;14:15–29.
- Ghafouri-Fard S, Ghafouri-Fard S. siRNA and cancer immunotherapy. Immunotherapy 2012;4:907–17.
- Naidu MD, Mason JM, Pica RV, et al. Radiation resistance in glioma cells determined by DNA damage repair activity of Ape1/Ref-1. J Radiat Res 2010; 51:393–404.
- Higgins GS, Prevo R, Lee YF, et al. A siRNA screen of genes involved in DNA repair identifies tumour specific radiosensitisation by POLQ knockdown. Cancer Res 2010;70:2984–93.
- Mehta M, Basalingappa K, Griffith JN, et al. HuR silencing elicits oxidative stress and DNA damage and sensitizes human triple-negative breast cancer cells to radiotherapy. Oncotarget 2016;7:64820–35.
- Jin H, Gao S, Guo H, et al. Re-sensitization of radiation resistant colorectal cancer cells to radiation through inhibition of AMPK pathway. Oncol Lett 2016;11:3197–201.
- Gong X, Li X, Zhou C. 81P radiation resistance induced immunity evasion by evoking PD-L1 expression. J Thorac Oncol 2016;11:S57–S166.
- Retif P, Pinel S, Toussaint M, et al. Nanoparticles for radiation therapy enhancement: the key parameters. Theranostics 2015;5:1030–44.
- Dexter DL, Leith JT. Tumor heterogeneity and drug resistance. J Clin Oncol 1986;4:244–57.
- Tekedereli I, Alpay SN, Akar U, et al. Therapeutic silencing of Bcl-2 by systemically administered siRNA nanotherapeutics inhibits tumor growth by autophagy and apoptosis and enhances the efficacy of chemotherapy in orthotopic xenograft models of ER (−) and ER (+) breast cancer. Mol Ther Nucleic Acids 2013;2:e121.
- Shen Y, Wang J, Li Y, et al. Co-delivery of siRNA and paclitaxel into cancer cells by hyaluronic acid modified redox-sensitive disulfide-crosslinked PLGA–PEI nanoparticles. RSC Adv 2015;5:46464–79.
- Creixell M, Peppas NA. Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance. Nano Today 2012;7:367–79.
- Godsey ME, Suryaprakash S, Leong KW. Materials innovation for co-delivery of diverse therapeutic cargos. RSC Adv 2013;3:24794–811.
- Kang L, Gao Z, Huang W, et al. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B 2015;5:169–75.
- Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine 2015;10:975–99.
- Shim G, Kim MG, Park JY, et al. Application of cationic liposomes for delivery of nucleic acids. J Pharm Sci 2013;8:72–80.
- Bender HR, Kane S, Zabel MD. Delivery of therapeutic siRNA to the CNS using cationic and anionic liposomes. J Vis Exp 2016;113:e54106. doi: 10.3791/54106
- Ko YT, Bickel U. Liposome-encapsulated polyethylenimine/oligonucleotide polyplexes prepared by reverse-phase evaporation technique. AAPS PharmSciTech 2012;13:373–8.
- Zhang Y, Peng L, Mumper RJ, et al. Combinational delivery of c-Myc siRNA and nucleoside analogues in a single, synthetic nanocarrier for targeted cancer therapy. Biomaterials 2013;34:8459–68.
- Qu MH, Zeng RF, Fang S, et al. Liposome-based co-delivery of siRNA and docetaxel for the synergistic treatment of lung cancer. Int J Pharm 2014;474:112–22.
- Peng Z, Wang C, Fang E, et al. Co-delivery of doxorubicin and SATB1 shRNA by thermosensitive magnetic cationic liposomes for gastric cancer therapy. PLoS One 2014;9:e92924. doi: 10.1371/journal.pone.0092924
- Yao Y, Su Z, Liang Y, et al. pH-Sensitive carboxymethyl chitosan-modified cationic liposomes for sorafenib and siRNA co-delivery. Int J Nanomedicine 2015;10:6185–97.
- Oh HR, Jo HY, Park JS, et al. Galactosylated liposomes for targeted co-delivery of doxorubicin/vimentin siRNA to hepatocellular carcinoma. Nanomaterials 2016;6:141.
- Yang T, Li B, Qi S, et al. Co-delivery of doxorubicin and Bmi1 siRNA by folate receptor targeted liposomes exhibits enhanced anti-tumor effects in vitro and in vivo. Theranostics 2014;4:1096–11.
- Yang ZZ, Li JQ, Wang ZZ, et al. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials 2014;35:5226–39.
- Lecaros RLG, Huang L, Lee TC, et al. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol Ther 2016;24:106–16.
- Kang SH, Cho HJ, Shim G, et al. Cationic liposomal co-delivery of small interfering RNA and a MEK inhibitor for enhanced anticancer efficacy. Pharm Res 2011;28:3069–78.
- Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond) 2008;3:761–76.
- Ha J, Cho SK, Park ES, et al. Enhanced stability of hydroxyapatite-coated liposomes for ultrasound-triggered drug release. Bull Korean Chem Soc 2015;36:83–7.
- Sévin DC, Sauer U. Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nat Chem Biol 2014;10:266–72.
- Hernández AV, Eriksson EK, Edwards K. Ubiquinone-10 alters mechanical properties and increases stability of phospholipid membranes. Biochim Biophys Acta 2015;1848:2233–43.
- Kang SN, Honga S-S, Kima S-Y, et al. Enhancement of liposomal stability and cellular drug uptake by incorporating tributyrin into celecoxib-loaded liposomes. Asian J Pharm Sci 2013;8:128–33.
- Pawar S, Shevalkar G, Vavia P. Glucosamine-anchored doxorubicin-loaded targeted nano-niosomes: pharmacokinetic, toxicity and pharmacodynamic evaluation. J Drug Target 2016;8:730–43.
- Sharma V, Anandhakumar S, Sasidharan M. Self-degrading niosomes for encapsulation of hydrophilic and hydrophobic drugs: an efficient carrier for cancer multi-drug delivery. Mater Sci Eng C Mater Biol Appl 2015;56:393–400.
- Namdeo A, Jain NK. Niosomal delivery of 5-fluorouracil. J Microencapsul 1999;16:731–40.
- Uchegbu IF, Double JA, Turton JA, et al. Distribution, metabolism and tumoricidal activity of doxorubicin administered in sorbitan monostearate (Span 60) niosomes in the mouse. Pharm Res 1995;12:1019–24.
- Uchegbu IF, Double JA, Kelland LR, et al. The activity of doxorubicin niosomes against an ovarian cancer cell line and three in vivo mouse tumour models. J Drug Target 1996;3:399–409.
- Huang Y, Chen J, Chen X, et al. PEGylated synthetic surfactant vesicles (Niosomes): novel carriers for oligonucleotides. J Mater Sci Mater Med 2008;19:607–14.
- Tavano L, Muzzalupo R, Mauro L, et al. Transferrin-conjugated pluronic niosomes as a new drug delivery system for anticancer therapy. Langmuir 2013;29:12638–46.
- Grijalvo S, Alagia A, Puras G, et al. Cationic vesicles based on non-ionic surfactant and synthetic aminolipids mediate delivery of antisense oligonucleotides into mammalian cells. Colloids Surf B Biointerfaces 2014;119:30–7.
- Rajput S, Puvvada N, Kumar BN, et al. Overcoming Akt induced therapeutic resistance in breast cancer through siRNA and thymoquinone encapsulated multilamellar gold niosomes. Mol Pharm 2015;12:4214–25.
- Tamura A, Nagasak Y. Smart siRNA delivery systems based on polymeric nanoassemblies and nanoparticles. Nanomedicine (Lond) 2010;5:1089–102.
- Shroff K, Vidyasagar A. Polymer nanoparticles: newer strategies towards targeted cancer therapy. J Phys Chem Biophys 2013;3:125.
- Zhang CG, Zhu WJ, Liu Y, et al. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci Rep 2016;6:23859.
- Hao S, Yan Y, Ren X, et al. Candesartan-graft-polyethyleneimine cationic micelles for effective co-delivery of drug and gene in anti-angiogenic lung cancer therapy. Biotechnol Bioprocess Eng 2015;20:550–60.
- Fu Y, Kao WJ. Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert Opin Drug Deliv 2010;7:429–44.
- Su WP, Cheng FY, Shieh DB, et al. PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomedicine 2012;7:4269–83.
- Patil YB, Swaminathan SK, Sadhukha T, et al. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials 2010;31:358–65.
- Wei W, Lv PP, Chen XM. Codelivery of mTERT siRNA and paclitaxel by chitosan-based nanoparticles promoted synergistic tumor suppression. Biomaterials 2013;34:3912–23.
- Alinejad V, Somi MH, Baradaran B, et al. Co-delivery of IL17RB siRNA and doxorubicin by chitosan-based nanoparticles for enhanced anticancer efficacy in breast cancer cells. Biomed Pharmacother 2016;83:229–40.
- Xu C, Tian H, Sun H, et al. A pH sensitive co-delivery system of siRNA and doxorubicin for pulmonary administration to B16F10 metastatic lung cancer. RSC Adv 2015;5:103380–5.
- Zhu C, Jung S, Luo S, et al. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials 2010;31:2408–16.
- Xu M, Qian J, Suo A, et al. Co-delivery of doxorubicin and P-glycoprotein siRNA by multifunctional triblock copolymers for enhanced anticancer efficacy in breast cancer cells. J Mater Chem B 2015;3:2215–28.
- Biswas S, Deshpande PP, Navarro G, et al. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials 2013;34:1289–301.
- Jiang Y, Huo S, Hardie J, et al. Progress and perspective of inorganic nanoparticle-based siRNA delivery systems. Expert Opin Drug Deliv 2016;13:547–59.
- Bishop CJ, Tzeng SY, Green JJ. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater 2015;11:393–403.
- Ding Y, Jiang Z, Saha K, et al. Gold nanoparticles for nucleic acid delivery. Mol Ther 2014;22:1075–83.
- Guo S, Huang Y, Jiang Q, et al. Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte. ACS Nano 2010;4:5505–11.
- Xiao Y, Jaskula-Sztul R, Javadi A, et al. Co-delivery of doxorubicin and siRNA using octreotide-conjugated gold nanorods for targeted neuroendocrine cancer therapy. Nanoscale 2012;4:7185–93.
- Esteban-Fernández AB, Angell C, Soto F, et al. Acoustically propelled nanomotors for intracellular siRNA delivery. ACS Nano 2016;10:4997–5005.
- Meng H, Liong M, Xia T, et al. Engineered design of mesoporous silica nanoparticles to deliver doxorubicin and P-glycoprotein siRNA to overcome drug resistance in a cancer cell line. ACS Nano 2010;4:4539–50.
- Hanafi-Bojd MY, Jaafari MR, Ramezanian N, et al. Co-delivery of epirubicin and siRNA using functionalized mesoporous silica nanoparticles enhances in vitro and in vivo drug efficacy. Curr Drug Deliv 2015;13:1176–82.
- Ma X, Zhao Y, Ng KW, et al. Integrated hollow mesoporous silica nanoparticles for target drug/siRNA co-delivery. Chemistry 2013;19:15593–603.
- Lee MS, Lee JE, Byun E, et al. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J Control Release 2014;192:122–30.
- Lee K, Oh MH, Lee MS, et al. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. Int J Pharm 2013;445:196–202.
- Cheng X, Kuhn L. Chemotherapy drug delivery from calcium phosphate nanoparticles. Int J Nanomedicine 2007;2:667–74.
- Jun W, Lin L, Yurong C, et al. Recent advances of calcium phosphate nanoparticles for controlled drug delivery. Mini Rev Med Chem 2013;13:1501–7.
- Zhang Y, Schwerbrock NM, Rogers AB, et al. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol Ther 2013;21:1559–69.
- Varkouhi AK, Foillard S, Lammers T, et al. SiRNA delivery with functionalized carbon nanotubes. Int J Pharm 2011;416:419–25.
- Siu KS, Zhang Y, Zheng X, et al. Non-covalently functionalized of single-walled carbon nanotubes by DSPE-PEG-PEI for SiRNA delivery. Methods Mol Biol 2016;1364:151–63.
- Pereira S, Lee J, Rubio N, et al. Cationic liposome-multi-walled carbon nanotubes hybrids for dual siPLK1 and doxorubicin delivery in vitro. Pharm Res 2015;32:3293–308.
- Kumar V, Mondal G, Slavik P, et al. Codelivery of small molecule hedgehog inhibitor and miRNA for treating pancreatic cancer. Mol Pharm 2015;12:1289–98.
- Chen Y, Zhu X, Zhang X, et al. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther 2010;18:1650–6.
- Ma X, Nguyen KT, Borah P, et al. Functional silica nanoparticles for redox-triggered drug/ssDNA co-delivery. Adv Healthc Mater 2012;1:690–7.
- Ren Y, Wang R, Gao L, et al. Sequential co-delivery of miR-21 inhibitor followed by burst release doxorubicin using NIR-responsive hollow gold nanoparticle to enhance anticancer efficacy. J Control Release 2016;28:74–86.
- Wang S, Zhang J, Wang Y, et al. Hyaluronic acid-coated PEI-PLGA nanoparticles mediated co-delivery of doxorubicin and miR-542-3p for triple negative breast cancer therapy. Nanomedicine 2016;12:411–20.
- Han Y, Zhang P, Chen Y, et al. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int J Mol Med 2014;34:191–6.
- Wang Y, Gao S, Ye WH, et al. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater 2006;5:791–6.
- Liang P, Wang CQ, Chen H, et al. Multi-functional heparin–biotin/heparin/calcium carbonate/calcium phosphate nanoparticles for targeted co-delivery of gene and drug. Polym Int 2015;64:647–53.
- Zhou Z, Kennella C, Lee JY, et al. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine 2017;2:403–10.
- Zhao D, Liu CJ, Zhuo RX, et al. Alginate/CaCO3 hybrid nanoparticles for efficient codelivery of antitumor gene and drug. Mol Pharm 2012;9:2887–93.
