Abstract
We investigated the reproductive strategy developed by the non-native fish Metynnis maculatus (Kner, 1858) to explain its success in establishing in a tropical reservoir in south-eastern Brazil. A total of 198 specimens were examined, 100 males (total length, TL = 110–172 mm) and 98 females (TL = 110–191 mm), collected using gill nets from September 2005 to August 2006. Sex ratio is balanced, although females significantly outnumber males in size larger than 160 mm TL. Six stages of oogenesis and five of spermatogenesis are described, based on staining aspect of the nucleus and cytoplasm structure with photonic microscopy. A protracted reproductive period from August to January was found with females producing several large clutches of small eggs. Oocyte mean diameter ranged from 19.6–48.3 μm in pre-vitellogenic ovarian stage to 83.4–149.4 μm in vitellogenic ovarian stage whereas in spawning stage ovary, oocyte diameter varied from 300 to 500 μm. In ripe stage, oocytes can reach diameter up to 2.00 mm. Batch fecundity ranged from 670 to 16,333 oocytes (mean fecundity = 7056 oocytes) for females having TL ranging from 145 to 191 mm and total weight ranging from 75 to 176 g. A tendency for opportunistic strategy was detected as indicated by a long spawning period, high fecundity, small oocytes and small body size that have proved to be effective in the Lajes reservoir.
Introduction
The knowledge of the reproductive strategy such as sex ratio, gonadal development, breeding season and fecundity is essential to obtain a comprehensive understanding of the population dynamics of a given species (Winemiller Citation1989; Lassala and Renesto Citation2007; Tamada Citation2009). Fish dynamically adjust their investment into reproduction according to the number of offspring in their brood, past investment, genetic relatedness and alternative mating opportunities, all of which affect the value of current offspring relative to potential future offspring (Shatunovskii et al. Citation1996; Gross Citation2005; Hardie et al. Citation2007). According to Winemiller (Citation1989), the ability to colonize in the face of unpredictable environmental condition is the major adaptative feature of the opportunist strategy.
Fish spawning time varies little from year to year (Vazzoler and Menezes Citation1992; Humphries Citation1995; Bailly et al. Citation2008). This means that the events of the reproductive cycle are locked to, and synchronized with the environmental changes that occur on a seasonal basis. Spawning in multiple batches is a mechanism that increases the reproductive effort and is not only the distribution throughout the reproductive period (Burt et al. Citation1988). Opportunistic species usually spawn many times during the course of a prolonged breeding season. Thus, batch spawning can be seen as a strategy to release eggs over a long period of time increasing the survival probability of offspring (Lambert and Ware Citation1984).
The spotted metynnis Metynnis maculatus (Kner, 1858) is a neotropical fish species from Characidae family (subfamily Serrasalminae) native to Pantanal Matogrossense, a large swamp in Central South-America (Zarske and Géry Citation1999). It is becoming widely spread in Brazilian rivers and reservoirs where it has been introduced for sport fishing purposes. Information on its life cycle and ecology is scarce. It was introduced one decade ago in the Lajes reservoir, a well preserved 30 km2 impoundment area in south-eastern Brazil, located in the upper slopes of Sea Mountains (southeast Brazilian Coast) and became common in this oligotrophic reservoir. Metynnis maculatus ranks among the most abundant species in the Lajes reservoir (Santos et al. Citation2008), suggesting that its reproductive strategy is effective in this reservoir constructed for hydroelectric purpose.
It is believed that M. maculatus has several attributes, such as rapid colonizing ability, early maturation, continuous reproduction and small eggs, which appear to correspond to the opportunist strategy sensu Winemiller (Citation1989). This study aims to test the hypothesis that M. maculatus exhibits opportunistic strategy, and to describe some tactics the species uses to succeed in the Lajes reservoir. To reach this goal, we have described the gametogenesis and characterized the germinative cells, determined the gonadal maturation stages and related some biological indexes (gonadosomatic, hepatosomatic and condition factor) with the spawning season and environmental factors.
Material and methods
Study area
The Lajes reservoir (22°42′–22°50′S; 43°53′–44°05′W) is a major impoundment in Rio de Janeiro State, located 415 m above mean sea level in the upper slopes of the Sea Mountains in south-eastern Brazil. It was built between 1905 and 1908 for hydroelectric purposes and has an area of 30 km2. This oligrotrophic system is remarkably well preserved (Soares et al. Citation2008) and has low concentrations of nitrogen ( < 10 μg l− 1), phosphate ( < 120 μg l− 1) and chlorophyll α ( < 2.5 μg l− 1). The water residence time (286 days) is high because only small streams drain into the reservoir (Santos et al. Citation2004). The study area has two distinct seasons: a wet season, from late spring to early autumn and a dry season, from late autumn to spring (Barbieri and Kronemberg Citation1994). Surface water temperature ranges from 15.3 to 30.6°C, pH between 6 and 8, dissolved oxygen is higher than 4.7 mg l− 1 and Secchi transparency averages 2.30 m (Araújo and Santos Citation2001). Fluctuation in rainfall combined with a regulated outflow results in peaks in water level (May–June) 1 or 2 months after the rainfall season, with differences among extremes of flood and drawdown events reaching up to 12 m (Santos et al. Citation2004).
Data collection and analysis
A total of 198 specimens (98 females and 100 males) were examined. Fish were collected monthly from September 2005 to August 2006 using gill nets (30 × 4 m, 3–12 cm stretched mesh size). All individuals were sexed and total body length (TBL ± 0.1 mm SE) and total body weight (TBW ± 0.1 g SE) were recorded. A Chi-square (χ2) test was used to assess differences in size structure between sexes and size classes.
Gonads were assigned to developmental stages, based on form, size, weight, colour and irrigation degree, and were ultimately classified as immature (juveniles), maturing, ripe, spawn/spent and resting. The diameter of oocytes was measured in a stereomicroscope using an ocular micrometer.
A cross-section from the gonads fixed in Bouin's solution was dehydrated in ascending solutions of alcohol and embedded in paraffin, and then 5 μm sections were cut using a microtome and followed by staining with haematoxylin and eosin. Oocytes were classified according to their morphology, their affinity for the dyes used and the presence of specific inclusions (lipid droplets, yolk granules and cortical alveoli). In five mature females from the monthly sample, the diameters of 30 oocytes randomly chosen among the largest were measured to the nearest 0.0001 mm. Identification of the ovarian and testicular stages was determined according to germinative cellular types for females (i.e. presence/absence of different types of oocytes; whether organized by ovarian lamellae or not) and males (presence/absence of seminiferous tubules, presence/absence of spermatozoa).
The annual gonadal cycle was determined by the variations of the gonadosomatic index (GSI) = 100 (GW × EBW− 1), where GW is total gonad weight and EBW is the eviscerated body weight. Condition factor (K) and hepatosomatic index (HSI) were calculated as indirect indices of energy status. The condition factor was calculated using a Fulton-type equation, K = 100 (EBW × TBL− 3). The HSI was calculated according to the following equation, HSI = 100 (LW × EBW− 1), where LW is the weight of the liver.
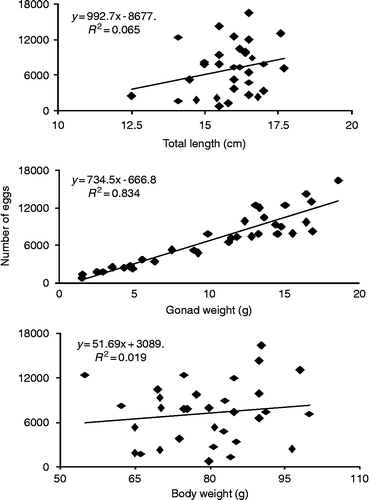
To estimate batch fecundity, a subsample was taken from random samples in mature ovaries, and advanced vitellogenic oocytes were counted. It was calculated as: F = (N × TGW) × SGW− 1, where F = fecundity, N = number of vitellogenic oocytes, TGW = total gonad weight and SGW = subsample gonadal weight. The relationship between fecundity versus eviscerated weight, gonad weight and TL was estimated by simple linear regression. Relationship between condition factor, GSI and HSI, was also assessed. Condition factor, gonad weight and liver weight means for each sex were compared among months by using one-way analysis of variance (ANOVA) and a post-hoc Tukey test (P < 0.05).
Results
Sex ratio and morphology of gonads
Size of M. maculatus ranged from 110 to 170 mm TL for males and from 110 to 191 mm TL for females. Males significantly outnumbered females in size class 140–150 mm TL (P < 0.05). Highly significant differences (P < 0.01) were found for larger sizes (TL>160 mm), where the females outnumbered males (Table ).
Table 1 χ2 Test for sex ratio comparisons by size classes of M. maculatus in the Lajes reservoir, 2005/06.
The ovaries are paired structures of different sizes, laterally compressed, located in the upper part of the coelomic cavity. The oviducts lead to a common orifice near to the anus. The left ovary is about two times larger than the right ovary in advanced stages. The gonads are separated by connective tissue located ventrally to the kidneys and swim bladder. Eggs were adhesive and agglutinated to each other forming a mass. The pair of testes is elongated, reaching the spermatic duct and have no morphological differences between each other. Metynnis maculatus also has an external sexual dimorphism with males showing a sinuous contour of the anal fin, whereas the females show a uniform contour.
Gonads development
Ovaries
Immature. Filiforms, slender, occupying less than one-third of the coelomic cavity, translucent with oocytes not visible to the naked eye.
Maturing. Lobular section, oocytes visible to the naked eye. Signs of enlargement take up half of coelomic cavity. Oocytes of pale cream colour in initial stages with mean diameter of 0.016 ± 0.004 mm. Oocytes in advanced stages with whitish-yellow colour, subtle granulation and mean diameter of 0.15 ± 0.08 mm.
Ripe. Voluminous with developed blood vessels. Ovaries occupy the entire coelomic cavity, with most of the oocytes visible to the naked eye. Mean yellowish oocyte diameters varied from 0.2 ( ± 0.08) to 2.0 ( ± 0.5) mm.
Spawn. Flaccid, wrinkled, occupying nearly half of the coelomic cavity. Darker colour than the anterior stage due to the haemorrhagic appearance. Heterogeneous oocytes, some residual or in degeneration. Residual oocyte diameters varied from 300 to 500 μm.
Testes
Immature. Filiforms, occupying less than one-third of the coelomic cavity, translucid.
Maturing. Larger than the anterior stage. Visible enlargement, occupying about one-third of the coelomic cavity. Milky aspect.
Ripe. Larger in length and width than the anterior stage, occupying two-thirds of the coelomic cavity, with creamy colouration. Release spermatic liquid when manipulated.
Spent. Flaccid and wrinkled occupying one-third of the coelomic cavity. Colouration – whitish.
Oocyte development
A description of the different stages of development of the oocytes follows (Figure ) the terminology proposed by Nuñez & Duponchelle (Citation2009):
Figure 1 Transversal section of ovarian tissue. (A) o, oogonia; eps, early perinucleolar stage; cn, chromatin nucleolar stage; (B) eps, early perinucleolar stage; yn, yolk nucleus; lps, late peri-nucleolar stage; n, nucleus; y, cortical alveoli formation; no, nucleoli; yn, yolk nucleus; l, lipid droplets; (C) v, vitellogenesis stage; n, nucleus; l, lipid droplets; (D) v, vitellogenesis stage; zr, zona radiata; l, lipid droplets; yg, yolk granules; (E) r, ripe stage; zr, zona radiata; (F) r, ripe stage; yg, yolk granules; pf, postovulatory follicles. Haematoxylin and eosin stain.
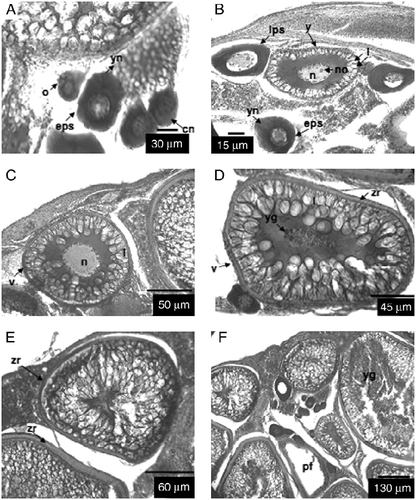
Oogonia. Spherical to slightly oval in shape. Diameter averaging 19.6 μm (range 16–34 μm). Cytoplasm lightly dyed. Very large nucleus (mean diameter = 14.2 μm, range 11–20 μm) with single very prominent nucleoli. Oogonia located on the periphery of the ovarian lamellae, isolated or forming cysts.
Chromatin nucleolar stage. Similar to oogonia, although somewhat larger (mean diameter = 27.9 μm, range 21–36 μm). Large nucleus (mean diameter = 14.4 μm, range 12–22 μm), with single nucleolus. Reduced cytoplasm, with little or no affinity with dyes used.
Perinucleolar stage. In the early stage, size increases (mean diameter = 33.3 μm, range 25–40 μm). Cytoplasm with strong affinity for haematoxylin. Nucleus evident (mean diameter = 14.7 μm, range 13–22 μm), with multiple nucleoli, generally peripheral, next to nuclear envelope. Yolk nucleus or Balbiani body (Wallace and Selman Citation1981) present in cytoplasm. Follicular layer present but difficult to observe. Late stage exhibits rapid growth (mean diameter = 48.3 μm, range 41–60 μm) and nucleus averages 24.0 μm (range 19–27 μm). There is progressive loss of affinity for haematoxylin. Disintegration of Balbiani body. Follicle or follicular layer is easier to observe, and consists of internal layer (granulosa layer) and an outer layer (theca) (Hunter and Macewicz Citation1985).
Cortical alveoli formation. Yolk vesicles present in cytoplasm. Vesicles increase progressively in both number and size. Mean diameter of oocyte is 83.4 μm (range 59–103 μm) and of nucleus is 29.4 μm (range 21–40 μm). Progressive loss of affinity by cytoplasm for haematoxylin. Follicular layer and zona radiata are visible, although zona radiata is not yet stained by eosin. Accumulation of lipid inclusions in cytoplasm has begun.
Vitellogenesis. In early stage, yolk granules containing yolk vesicles are present. Mean diameter of oocyte is 93.2 μm (range 72–195 μm) and of nucleus is 30.9 μm (range 25–48 μm). Yolk vesicles increase in size while the yolk granules grow. Zona radiata is stained with eosin. In late stage, perpendicular striations are apparent in zona radiata. Mean diameter of oocyte is 122.4 μm (range 104–202 μm) and the nucleus is often not visible because of its migration to the animal pole.
Ripe. Nucleus not visible. Mean diameter of oocyte is 979 μm (range 200–2000 μm).
Resting. The structure of the follicle is recognizable by its disorganized structure and abundant vacuoles.
Stages of espermatogenesis development
The spermatogenic cells appear in the interior of the seminiferous tubules at different stages during spermatogenesis (spermatogonia, primary and secondary spermatocytes, spermatids and spermatozoa), forming cysts (Figure (a)). Each cyst is bound by a layer of connective tissue and contains cells at the same stage of development. In mature testes, the seminiferous tubules are filled with spermatozoa.
Figure 2 Tubules containing stages of spermatogenesis. (A) s, spermatogonia; ps, primary spermatocytes; ss, secondary spermatocytes; st, spermatids; sz, spermatozoa; ta, tunica albuginea; cy, cysts; (B) ps, primary spermatocytes; ss, secondary spermatocytes; Haematoxylin and eosin stain.
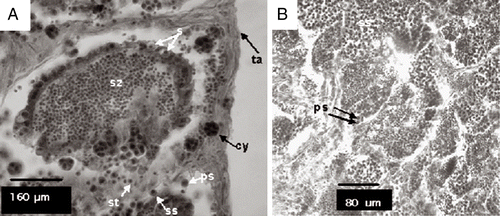
Spermatogonia. They could occur in isolate or in groups inside the cysts in the seminiferous tubules. They are spherical in form and do not hold colour well when colouration techniques are applied. The appearance of spermatogonia is generally associated with tunica albuginea (Figure ).
Primary spermatocytes. Smaller than spermatogonias, from which they originate.
Secondary spermatocytes. Present little morphologic variation and are, therefore, slightly different from primary spermatocytes. They are somewhat smaller than primary spermatocytes.
Spermatids. Occur in cysts and in the interior region of the seminiferous tubules. Nucleus is denser and has more uniform chromatin.
Spermatozoa. They occur in the interior region of the seminiferous tubules and sperm duct.
Spawning season
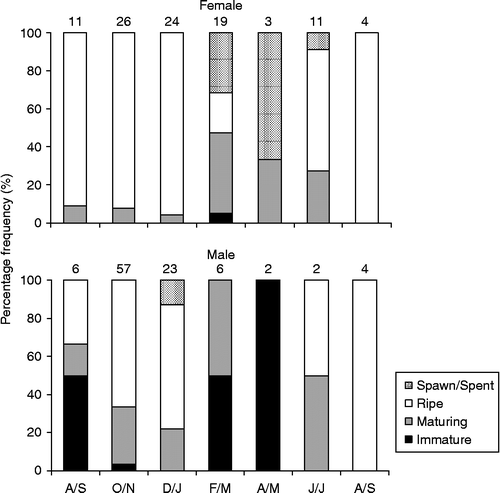
The percent of ovaries in the ripe stage was between 20% and 100% in all months, with exception of April/May when no ripe ovary was found, with the highest proportion being recorded between August and January (Figure ). On the other hand, the percent of testes in the ripe stage was between 33% and 100% during most periods of the year, with the exception of February to May when no fish were recorded as ripe (Figure ).
Figure 3 Temporal variation of ovarian (top) and testes (bottom) stages of M. maculatus in the Lajes reservoir. Number of examined individuals is indicated above the bars. A/S, August/September; O/N, October/November; D/J, December/January; F/M, February/March; A/M, April/May; J/J, June/July.
The highest GSI values for females (F = 12.06, P = 0.0001) were found between August and January, peaking during December/January (Figure ; Table ). A clear seasonal variation in GSI was not detected for males, probably because of the small sample size. The highest average temperature (Figure ) was recorded during December/March (overall average = 27°C) and the lowest during June/July (20°C). Rainfall was the highest between December and March and the lowest between June and September. The highest accumulated rainfall occurred during February/March (around 150 mm) and the lowest during June/July (around 100 mm).
Figure 4 Temporal variation in GSI of M. maculatus (top) and in water level (plus 400 m above sea level), temperature (°C, means+SE) and accumulated rainfall (mm) (bottom) in the Lajes reservoir, from August 2005 to September 2006. Codes of months and number of examined individuals according to Figure 3.
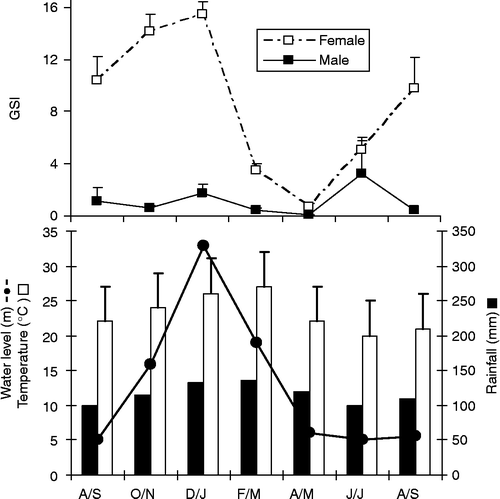
Table 2 F-Values from ANOVA for monthly comparisons of GSI, HSI and K for males and females M. maculatus in the Lajes reservoir.
The values of the condition factor (K) changed significantly for both sexes, with males having higher values during April/May than during the remaining months (F = 4.8, P = 0.0001). Females had the highest K values during February/March and lowest K values during June/July (F = 2.62, P = 0.02*) (Table ; Figure ).
Figure 5 Temporal variation in condition factor (K) and hepatossomatic index (HSI) for both sexes of M. maculatus in the Lajes reservoir. Codes of months and number of examined individuals according to Figure 3.
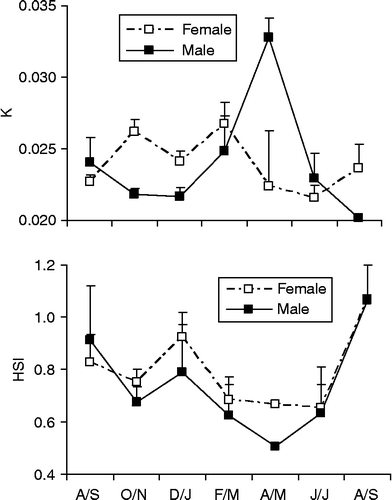
The HSI showed a similar pattern for both sexes with higher values being found during August/September, then decreasing to the lowest values during April/May. However, no significant differences in HSI were found among the studied period for both sexes (Table ; Figure ). No significant correlation was found between any pair of combination of GSI, HSI and K for both sexes.
Fecundity
Mean batch fecundity is 7056 oocytes ± 718 SE (relative fecundity = 92 ocytes g− 1) in the 33 examined females. The batch fecundity ranged from 670 to 16,333 oocytes for females having TL ranging from 145 to 191 mm and total weight ranging from 75 to 176 g. Fecundity tended to increase linearly with TL, total weight and gonad weight, but the only significant relationship (r 2 = 0.834; P < 0.01) was found between the batch fecundity and gonad weight (Figure ). The equation that relates these parameters is Fecundity = − 667+734.5 × gonad weight.
Discussion
The populations of M. maculatus showed a balanced sex ratio in the Lajes reservoir, although in size larger than 160 mm TL females outnumbered males, an indication that females reach larger sizes than males. Such differences have been attributed to differences between-sex growth rate, behaviour and mortality (Lambert and Ware Citation1984; Gross Citation2005). Such a strategy is mainly associated with selection to reach higher fecundity (Nikolsky Citation1963; Gross and Sargent Citation1985) since large females have a larger peritoneal cavity and, thus, can lay a larger number of eggs. Although fertility and the number of fertilized eggs increase with female body size, a general pattern in teleosts (Gross and Sargent Citation1985), we did not detect significant relationship between fecundity and female body size in this study. In addition, smaller male size may in part be a consequence of selection for early male maturation and reproductive effort, which reduces male growth compared to that of females (Andersson Citation1994).
Metynnis maculatus has adhesive eggs and like other Serrasalminae, the eggs are laid on littoral zones of the reservoir (Guimarães and Quagio-Grassiotto Citation2001; Quagio-Grassiotto and Guimarães Citation2003). Whyte (Citation1975) reported that most fishes in lakes need a firm substrate to spawn on, and that none of the fishes are completely independent of the shallows. In the Lajes reservoir, annual water level fluctuations averaged nearly 3.0 m between high and low water, but such difference can reach up to 12.0 m in years of extreme variation. High water levels have been associated with high food supply and shelter availability, favouring early life stages development, whereas low water levels are associated with eggs and larvae exposure triggering intense mortalities (Maceina and Stimpert Citation1998; Sammons et al. Citation1999).
The production of small eggs by M. maculatus (diameter = 200–2000 μm) from the Lajes reservoirs compared with other Serrassalminae species, such as Pygocentrus nattereri Kner, 1858, 1500–2300 μm (Duponchelle et al. Citation2007), could be a mechanism to take advantage of more suitable environmental conditions to spawn a large number of eggs, thus increasing the chance of offspring survival, since most of the spawnings occur during the rainy season, when a larger amount of allochthonous material is brought into the reservoir by runoff. The high water transparency of the Lajes reservoir enables increased primary productivity during the raining season (Soares et al. Citation2008). According to Duarte and Alcaraz (Citation1989) the reproductive success in fish should be more dependent on the survival of the individual larvae. Since the Lajes reservoir seems to offer favourable environmental conditions during the spawning period, the production of a large number of small eggs should be more advantageous than to produce a smaller number of large eggs.
The breeding season spanned between August and January as indicated by the trend shown in the GSI variation and the proportion of mature stages. The high percentage of spent ovaries observed during February–May indicates that most of the females have concluded their reproductive cycle between late summer and early autumn. The reproductive period, from August to January, seems to be more associated with the highest water level, increasing temperature and rainfall. In the Lajes reservoir, the water level peaked during December/January coinciding with the highest GSI, whereas the temperature and rainfall reached the maximum values during February/March. However, overall most of tropical fishes spawning season is associated with increasing temperature, rainfall and water level (Lamas and Godinho Citation1996; Suzuki et al. Citation2000).
No clear significant relationship was found between the HSI and condition factor (K) indices with the GSI. The K and HSI have been applied as surrogate to assess the reproductive period in some species, since they can be related to the mobilization of energetic reserves related to the vitellogenesis process (Payne Citation1975; Nuñez and Duponchelle Citation2009). Therefore, for M. maculatus, the vitellogenesis and/or the gametogenesis seemed not to be related to the depletion of hepatic reserves or to the decreased condition factor, although the lowest HSI occurred between February and June for both sexes, coinciding with the lowest GSI. We found a trend of inverse relationship between K and GSI for both sexes, although no significant correlations were detected. Males had the highest K values in April/May during the resting season. Females had their highest K values coinciding with a sharp decrease in GSI in February/March, when the spawning was complete. This suggests a recovery in body fitness after the reproductive effort by both sexes.
The reproductive strategy of M. maculatus is to produce large number of small eggs. On the other hand Bailly et al. (Citation2008) found parental care in some Serrasalminae species such as Serrassalmus spp. and P. nattereri that have comparatively lower fecundity and larger egg size. Similarly to those Serrasalminae studied by several workers (Rodrigues et al. Citation1978; Leão et al. Citation1991; Vazzoler and Menezes Citation1992; Bailly et al. Citation2008), M. maculatus is a small-sized non-migratory fish which has a long reproductive period that coincides with the rainy season. Overall, Serrasalminae species are well adapted to lentic environments, frequently have continuous non-seasonal reproduction and asynchronous ovarian development with multiple spawnings (Paiva Citation1958; Lamas and Godinho Citation1996; Teles and Godinho Citation1997). This reproductive strategy has been considered to be selectively advantageous, as it results in an increase of genetic variability within the population (Burt et al. Citation1988) or avoids competition for oviposition sites (Leão et al. Citation1991; Albieri et al. Citation2010).
Occurrences of ripe individuals all over the year, in addition to a variety of developing stages and groups of oocytes size inside ovary, point out to a partial spawning type for this species. In this type of spawning, besides large number of reserve oocytes, evidence of oocytes in distinct stages of development is found Vazzoler (Citation1996). The spawning of multiple batches increases the fecundity, enhancing the reproductive success irrespective of changes in environmental conditions (Burt et al. Citation1988; Vazzoler Citation1996). Multiple spawning also reduces the number of coexistent, high volume and preovulatory hydrated oocytes. Other Serrasalminae such as Serrasalmus spilopleura Kner, 1858, S. marginatus Valenciennes, 1837 and Myleus micans Lütken, 1875 also present batch spawning (Vazzoler Citation1996), indicating that this could be a prevailing pattern in this subfamily.
The efficient use of the Lajes reservoir, constructed about one century ago, by the non-native M. maculatus, which was introduced in this system in the early 1980s, is probably associated with its opportunistic strategy characterized by a long reproductive period, batch spawning, with batches relatively small, small oocytes and body size relatively small. In the Lajes reservoir, M. maculatus ranks among the most abundant species (Santos et al. Citation2008) and seems to be well adapted having occupied empty niches successfully (Herbold and Moyle Citation1986), forming a population of small-sized fish with high-reproductive compensation.
Acknowledgements
This study was part of the activities of Pisces Project – financially supported by LIGHT Serviços de Eletricidade S/A, through their Program for Research and Development. The authors thank the technician Paulo César da Silva and several undergraduate students from Fish Ecology Laboratory of UFRuralRJ for helping with the fieldwork.
References
- Albieri , RJ , Araújo , FG and Uehara , W . 2010 . Differences in reproductive strategies between two co-occurring mullets Mugil curema Valenciennes 1836 and Mugil liza Valenciennes 1836 (Mugilidae) in a tropical bay . Tropical Zoology , 23 ( 1 ) : 51 – 62 .
- Andersson , M , ed. 1994 . Sexual selection. Monographs in behavior and ecology , 624 Princeton (NJ) : Princeton University Press .
- Araújo , FG and Santos , LN . 2001 . Distribution of fish assemblages in Lajes reservoir, Rio de Janeiro, Brazil . Brazilian Journal of Biology , 61 ( 4 ) : 563 – 576 .
- Bailly , D , Agostinho , AA and Suzuki , HI . 2008 . Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiabá river, upper Pantanal, Brazil . River Research and Applications , 24 : 1218 – 1229 .
- Barbieri , EB and Kronemberg , DMP . 1994 . Climatologia do litoral sul-sudeste do Estado do Rio de Janeiro . Cadernos de Geociências , 12 : 57 – 73 .
- Burt , A , Krammer , DL , Nakatsuru , K and Spry , C . 1988 . The tempo of reproduction in Hyphessobrycon pulchripinnis (Characidae) with a discussion on the biology of ‘multiple spawning’ in fishes . Environmental Biology of Fishes , 22 ( 1 ) : 15 – 27 .
- Duarte , CM and Alcaraz , M . 1989 . To produce many small or few large eggs: a size-independent reproductive tactic of fish . Oecologia , 80 ( 3 ) : 401 – 404 .
- Duponchelle , F , Lino , F , Hubert , N , Panfili , J , Renno , JF , Baras , E , Torrico , JP , Dugué , R and Nuñez , J . 2007 . Environment-related life history trait variations of the red-bellied piranha, Pygocentrus nattereri, in two river basins of the Bolivian Amazon . Journal of Fish Biology , 71 ( 4 ) : 1113 – 1134 .
- Gross , MR . 2005 . The evolution of parental care . Quaterly Reveiw of Biology , 80 : 37 – 45 .
- Gross , MR and Sargent , RC . 1985 . The evolution of male and female parental care in fishes . American Zoologist , 25 ( 3 ) : 807 – 822 .
- Guimarães , ACD and Quagio-Grassiotto , I . 2001 . Ultrastructural aspects of oogenesis and oocyte primary growth in Serrasalmus spilopleura (Teleostei, Characiformes, Characidae) . Tissue and Cell , 33 ( 3 ) : 241 – 248 .
- Hardie , SA , White , RWG and Barmuta , LA . 2007 . Reproductive biology of the threatened golden galaxies Galaxias auratus Johnston and the influence of lake hydrology . Journal of Fish Biology , 71 ( 6 ) : 1820 – 1840 .
- Herbold , B and Moyle , PB . 1986 . Introduced species and vacant niches . The American Naturalist , 128 ( 5 ) : 751 – 760 .
- Humphries , P . 1995 . Life history, food and habitat of southern pygmy perch, Nannoperca australis, in the Macquarie River, Tasmania . Marine and Freshwater Research , 46 ( 8 ) : 1159 – 1169 .
- Hunter , JR and Macewicz , BJ . 1985 . Rates of atresia in the ovary of captive and wild northern anchovy . Engraulis mordax. Fishery Bulletin , 83 ( 2 ) : 119 – 136 .
- Lamas , IR and Godinho , AL . 1996 . Reproduction in the piranha Serrasalmus spilopleura, a neotropical fish with an usual pattern of sexual maturity . Environmental Biology of Fishes , 45 ( 2 ) : 161 – 168 .
- Lambert , TC and Ware , DM . 1984 . Reproductive strategies of demersal and pelagic spawning fish . Canadian Journal of Fisheries and Aquatic Science , 41 ( 11 ) : 1565 – 1569 .
- Lassala , MDP and Renesto , E . 2007 . Reproductive strategies and genetic variability in tropical freshwater fish . Genetics and Molecular Biology , 30 ( 3 ) : 690 – 697 .
- Leão , ELM , Leite , RG , Chaves , PTC and Ferraz , E . 1991 . Aspectos da reprodução, alimentação e parasitofauna de uma espécie rara de piranha, Serrasalmus altuvei Ramírez, 1956 (Pisces, Serrasalmidae) do baixo rio Negro . Brazilian Journal of Biology , 51 : 545 – 553 .
- Maceina , MJ and Stimpert , MC . 1998 . Relations between reservoir hydrology and crappie recruitment in Alabama . North American Journal of Fisheries Management , 18 : 104 – 113 .
- Nikolsky , GV , ed. 1963 . The ecology of fishes , 352 London : Academic Press .
- Nuñez , J and Duponchelle , F . 2009 . Towards a universal scale to assess sexual maturation and related life history traits in oviparous teleost fishes . Fish Physiology and Biochemistry , 35 : 167 – 180 .
- Paiva , MP . 1958 . Sobre o controle da pirambeba Serrasalmus rhombeus (L. 1766) Lacépède, 1803, no Açude Lima Campos (Icó, Ceará) através da pesca seletiva . Brazilian Journal of Biology , 18 : 251 – 266 .
- Payne , AI . 1975 . The reproductive cycle, condition and feeding in Barbus liberiensis, a tropical stream-dwelling cyprinid . Journal of Zoology , 176 ( 2 ) : 247 – 269 .
- Quagio-Grassiotto , I and Guimarães , ACD . 2003 . Follicular epithelium, theca and egg envelope formation in Serrasalmus spilopleura (Teleostei, Characiformes, Characidae) . Acta Zoologica , 84 ( 2 ) : 121 – 129 .
- Rodrigues , JD , Mota , A , Moraes , MN and Ferreira , AE . 1978 . Curvas de maturação gonadal e crescimento de fêmeas de pirambeba, Serrasalmus spilopleura (Kner 1859) (Pisces, Cypriniformes) . Boletim do Instituto de Pesca , 5 : 51 – 63 .
- Sammons , SM , Dorsey , LG , Bettoli , PW and Fiss , FC . 1999 . Effects of reservoir hydrology on reproduction by largemouth bass and spotted bass in Normandy reservoir, Tennessee . North American Journal of Fisheries Management , 19 : 78 – 88 .
- Santos , LN , Araújo , FG , Brotto , DS and Araújo , FG . 2008 . Artificial structures as tools for fish habitat rehabilitation in a neotropical reservoir . Aquatic Conservation: Marine and Freshwater Ecosystems , 18 ( 6 ) : 896 – 908 .
- Santos , AFGN , Santos , LN and Araújo , FG . 2004 . Water level influences on body condition of Geophagus brasiliensis (Perciformes, Cichlidae) in a Brazilian oligotrophic reservoir . Neotropical Ichthyology , 2 ( 3 ) : 151 – 156 .
- Shatunovskii , MI , Akimova , NV and Ruban , GI . 1996 . Response of reproductive systems of fishes to anthropogenic impacts . Voprosy Ikhtiologii , 36 : 247 – 256 .
- Soares , MCS , Marinho , MM , Huszar , VLM , Branco , CWC and Azevedo , SMFO . 2008 . The effects of water retention time and watershed features on the limnology of two tropical reservoirs in Brazil . Lakes & Reservoir: Research and Management , 13 ( 4 ) : 257 – 269 .
- Suzuki , HI , Agostinho , AA and Winemiller , KO . 2000 . Relationship between oocyte morphology and reproductive strategy in loricariid catfishes of the Parana River, Brazil . Journal of Fish Biology , 57 ( 3 ) : 791 – 807 .
- Tamada , K . 2009 . Variations in clutch and egg sizes in the amphidromous goby Rhinogobius sp. CB along a river course and within a spawning season . Ichthyological Research , 56 : 69 – 75 .
- Teles , MEO and Godinho , HP . 1997 . Ciclo reprodutivo da pirambeba Serrasalmus branditii (Teleostei, Characidae) na Represa de Três Marias, Rio São Francisco . Brazilizan Journal of Biology , 57 : 177 – 184 .
- Vazzoler , AEAM , ed. 1996 . Biology of reproduction of teleosteans: theory and practice , XVIII+169 Maringá : EDUEM .
- Vazzoler , AEAM and Menezes , NA . 1992 . Síntese do conhecimento sobre o comportamento reprodutivo dos Characiformes da América do Sul (Teleostei, Ostariphysi) . Brazilian Journal of Biology , 52 : 627 – 640 .
- Wallace , RA and Selman , K . 1981 . Cellular and dynamic aspects of oocyte growth in teleosts . American Zoologist , 21 ( 2 ) : 325 – 343 .
- Whyte , SA . 1975 . Distribution, trophic relationships and breeding habits of the fish populations in a tropical lake basin (Lake Bosumtwi-Ghana) . Journal of Zoology (London) , 177 : 25 – 56 .
- Winemiller , KO . 1989 . Patterns of variation in life history among South American fishes in seasonal environments . Oecologia , 81 ( 2 ) : 225 – 241 .
- Zarske , A and Géry , K . 1999 . Revision der neotropischen Gattung Metynnis Cope, 1878. 1. Evaluation der Typusexemplare der nominellen Arten (Teleostei: Characiformes: Serrasalmidae) . Zoologische Abhandlungen (Dresden) , 50 ( 2 ) : 169 – 216 .