Abstract
As a group of freshwater invertebrates, dragonflies (Odonata) are commonly used as ecological indicators of freshwater ecosystems. Despite earlier studies suggesting that adult odonates may be good indicators for complex changes in a landscape, the utility of odonates as suitable indicators to indicate health of non-aquatic (forest) habitats remains poorly understood. This study analyses the adult dragonfly assemblage pattern against spatial and temporal disturbance characteristics in Indonesia's Sungai Wain Protection Forest. The core of this reserve comprises one of the few remaining fragments of primary rain forest along the East Kalimantan coast, whereas the rest of the reserve is covered by secondary forest, scrub, grassland, and farmland. Adult dragonfly assemblages at individual sampling sites were analysed in relation to (1) their intensity, (2) frequency of human-caused disturbances, and (3) the time since the last such disturbance, while controlling random variables (type of aquatic and terrestrial habitat) were removed. This study tests the effect of these factors on (1) species richness, (2) proportion of Zygoptera, (3) proportion of forest specialists, and (4) proportion of Borneo's endemics. The human-induced disturbances in the rain forest resulted in pronounced changes in the taxonomical composition and functional diversity of the odonate fauna. Results reported here demonstrate that gradual changes in the odonate assemblages correspond to the degree of anthropogenic influences on forest environments. Adult odonates comprise an appropriately sensitive and versatile indicator group for identifying changes in terrestrial forest environments as well as in freshwater habitats.
Introduction
From a global perspective, odonates (dragonflies and damselflies) are among the best-known insect groups with respect to taxonomy and distribution, and, apart from butterflies, probably no other group of insects receives so much positive attention from the general public (Kalkman et al. Citation2008; Barua et al. Citation2012). Odonates are often used in both basic and applied research because of the relative ease with which they can be observed or found (due to their size and colour), their broad array of behaviours (diurnal and often conspicuous activity), and their relative ease of identification (as reliable identification literature is available) (Kalkman et al. Citation2008). Odonata are currently the only insect group for which a representative global assessment of conservation status has been completed and analysed. This assessment succeeds in providing an indication as to the level of global threat across a freshwater invertebrate group (Clausnitzer et al. Citation2009). Odonates have proven useful for nature management and conservation, and recently there is increased effort to make information on dragonflies available for both scientists and policymakers (Kalkman et al. Citation2008).
The great majority of the world's terrestrial biological diversity is found in the tropics (Jenkins Citation2003), while the same applies to odonates (Kalkman et al. Citation2008). The Indomalaya and Neotropics are by far the most species-rich areas of the world's eight biogeographic regions (Kalkman et al. Citation2008). While at least 70% of Bornean odonate fauna is presently confined to forest habitats and ultimately depends on the forest for its survival (Orr Citation2006), to date the requirements of the individual species and the species diversity of odonates in Southeast Asian rainforests, and particularly in the Indo-Malayan realm, are largely unknown (Orr Citation2004; Corbet Citation2006).
Deforestation pressure will remain high for the immediate future in a number of other tropical developing countries, including those, such as Indonesia, Madagascar and the Philippines, that are home to many endemic forest-dependent species (Jenkins Citation2003). Deforestation in the tropical rain forest environment will also have a particularly strong impact on biodiversity. Frequent anthropogenic disturbances here lead to immediate reduction in diversity and extinction of many arthropods (Lawton et al. Citation1998; Liow et al. Citation2001; Clausnitzer Citation2003; Hanski et al. Citation2007; Clausnitzer et al. Citation2009; Sodhi et al. Citation2009). These disturbances are imminently the most threatening to odonate diversity and will potentially result in extinction of numerous species (Orr Citation2004; Clausnitzer et al. Citation2009).
The use of insect bioindicators is generally restricted to their preferred habitat type or the habitat type essentially used for larval development (McGeoch Citation1998). For example, several groups of aquatic insects (e.g. mayflies, caddisflies or stoneflies, but also dragonflies) are used as indicators of pollution in streams or lakes, whereas terrestrial insects (beetles, ants or butterflies) are widely used as ecological indicators of terrestrial habitats (da Rocha et al. Citation2010). The Odonata, as a group of freshwater invertebrates closely linked to specific freshwater habitat conditions, are widely used as ecological indicators of habitat quality and the integrity of freshwater ecosystems (Hardersen Citation2000; Sahlén and Ekestubbe Citation2001; Smith et al. Citation2007; Silva et al. Citation2010; Arimoro et al. Citation2011; Simaika and Samways Citation2011; Dolný and Harabiš Citation2012). Some studies (e.g. Samways and Steytler Citation1996; Oppel Citation2006a; Silva et al. Citation2010) have pointed out that odonates may also serve as a good indicator for complex changes in landscapes. While Oppel (Citation2006a) compared a natural tropical rainforest and a nearby village, likewise Samways and Steytler (Citation1996) compared four habitat sites: plantation forest, residential area, parkland, and industrial area. Both studies suggested that odonates reflect large integral changes in aquatic as well as in terrestrial environments. Nevertheless, these studies have not convincingly explained how odonates respond to minor anthropogenic disruption of the integrity of terrestrial (rainforest) ecosystems. Such disruption may not yet be associated with direct impact on the aquatic environment. It is not well known if even such small interventions as logging in the forests without any direct disturbance to aquatic habitats have a direct effect on diversity of odonates and whether the response of dragonflies and damselflies is equivalent in this respect to those of terrestrial indicators already widely used.
The response of individual dragonfly species to habitat disturbances in forests can vary significantly depending on their degree of specialization (Sahlén and Ekestubbe Citation2001; Clausnitzer Citation2003). Generally, the niche partitioning of odonates under primary forest canopy is more complex than in more open landscapes within secondary forest (Dijkstra and Lempert Citation2003; Oppel Citation2005). Low insolation in forest habitats and interspecific competition are key factors segregating forest and non-forest species (Dijkstra and Clausnitzer Citation2006). Since it is considerably cooler inside the forest canopy than in adjacent open habitats (Turton and Freiburger Citation1997), the conditions can support slower life history and therefore a longer development cycle (Heinrich and Casey Citation1978; Benstead and Pringle Citation2004; Corbet Citation2006). In general, forest species are habitat specialists with limited dispersal ability and strong competitiveness under their preferred conditions, but they have low competitiveness outside their optimum ranges in comparison to more aggressive eurytopic generalists linked to more open landscapes (Samways and Steytler Citation1996; Dijkstra and Clausnitzer Citation2006; Stewart and Samways Citation2008). The penetration of habitat generalists from non-forest habitats into a more open secondary forest could considerably increase interspecific competition and may result in local extinctions of many habitat specialists and homogenization of dragonfly assemblages (Dijkstra and Clausnitzer Citation2006; Oppel Citation2006a). This was also our main assumption.
We assumed gradual changes in the odonate assemblages corresponding to the degree of anthropogenic influences on forest environments. Therefore, we analysed the effect of disturbance in terms of the extent of intervention and its overall character. A second objective of the study was to identify how changes in dragonfly assemblages correspond to different levels of anthropogenic impacts, as measured by spatial and temporal attributes of disturbance along a gradient, as well as whether there is a significant effect on assemblages even at low levels of anthropogenic disturbance (such as from selective logging or ecotourism). Since many authors simplify disturbance to the final result of perturbation, our research was designed to take into account also temporal patterns and intensity of disturbance.
We hypothesized that disturbance would affect (1) number of species, (2) proportion of Zygoptera (suborder comprised of damselflies) relative to Anisoptera (dragonfly suborder), (3) proportion of habitat (forest) specialists, and (4) proportion of Borneo's endemics. We used three different components (or characteristics) of disturbance as response variables: the intensity of disturbance, frequency of disturbance, and time since the disturbance.
Methods
Study area
Sungai Wain Protection Forest (SWPF) is a water catchment reserve (first gazetted in 1934) located within the administrative area of Balikpapan, East Kalimantan, Indonesia (01°02′–01°10′S, 116°47′–116°55′E). The reserve itself covers an area of less than 100 km2, and the research area covers approximately 20 km2. The core of the reserve comprises one of the few remaining fragments of primary rain forest found along the East Kalimantan coast, while the rest of the reserve, covered with secondary forest, scrub, grassland, and farmland, represents the dominant types of landscape found today in lowland of the East Kalimantan coast. There are no large rivers in the reserve, but there are numerous smaller rivers and streams. Swamps comprise approximately 5.9% of the primary forest, have no significant peat layer, and are semi-permanent, drying out several times per year. There had been human settlements and farms in the south-western part of the reserve until as recently as the 1980s. These settlements were gradually relocated to what is today known as Sungai Wain village and were replaced with a secondary forest containing a large proportion of plantation (such as fruit and rubber) trees. More recently, and following construction of a provincial road, the north-eastern part of the reserve has also been converted into farms and settlements.
Most of the reserve was affected by prolonged drought from 1997 through 1998 in relation to the El Niño–Southern Oscillation, which resulted in increased tree mortality in the primary forest and, during March 1998, in forest fires. These fires were most likely anthropogenic in origin. They remained mainly in the undergrowth and caused high tree mortality along dry ridges, but less so along more humid valleys. They were eventually stopped by firebreaks, but more than 50% of the reserve was nevertheless affected (Fredriksson Citation2002). The area of unburned forest in the central part of the reserve is approximately 4000 ha and currently is the largest fragment of primary rainforest in the Balikpapan–Samarinda area.
SWPF is an ideal place for comparing the effects of disturbance. Four basic types of terrestrial environments are located close together here and on a relatively large area: (1) primary forest, which is unburned forest unaffected (or negligibly affected) by past logging but disturbed by increased tree mortality following the drought in 1997–1998; (2) slightly disturbed forest, which is old-growth secondary forest or primary forest unaffected by fire in 1998 (but affected by drought in 1997–1998), as well as forest disturbed by past logging and small-scale clearing (which largely ceased by the late 1990s); (3) secondary forest, regenerating after the fires in 1998 (including small patches of unburned forest in a burned forest matrix) and forest directly affected, but not destroyed, by construction and maintenance of a dam; and (4) non-forest, which is deforested shrub and grassland, cropland, and open wetlands created by construction and maintenance of the dam. The study area used in this study (including a map of all research localities) and the history of human-caused disturbances are described in greater detail in Dolný et al. (Citation2011).
Localities and habitat types
Adult odonates were sampled at 31 sites of the different habitat types and combined into six broader categories: (1) running waters (RW) in closed canopy conditions, (2) still waters (SW) in closed canopy conditions, (3) terrestrial sites (T) in closed canopy conditions, (4) running waters in open canopy conditions, (5) still waters in open canopy conditions, and (6) terrestrial sites in open canopy conditions. The spatial arrangement of study sites, including a map of all localities and GPS coordinates, are described in greater detail in Dolný et al. (Citation2011).
We analysed the adult odonate assemblages in individual sampling sites within SWPF in relation to the intensity and frequency of human-caused disturbances, as well as the time since the last such disturbance. We used a four-level ordinal scale to classify the intensity, frequency, and time since the last disturbance, with the value of 1 representing a virtual absence of human-caused disturbances and the value 4 representing the highest disturbance level (Tables and ). We based our evaluation on knowledge of SWPF's recent history (derived from interviews with the reserve staff and representatives of the local community), inspection of recent satellite imagery (where primary or old-growth forest, burnt forest, and open scrub can be distinguished), and on our own experience.
Table 1 Disturbance classification scheme for Sungai Wain Protection Forest.
Table 2 Sample sites, multiple-scale assessment of human disturbance, and sampling effort in person-hours (p-h).
Odonate sampling
We sampled odonates at SWPF during both the drier (from 29 July to 16 August 2008) and wetter seasons (from 11–26 January 2010). Sampling was carried out for approximately equal time periods throughout the season for the four habitat types, as measured in person-hours of sampling effort per habitat type (Table ). All localities were visited on at least two different days in each season. Sampling effort depended on the specific conditions of the individual sites and therefore differed between the localities. To allocate the sampling time, we considered the extent and structure of the available habitat, ease of surveying and the number of dragonfly individuals and species encountered (in accordance with the variation in detectability of odonate individuals across habitats). The ultimate criterion for time allocation was to continue the survey as long as we were encountering new species at a given location. We discontinued our inventory of a given site after finding no new species for 0.5 h. Adult odonates were caught with butterfly nets. Any identities that were unclear were later confirmed by R.A. Dow (National Museum of Natural History Naturalis, Leiden, The Netherlands).
Habitat preference was determined for each species (see Appendix 1) using the classification of Orr (Citation2001, Citation2006) and Dow (cited Citation2009). To categorize the affinity for forest habitat type, we applied the classification from Orr (Citation2006) to distinguish (1) forest species (lowland mixed dipterocarp, freshwater swamp forest, peat swamp forest, kerangas, mangrove, and secondary mixed dipterocarp species) and (2) non-forest species. All forest species observed at SWPF showed closer links to specific forest environments, whereas all species found at open sites were habitat generalists (species with no significant habitat affinity). Therefore, this classification can also be generally used for the separation of habitat specialists and generalists.
Data analysis
We tested relationships between assemblage pattern and disturbance attributes (time since disturbance, frequency, and intensity of disturbance) using a linear model for species richness and generalized linear models (binomial family, logit link) for proportions of Zygoptera, specialists, and Borneo's endemic species (classification according to Orr Citation2003, and Dow et al. Citation2012). Proportions for each site were weighted by total numbers of species in the GLM. We tested significance of the models against the null model using an F test for the linear model and χ2 test for the GLM, and factors were added to the model sequentially. As we tested disturbance attributes, the effects of canopy openness and type of aquatic habitat were used as a covariate in a final model (only when the factor had significant effect). Test of correlation coefficient was calculated performing t-test with H 0 r = 0. All statistical analyses were performed using R software (R Development Core Team [cited Citation2012]).
Results
During this study, a total of 5360 individuals from 88 species and belonging to 14 families were sampled at SWPF. More than 2600 individuals representing 72 species were found during the drier season (during July and August) while more than 2760 individuals from 78 species were found during the wetter season (January). A total of 34 (39%) of the sampled species belonged to the suborder Zygoptera and 54 (61%) species belong to the suborder Anisoptera. The most widely abundant family, Libellulidae, was represented by 38 species (43%) and over 2420 individuals (45%). Majority of species (63%) from SWPF is generally considered as forest specialist, 34% represented non-forest species and only three species (3%) were listed as data deficient (Appendix 1). The highest species richness, with more than 60% of species (53 species from 40 genera and 13 families) and 70% of Borneo's endemic species (7 species), was observed in undisturbed primary forest. (For a complete species list, see Appendix 1; for a number of individuals per species, see Dolný et al. Citation2011).
Habitat type, including closed canopy and open habitats, affected both species richness and species composition. There was no significant trend for species richness (F = 0.620, p = 0.437; Figure (a)); however, all metrics of community composition show significant trend (Figure (b)–(d)). The proportion of Zygoptera (F = 6.373, p = 0.017), proportion of habitat (forest) specialists (F = 15.176, p < 0.001), and presence of endemics (F = 4.588, p = 0.041) were significantly higher in closed canopy habitats (Table ).
Figure 1 Overall species richness (a), proportion of Zygoptera species (b), proportion of forest specialists (c), and proportional representation of Borneo's endemic species (d) in odonate assemblages of the different habitat categories. RW = running waters, SW = still waters, T = terrestrial sites, closed = closed canopy conditions, open = open canopy conditions. Box and whiskers plots: bold line = mean, box = upper and lower quartiles, dashed lines = range, open circles = outliers
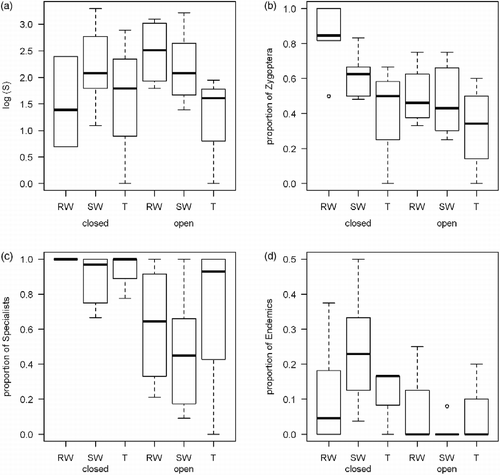
Table 3 Results of the model analysis (χ2 test where the terms were added sequentially) and estimated coefficients for intensity.
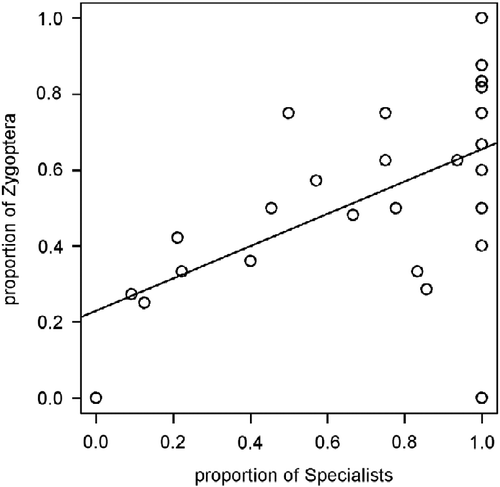
The effect of all evaluated parameters of human disturbance (intensity, frequency, and time) of habitat specialists strongly correlated with the proportion of Zygoptera at each site and habitat (Figure ), and therefore, we utilized only the model analysing the effect of human-induced disturbances (three parameters) upon the proportion of Zygoptera and endemics. The correlation between the proportion of specialists and Zygoptera species was significant (t-test for Pearson's r = 0.55, t = 3.58, p = 0.001; Figure ).
Figure 2 Correlation of habitat specialists with the proportion of Zygoptera species in adult odonate assemblages.
The significance of disturbance components' effects was tested by comparison of the null model with the model including the habitat characteristics (RW, SW, T) and forest canopy (open/closed). Intensity was the only one of the three evaluated parameters of human-caused disturbances with significant effect on distribution of Borneo's endemic, habitat specialists, and Zygoptera, while the frequency of disturbances had significant effect only on the distribution of endemic species. The negative effect of disturbances on local populations was significant after removing the effect of habitat characteristics (Table ; Figures and ).
Figure 3 Changes in proportional representation of Zygoptera in odonate assemblages in relation to the intensity of human-induced habitat disturbance. Right figure shows change after the effect of habitat and canopy was removed.
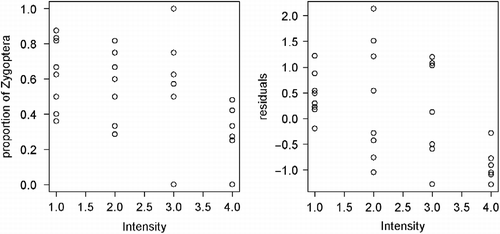
Figure 4 Changes in proportional representation of Borneo's endemic species in odonate assemblages in relation to the intensity of human-induced habitat disturbance. Right figure shows change after the effect of habitat and canopy was removed.
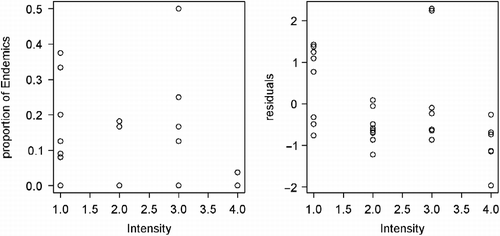
Generally, both habitat characteristics were significant for proportion of Zygoptera while only forest canopy was significant for proportion of endemics (Table ). The species endemic to Borneo preferred forest canopies (i.e. less illuminated habitats) whereas a smaller proportion of endemics occurred in strongly disturbed sites (Figure ; Table ). There were significantly higher proportions of habitat specialists (Zygoptera) in streams and rivers and in habitats inside forest canopy, whereas Anisoptera (in particular family Libellulidae) prevailed in environments affected by large-scale and frequent disturbances (Figure ; Table ).
Discussion
Our findings suggest that odonates not only are ecological indicators of freshwater ecosystems but also are suitable and sensitive indicators of human-induced changes in tropical forests, whose diversity has been shown to be negatively affected by forest disturbances and turnover to secondary habitats (mainly secondary forests or open plains). The adult odonates also reflect minor anthropogenic intervention into the integrity of terrestrial ecosystems, such as logging in the forests without any direct disturbance to aquatic habitats. Several changes in the proportion of Zygoptera, forest specialists, and Borneo's endemics can be seen already in forests slightly affected by past logging or by riverbank weeding and cleaning – all without causing major gaps in the forest canopy (Figures and ; Table ). Thus, dragonflies and damselflies are sensitive to changes in terrestrial (forest) environments and can be equivalent to widely used terrestrial indicators in this respect.
Orr (Citation2003) in the checklist of the Borneo species listed 275 species from Borneo, and later records (Orr Citation2006) bring this total to 280 known species. Approximately 32% of Bornean odonate taxa have been recorded in SWPF, i.e. in an area covering only about 20 km2. Level of endemicity in SWPF was relatively low, only 15% of forest species are considered among Borneo endemics. For comparison, 53% of forest species found in Brunei were Borneo endemics (Orr Citation2006). However, this information may be distorted, because many odonate species previously considered as Borneo endemic, e.g. Tyriobapta laidlawi Ris, 1919, Brachygonia ophelia Ris, 1910, Elattoneura coomansi Lieftinck, 1937, and Elattoneura longispina Lieftinck, 1937, were also found outside Borneo (Dow et al. Citation2012).
Although there was no consistent change in the species richness in relation to the level of disturbance, we were able to identify significant changes in community structure. The proportion of specialists was negatively affected by the intensity of disturbance. Intensity of disturbance had a negative effect on the proportion of Zygoptera, as well as on habitat specialists, because the responses of the two groups were correlated. This is mainly because the majority of species from the suborder Zygoptera have very specific life history requirements (Corbet Citation2006; Sahlén Citation2006). Generally, Zygoptera can be described as “perchers” sensu Corbet and May (Citation2008). This strategy is advantageous in dense primary forest because “perchers” have better motion ability and can be active at lower temperatures compared to “fliers” (Heinrich and Casey Citation1978; Corbet Citation2006). On the other hand, Corbet (Citation2006) suggested that “fliers”, which require more heat energy, may outcompete the “perchers” in more open habitats. It seems that the ratio of Zygoptera/Anisoptera represents a comprehensive parameter that could be used for similar ecological studies instead of the ratio of specialists/generalists – a parameter with less predictive value. Intensive disturbances such as large-scale fires may cause local extinctions of forest species having limited dispersal ability (Cleary et al. Citation2004; Sahlén Citation2006).
Disturbance refers to environmental change, both natural and anthropogenic (Connell Citation1978). The natural disturbances can have similar impact on odonates as do anthropogenic disturbances, e.g. a significant reduction in the proportion of Zygoptera (Dolný et al. Citation2011). Although “natural” disturbances in the tropical rain forest are relatively common, and many forest species are able to escape from disturbed habitats through dispersal, the niche segregation in tropical rain forest is structured horizontally and vertically in a very complex way (Sahlén Citation2006). Many stenotopic species linked to specific microhabitats have very limited dispersal ability (Corbet Citation1999; Sahlén Citation2006). Their probability of finding suitable habitat in frequently disturbed and often fragmented areas is very low. They are unable to persist in long-term suboptimal conditions and are prone to local extinctions (Korkeamäki and Suhonen Citation2002).
Many invertebrate species may profit from better-illuminated habitats in places where trees have disappeared (Cleary et al. Citation2004; Sahlén Citation2006). Several authors have pointed out that species richness in disturbed forests may be increased by the generalists penetrating into the more open parts of secondary forests in disturbed areas (Cleary Citation2003; Dijkstra and Lempert Citation2003; Sahlén Citation2006). In addition, undisturbed forest may provide shadiness (limited illumination), which in its final consequences segregates specialized forest species (mainly Zygoptera) and generalists (Anisoptera) from open, non-forest habitats (Dijkstra and Clausnitzer Citation2006; Orr Citation2006). Although there is very little evidence of this from SWPF, it can be assumed that the intrusion of species with more competitive ability as a consequence of intense disturbances may result in increased competition and predation pressure (Sodhi et al. Citation2010) and may lead to the degradation of forest canopy micro-habitats and subsequent local extinctions of forest species accompanied by the significant decrease of diversity and total species richness at a higher scale (Hamer and Hill Citation2000; Dijkstra and Clausnitzer Citation2006).
There is no doubt that large, anthropogenic disturbances have a negative effect on the richness of tropical invertebrate species (Lawton et al. Citation1998; Liow et al. Citation2001; Hanski et al. Citation2007; Clausnitzer et al. Citation2009; Sodhi et al. Citation2009). However, overall species richness may not change at the local scale or can be even higher with growing intensity of disturbance (Hamer et al. Citation1997; Shahabuddin et al. Citation2010). Human-induced disturbances in the rain forest therefore result in pronounced changes in species composition and an overall reduction in species diversity. The proportion of habitat (forest) specialists and endemics strongly decreased along the disturbance gradient from intact primary forest to heavily degraded rain forest and non-forest. The ecological consequences of single anthropogenic disturbances on the diversity in tropical ecosystems cannot be evaluated directly from diversity indices that are based on the species richness.
The intermediate disturbance hypothesis (Connell Citation1978; Huston Citation1979) assumes that maximum diversity is reached between the two extremes of low and high stress, which mainly refers to disturbances, provided that high diversity can be maintained in a non-equilibrium state by frequent disturbances, gradual changes in climate, or equivalence among species in competitive ability (Sheil and Burslem Citation2003). A positive effect of minor disturbances on diversity and species richness has been recorded in odonates of streams and rivers in more open, savanna landscapes (Samways and Grant Citation2008; Stewart and Samways Citation2008). This phenomenon was caused mainly by removing the negative effects of invasive plants (Samways et al. Citation2005; Samways and Grant Citation2008). In contrast, the impact of disturbances affecting the complex structure of forest canopy on species richness in primary rain forest was markedly negative (Orr Citation2004; Sahlén Citation2006). The immediate changes in diversity caused by rain forest modifications generally indicate a strong negative effect on biodiversity (Oppel Citation2005; Orr Citation2006).
Overall species richness did not change with growing intensity of disturbance, and therefore, it will be more efficient to concentrate on taxa that are important in ecological or conservation terms. We must take into consideration the fact that even with uniform sampling effort, the detectability of odonate individuals differs across habitats and it is very difficult to quantify the total diversity of odonate assemblages in a given area featuring different habitats (Oppel Citation2006b). The unequal detectability across different habitats is a problem that can induce severe bias and undermine ecological inferences. The sampling effort required to obtain odonates in a tropical rainforest habitat is very high (Oppel Citation2006b). Another pitfall of implementing absolute species richness is that it is dependent upon sampling effort and area, and the number of species may be affected by the number of individuals in each sample (i.e. the more the organisms in a sample, the more likely an increase in species). However, other biodiversity metrics, such as functional diversity, are independent of sampling effort, require low identification effort, are easy to calculate, and allow comparisons among sites of different taxonomic composition (Gallardo et al. Citation2011). We focused, therefore, on the proportional occurrences of ecologically important odonate species groups (e.g. Zygoptera or endemic) in assemblages, which remain constant regardless of the variable detectability of odonates in different habitats.
In conclusion, human-caused degradation results in pronounced changes in the taxonomical composition and functional diversity of the odonate fauna. Losses of Zygoptera species reflect the changes from disturbances even in terrestrial habitats. In addition to the negative impact of other factors (stressors) such as climate change, this may be indicative of global diversity crises for many invertebrates (Brook et al. Citation2006; Clausnitzer et al. Citation2009). Adult odonates are equally sensitive and applicable as ecological indicator groups for identifying these changes in terrestrial habitats, and particularly in forest environments.
Acknowledgements
This paper was written in connection with the Institute of Environmental Technologies project, Reg. No. CZ.1.05/2.1.00/03.0100, supported by the Research and Development for Innovations Operational Programme financed by the Structural Funds of the Europe Union. The fieldwork was supported by Grant No. SM/6/104/05 from the Ministry of the Environment of the Czech Republic and Grant No. 206/07/0811 from the Grant Agency of Czech Republic. We would also like to thank Pilsen, a European Capital of Culture 2015, for its support. Stanislav Lhota has been supported by Grant No. MSMT 6007665801 from the Ministry of Education, Youth and Sports of the Czech Republic. We would like to thank Muhalir and Muhammad Fitriadi for field assistance (Sungai Wain, Balikpapan), as well as Agusdin, Rusdianto, Purwanto, and Gabriella Fredriksson for additional facilitation of the fieldwork, and Rory A. Dow for help with determination. English Editorial Services edited the manuscript in its near-final form.
References
- Arimoro , FO , Nwadukwe , FO and Mordi , KI . 2011 . The influence of habitat and environmental water quality on the structure and composition of the adult aquatic insect fauna of the Ethiope River, Delta State, Nigeria . Tropical Zoology , 24 ( 2 ) : 159 – 171 .
- Barua , M , Gurdak , DJ , Ahmed , RA and Tamuly , J . 2012 . Selecting flagships for invertebrate conservation . Biodiversity and Conservation , 21 ( 6 ) : 1457 – 1476 .
- Benstead , JP and Pringle , CM . 2004 . Deforestation alters the resource base and biomass of endemic stream insects in eastern Madagascar . Freshwater Biolology , 49 ( 4 ) : 490 – 501 .
- Brook , BW , Bradshaw , CJA , Koh , LP and Sodhi , NS . 2006 . Momentum drives the crash: mass extinction in the tropics . Biotropica , 38 ( 3 ) : 302 – 305 .
- Clausnitzer , V . 2003 . Odonata of African humid forests – a review . Cimbebasia , 18 : 173 – 190 .
- Clausnitzer , V , Kalkman , VJ , Ram , M , Collen , B , Baillie , JEM , Bedjanič , M , Darwall , WRT , Dijkstra , K-DB , Dow , R Hawking , J . 2004 . Odonata enter the biodiversity crisis debate: the first global assessment of an insect group . Biological Conservation , 142 ( 8 ) : 1864 – 1869 .
- Cleary , DFR . 2003 . An examination of scale of assessment, logging and ENSO-induced fires on butterfly diversity in Borneo . Oecologia , 135 ( 2 ) : 313 – 321 .
- Cleary , DFR , Mooers , AØ , Eichhorn , KAO , van Tol , J , de Jong , R and Menken , SBJ . 2004 . Diversity and community composition of butterflies and odonates in an ENSO-induced fire affected habitat mosaic: a case study from East Kalimantan, Indonesia . Oikos , 105 ( 2 ) : 426 – 448 .
- Connell , JH . 1978 . Diversity in tropical rain forests and coral reefs . Science , 199 ( 4335 ) : 1302 – 1310 .
- Corbet , PS . 1999 . Dragonflies: behaviour and ecology of Odonata , 829 p Colchester : Harley Books .
- Corbet , PS . 2006 . “ Forests as habitats for dragonflies (Odonata) ” . In Forest and dragonflies: Proceedings of the 4th WDA International Symposium of Odonatology; 2005 July; Pontevedra, Spain , Edited by: Rivera , AC . 13 – 36 . Sofia-Moscow : Pensoft .
- Corbet , PS and May , M . 2008 . Fliers and perchers among Odonata: dichotomy or multidimensional continuum? A provisional reappraisal . International Journal of Odonatology , 11 ( 2 ) : 155 – 172 .
- da Rocha , MJR , De Almeida , JR , Lins , GA and Durval , A . 2010 . Insects as indicators of environmental changing and pollution: a review of appropriate species and their monitoring . Holos environment , 10 ( 2 ) : 250 – 262 .
- Dijkstra , K-DB and Clausnitzer , V . 2006 . “ Thoughts from Africa: how can forest influence species composition, diversity and speciation in tropical Odonata? ” . In Forest and dragonflies. Proceedings of 4th WDA International Symposium of Odonatology; 2005 July; Pontevedra, Spain , Edited by: Rivera , AC . 127 – 151 . Sofia-Moscow : Pensoft .
- Dijkstra , K-DB and Lempert , J . 2003 . Odonate assemblages of running waters in the Upper Guinean forest . Archiv für Hydrobiologie , 157 ( 3 ) : 397 – 412 .
- Dolný , A , Bárta , D , Lhota , S and Rusdianto, Drozd , P . 2011 . Dragonflies (Odonata) in the Bornean rain forest as indicators of changes in biodiversity resulting from forest modification and destruction . Tropical Zoology , 24 : 63 – 86 .
- Dolný , A and Harabiš , F . 2012 . Underground mining can contribute to freshwater biodiversity conservation: Allogenic succession forms suitable habitats for dragonflies . Biological Conservation , 145 : 109 – 117 .
- Dow , RA . cited 2009 . Odonata Red List. IUCN red list of threatened Ssecies [Internet] , Cambridge (UK) : IUCN . Available from: www.iucnredlist.org
- Dow , RA , Ng , YF and Choong , CY . 2012 . Odonata of Sungai Bebar, Pahang, Malaysia, with four species recorded for the first time from mainland Asia . Journal of Threatened Taxa , 4 ( 3 ) : 2417 – 2426 .
- Fredriksson , GM . 2002 . “ Extinguishing the 1998 forest fires and subsequent coal fires in the Sungai Wain Protection Forest, East Kalimantan, Indonesia ” . In Communities in flames: Proceedings of an International Conference on Community Involvement in Fire Management , Edited by: Moore , P , Ganz , D , Tan , LC , Enters , T and Durst , PB . 74 – 81 . Bangkok : FAO .
- Gallardo , B , Gascón , S , Quintana , X and Comín , FA . 2011 . How to choose a biodiversity indicator - redundancy and complementarity of biodiversity metrics in a freshwater ecosystem . Ecological Indicators , 11 ( 5 ) : 1177 – 1184 .
- Hamer , KC and Hill , JK . 2000 . Scale-dependent effects of habitat disturbance on species richness in tropical forests . Conservation Biology , 14 ( 5 ) : 1435 – 1440 .
- Hamer , KC , Hill , JK , Lace , LA and Longan , AM . 1997 . Ecological and biogeographical effects of forest disturbance on tropical butterflies of Sumba, Indonesia . Journal of Biogeography , 24 : 67 – 75 .
- Hanski , I , Koivulehto , H , Cameron , A and Rahagalala , P . 2007 . Deforestation and apparent extinctions of endemic forest beetles in Madagascar . Biology Letters , 3 ( 3 ) : 344 – 347 .
- Hardersen , S . 2000 . The role of behavioural ecology of damselflies in the use of fluctuating asymmetry as a bioindicator of water pollution . Ecological Entomology , 25 : 45 – 53 .
- Heinrich , B and Casey , TM . 1978 . Heat transfer in dragonflies: ‘fliers’ and ‘perchers’ . Journal of Experimental Biology , 74 : 17 – 36 .
- Huston , M . 1979 . A general hypothesis of species diversity . American Naturalist , 113 : 81 – 101 .
- Jenkins , M . 2003 . Prospects for biodiversity . Science , 302 ( 5648 ) : 1175 – 1177 .
- Kalkman , VJ , Clausnitzer , V , Dijkstra , KDB , Orr , AG , Paulson , D and van Tol , J . 2008 . Global diversity of dragonflies (Odonata) in freshwater . Hydrobiologia , 595 : 351 – 363 .
- Korkeamäki , E and Suhonen , J . 2002 . Distribution and habitat specialization of species affect local extinction in dragonfly Odonata populations . Ecography , 25 ( 4 ) : 459 – 465 .
- Lawton , JH , Bignell , DE , Bolton , B , Bloemers , GF , Eggleton , P , Hammond , PM , Hodda , M , Holt , RD , Larsen , TB and Mawdsley , NA . 1998 . Biodiversity inventories, indicator taxa and effects of habitat modification in tropical forest . Nature , 391 : 72 – 76 .
- Liow , LH , Sodhi , NS and Elmquist , T . 2001 . Bee diversity along a disturbance gradient in tropical lowland forests of south-east Asia . Journal of Applied Ecology , 38 : 180 – 192 .
- McGeoch , MA . 1998 . The selection, testing and application of terrestrial insects as bioindicators . Biological Reviews , 73 ( 2 ) : 181 – 201 .
- Oppel , S . 2005 . Habitat associations of an Odonata community in a lower montane rainforest in Papua New Guinea . International Journal of Odonatology , 8 ( 2 ) : 243 – 257 .
- Oppel , S . 2006a . Comparison of two Odonata communities from a natural and a modified rainforest in Papua New Guinea . International Journal of Odonatology , 9 : 89 – 102 .
- Oppel , S . 2006b . Using distance sampling to quantify Odonata density in tropical rainforests . International Journal of Odonatology , 9 : 81 – 88 .
- Orr , AG . 2001 . An annotated checklist of the Odonata of Brunei with ecological notes and descriptions of hither to unknown males and larvae . International Journal of Odonatology , 4 ( 2 ) : 167 – 220 .
- Orr , AG . 2003 . A guide to the dragonflies of Borneo: their identification and biology , Kota Kinabalu (Sabah) : Natural History Publications .
- Orr , AG . 2004 . Critical species of Odonata in Malaysia, Indonesia, Singapore and Brunei . International Journal of Odonatology , 7 ( 2 ) : 371 – 384 .
- Orr , AG . 2006 . “ Odonata in Bornean tropical rain forest formations: diversity, endemicity and implications for conservation management ” . In Forest and dragonflies: Proceedings of the 4th WDA International Symposium of Odonatology; 2005 July; Pontevedra, Spain , Edited by: Rivera , AC . 51 – 78 . Sofia-Moscow : Pensoft .
- R Development Core Team . [cited 2012 Jun 22] . R: The R project for statistical computing [Internet] , Vienna (Austria) : WU . Available from: http://www.R–project.org
- Sahlén , G . 2006 . “ Specialists vs. generalists in the Odonata – the importance of forest environments in the formation of diverse species pools ” . In Forest and dragonflies: Proceedings of the 4th WDA International Symposium of Odonatology; 2005 July; Pontevedra, Spain , Edited by: Rivera , AC . 153 – 179 . Sofia-Moscow : Pensoft .
- Sahlén , G and Ekestubbe , K . 2001 . Identification of dragonflies (Odonata) as indicators of general species richness in boreal forest lakes . Biodiversity and Conservation , 10 ( 5 ) : 673 – 690 .
- Samways , MJ and Grant , PBC . 2008 . Elephant impact on dragonfly assemblages . Journal of Insect Conservation , 12 ( 5 ) : 493 – 498 .
- Samways , MJ and Steytler , NS . 1996 . Dragonfly (Odonata) distribution patterns in urban and forest landscapes, and recommendations for riparian management . Biological Conservation , 78 ( 3 ) : 279 – 288 .
- Samways , MJ , Taylor , S and Tarboton , W . 2005 . Extinction reprieve following alien removal . Conservation Biology , 19 ( 4 ) : 1329 – 1330 .
- Shahabuddin , Hidayat , P , Manuwoto , S , Noerdjito , WA , Tscharntke , T and Schulze , CH . 2010 . Diversity and body size of dung beetles attracted to different dung types along a tropical land-use gradient in Sulawesi, Indonesia . Journal of Tropical Ecology , 26 : 53 – 65 .
- Sheil , D and Burslem , DFRP . 2003 . Disturbing hypotheses in tropical forests . Trends in Ecology & Evolution , 18 : 18 – 26 .
- Silva , D , De Marco , P and Resende , DC . 2010 . Adult odonate abundance and community assemblage measures as indicators of stream ecological integrity: a case study . Ecological Indicators , 10 ( 3 ) : 744 – 752 .
- Simaika , JP and Samways , MJ . 2011 . Comparative assessment of indices of freshwater habitat conditions using different invertebrate taxon sets . Ecological Indicators , 11 ( 2 ) : 370 – 378 .
- Smith , J , Samways , MJ and Taylor , S . 2007 . Assessing riparian quality using two complementary sets of bioindicators . Biodiversity and Conservation , 16 ( 9 ) : 2695 – 2713 .
- Sodhi , NS , Lee , TM , Koh , LP and Brook , BW . 2009 . A meta-analysis of the impact of anthropogenic forest disturbance on Southeast Asia's biotas . Biotropica , 41 : 103 – 109 .
- Sodhi , NS , Wilcove , DS , Subaraj , R , Yong , DL , Lee , TM , Bernard , H and Lim , SLH . 2010 . Insect extinctions on a small denuded Bornean island . Biodiversity and Conservation , 19 ( 2 ) : 485 – 490 .
- Stewart , DAB and Samways , MJ . 2008 . Conserving dragonfly (Odonata) assemblages relative to river dynamics in an African savanna game reserve . Conservation Biology , 12 ( 3 ) : 683 – 692 .
- Turton , SM and Freiburger , H-J . 1997 . “ Edge and aspect effects on the microclimate of a small tropical forest remnant on the Atherton Tableland, northeastern Australia ” . In Tropical forest remnants , Edited by: Laurance , WF and Bierregaard , RO . 45 – 54 . Chicago (IL) : The University of Chicago Press .
Appendix 1
Appendix 1 Table A1 shows the total matrix species: the presence and/or absence of species at individual sites, including habitat specificity (based on data from Orr 2001, 2006 and Dow [cited 2009]); and the total number of sites occupied by species (Loc. total).
Table A1. Total matrix species.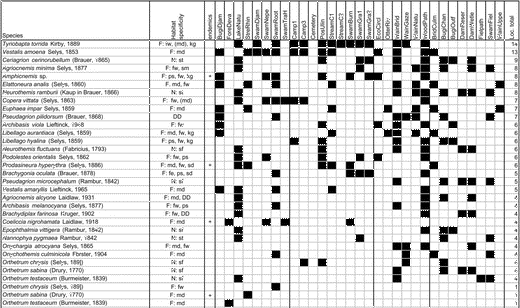
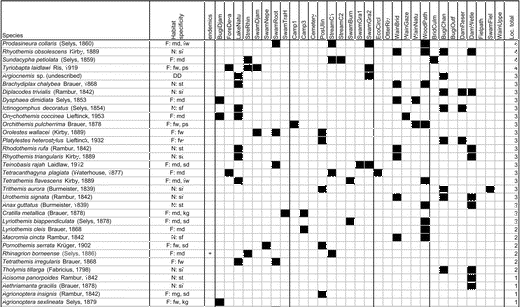
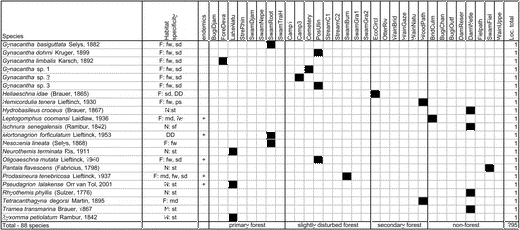
Note: F = forest species (md = lowland mixed dipterocarp, fw = freshwater swamp forest, ps = peat swamp forest, kg = kerangas, mg = mangrove, sd = secondary mixed dipterocarp species), N = non-forest species (st = a wide variety of standing-water habitats, sm = shallow marshes, sf = a wide variety of standing- and floating-water habitats), DD = data deficient.