Abstract
Opportunistic nocturnal catch-and-release turtle surveys were conducted on three nearshore, shallow coral reefs that fringe the main navigation channel to Cockburn Harbour, South Caicos, between 2005 and 2015. A total of 117 captures were made, representing 73 individual turtles (32 green, 41 hawksbill). Almost half of the green turtles were recaptured on at least one occasion, with a maximum time at liberty of 574 days (median = 94 days). Only 20% of hawksbill turtles were recaptured, with a maximum time at liberty of 587 days (median = 120 days). These data suggest that the study sites are utilised by a combination of transient visitors and short to medium-term resident turtles. However, no evidence of long-term nocturnal site fidelity was found. The size ranges observed in both species indicate that all turtles were immature, and the minimum sizes are consistent with previously reported settlement sizes from the region. Despite the lack of long-term fidelity, turtles were regularly encountered over the course of the study, indicating that these reefs are an important nocturnal refugium for local juvenile turtles in general.
Introduction
The green sea turtle Chelonia mydas (Linnaeus, 1758) and the hawksbill sea turtle Eretmochelys imbricata (Linnaeus, 1766) are distributed circumglobally, primarily in tropical and subtropical areas (Márquez Citation1990). Their populations have been in decline due to anthropogenic impacts, and in 1986 they were added to the International Union for the Conservation of Nature (IUCN) Red List of Threatened Species. The classification of C. mydas remains Threatened, while E. imbricata was elevated to Critically Endangered in 1994 (IUCN Citation2016). Although both species are listed on Appendix I of the Convention on the International Trade in Endangered Species (CITES), domestic fisheries and other anthropogenic impacts remain matters for concern (Hazel and Gyuris Citation2006; McClenachan et al. Citation2006; Richardson et al. Citation2006; Hazel et al. Citation2007).
As with all sea turtles, these two species initially undertake a pelagic existence before moving to inshore foraging grounds where they gradually attain maturity (Bolten Citation2003). Although the species are omnivorous and carnivorous, respectively, during their pelagic phase (Bolten Citation2003), green turtles become largely herbivorous once they settle inshore (Bjorndal Citation1997; Limpus and Limpus Citation2000; Fuentes et al. Citation2007; Arthur et al. Citation2008). Consequently, green turtles primarily forage in areas with dense plant or algal growth, such as seagrass meadows and alga-rich reefs (Carr and Ogren Citation1960; Bjorndal Citation1980; Musick and Limpus Citation1997; Makowski et al. Citation2006), while hawksbill turtles more commonly associate with coral reefs and other hard substrates supporting abundant sponge growth (Musick and Limpus Citation1997; Houghton et al. Citation2003). However, it should be noted that post-settlement feeding on animal matter has also been reported in green turtles (Heithaus et al. Citation2002; Carrión-Cortez et al. Citation2010; Carman et al. Citation2014), while algivory has been reported in hawksbills (Bell Citation2013).
Juvenile green and hawksbill turtles have been shown to utilise relatively small home ranges (van Dam and Diez Citation1998; Makowski et al. Citation2006; Cuevas et al. Citation2007; Blumenthal et al. Citation2009), and both species exhibit distinct diurnal foraging and nocturnal resting cycles (Carr and Ogren Citation1960; Ogden et al. Citation1983; Houghton et al. Citation2003; Makowski et al. Citation2006; Blumenthal et al. Citation2009, 2010); although, continuous diel activity has also been reported in green turtles (Senko et al. Citation2010). In the case of hawksbills, foraging and resting occur within the same general area (van Dam and Diez Citation1997; Blumenthal et al. Citation2009), but studies on green turtles have found locale-specific variability in this regard. In St. Croix, United States Virgin Islands (Ogden et al. Citation1983), and Mayotte Island in the Indian Ocean (Taquet et al. Citation2006), the turtles moved between distinct seagrass foraging areas and reef resting areas. Similarly, Mendonça (Citation1983) found that juvenile green turtles in Mosquito Lagoon, Florida, utilised distinct foraging and resting areas. Contrasting results have been reported from Palm Beach, Florida (Makowski et al. Citation2006), and north-eastern Australia (Hazel et al. Citation2013), where foraging and resting largely occurred within the same area. Regardless of whether resting sites are within or outside the diurnal foraging area, previous studies are in agreement that individual green turtles exhibit a high degree of fidelity to their resting site (Mendonça Citation1983; Makowski et al. Citation2006) – a situation also reported for hawksbills (van Dam and Diez Citation1997).
Turtle populations within the Wider Caribbean Region have been hit particularly hard by human exploitation, with estimated declines up to 95% (Berube et al. Citation2012). While efforts to effectively manage national fisheries are ongoing (Richardson et al. Citation2006), habitat disturbance through increased in-water human activities and coastal development is another issue that needs to be addressed – particularly given the site fidelity displayed by the turtles. A thorough understanding of turtle habitat use and dependence is highly desirable in order to inform conservation strategies and management efforts, particularly in the case of islands which are undergoing development.
A case in point is the island of South Caicos, in the Turks and Caicos Islands (TCI). Although it is the main centre of fishing in the TCI, it has a small population and has lagged behind other populated islands in the country with regard to tourism development. However, the first large development on the island has recently opened, and there have been proposals to build an accompanying marina, as well as to dredge a channel to Cockburn Harbour, facilitating access for larger vessels. Given the proximity of coral reefs to these sites, any such developments could potentially impact turtle populations in the area. Therefore, the present study was undertaken to assess the extent to which shallow, nearby coral reefs are utilised by resting marine turtles.
Materials and methods
Study site
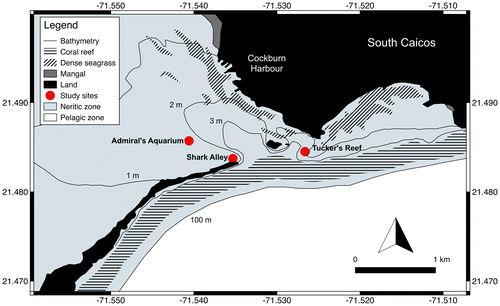
South Caicos is located at the eastern margin of the Caicos Bank, which lies at the southern end of the Lucayan Archipelago. The island is fringed by coral reef to the east and south, and by shallow sand banks supporting patchy seagrass to the west and north. Three distinct nearshore reefs were the focus of the present study, based on their proximity to current and proposed coastal development sites, and their accessibility at night. Admiral’s Aquarium is a 1311 m2 patch reef surrounded by a 2.5 m deep sandy bottom with sparse seagrass cover, lying between South Caicos and the neighbouring Long Cay; Shark Alley is a 2848 m2 area of fringing reef at the northern tip of Long Cay, which is adjacent to a 5 m deep sandy bottom dominated by octocorals; Tucker’s Reef is a 2568 m2 area of fringing reef bordered to the north by a similar substrate as Admiral’s Aquarium, and by a deeper channel (4 m) to the west (Figure ).
Data collection
The three study sites were visited at night (approximately 20:00) on an opportunistic rotating basis, with each site being sampled approximately once per month from October 2005 to July 2006 and from April 2012 to February 2015. Where circumstances permitted, the study sites were occasionally visited more frequently.
Each sampling session lasted 20 min and consisted of 12 in-water sampling personnel and one boat-based operator. Buddy-pairs used snorkel equipment to search the study site for turtles resting on the reef or in reef crevices/overhangs, which were then captured by hand and brought to the surface. Captured turtles were subsequently brought to the boat, where they were placed carapace-down in a polyethylene foam cradle which kept the turtle in a horizontal position and prevented excessive rocking. Once the sampling session was completed, any turtles that had been captured were identified to species, measured to the nearest 0.1 cm (curved carapace length – CCL) and double-tagged with numbered metal Inconel tags. When a tagged turtle was recaptured, the tag numbers were noted and CCL was recorded. The maximum time between capture and release for any turtle was 50 min, and all turtles were released at their site of capture.
Data analysis
Relative abundance was assessed via catch per unit effort (CPUE), which was calculated as the number of turtles caught per buddy pair per hour (turtles/buddy pair/hour). All statistical analyses were conducted with the JMP software package (SAS Institute Inc.), employing non-parametric tests due to the relatively small sample size involved.
Results
A total of 117 turtle captures were performed during the course of the study, representing 73 individual turtles (32 greens, 41 hawksbill). Green turtles ranged in size from 23.1 to 74.0 cm CCL (median = 40.6 cm), while hawksbills ranged from 21.3 to 75.4 cm CCL (median = 39.5 cm), but the difference in size was not found to be statistically significant (Mann–Whitney U-test, 1 d.f., p > 0.05). The size characteristics of both species were variable across the three study sites (Figure ), but again, these differences were not statistically significant (Kruskal–Wallis test, 2 d.f., p > 0.05 for both species). The two species were similarly abundant at Admiral’s Aquarium, which was the site of highest abundance for green turtles (Figure ). However, hawksbill turtles were notably more abundant at Tucker’s Reef, with both species displaying their lowest abundance at Shark Alley (Figure ).
Figure 2. Size distributions of green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles captured during nocturnal surveys at Tucker’s Reef, Shark Alley and Admiral’s Aquarium.
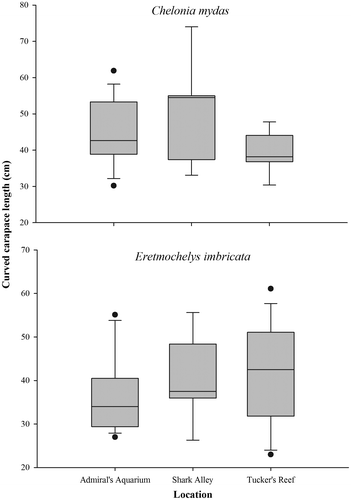
Figure 3. Catch per unit effort (CPUE) of green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles during nocturnal surveys at Tucker’s Reef, Shark Alley and Admiral’s Aquarium.
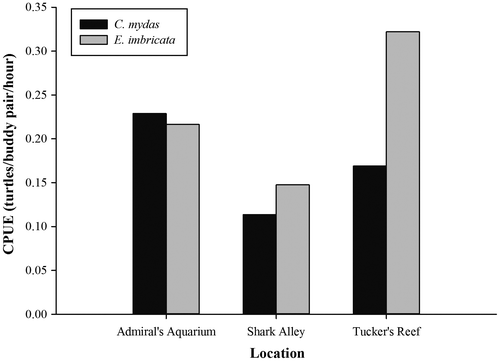
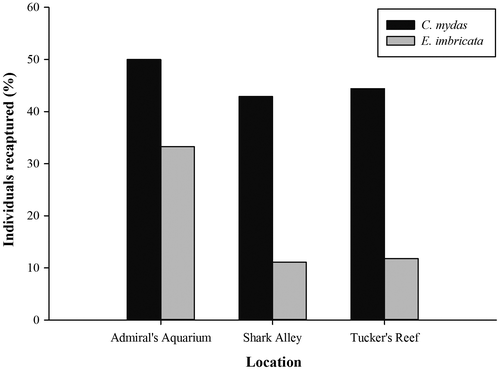
Despite the fact that a higher number of individual hawksbills were encountered during the study, 15 individual green turtles were recaptured on at least one occasion, compared to only eight hawksbills. The number of times an individual was captured varied from 1 to 7 and 1 to 4 for greens and hawksbills, respectively, with a total of 44 recapture events (22 greens and 12 hawksbills). Although a single capture was the most common situation in both species (Figure ), recapture frequency was significantly higher in the case of green turtles (Mann–Whitney U-test, 1 d.f., p < 0.05). In all but one case, the turtles were recaptured at the same site as their original capture; the exception being a 27 cm CCL hawksbill that was originally captured at Admiral’s Aquarium and subsequently recaptured three times at Shark Alley over the course of 587 days. Admiral’s Aquarium yielded the highest proportion of recaptures for both species, and although green turtles displayed a broadly similar proportion of recaptures at Shark Alley and Tucker’s Reef, the proportion of hawksbills that were recaptured at these locations was notably lower (Figure ).
Figure 4. Capture frequency of individual green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles during the present study.
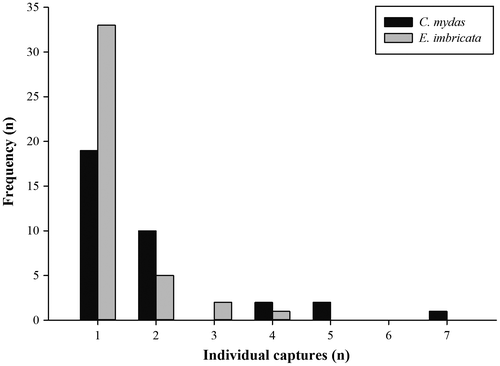
Figure 5. The percentage of tagged green (Chelonia mydas) and hawksbill (Eretmochelys imbricata) turtles that were recaptured during the present study.
Time at liberty, from first capture to final recapture, ranged from 2 to 574 days (median = 94 days) and 35 to 587 days (median = 120 days) for greens and hawksbills, respectively. Although hawksbills tended to be at liberty for longer periods, the median time at liberty did not differ between the species (Mann–Whitney U-test, 1 d.f., p > 0.05). There was a significant correlation between frequency of recapture and overall time at liberty (Spearman rank correlation, 22 d.f., p < 0.05), i.e. there was a tendency for multiple recapture turtles to be encountered over a broader time frame than single-recapture turtles.
Discussion
Comparison of the current size data with minimum size at maturity data from previous studies strongly indicates that all of the turtles encountered during this study were immature (Carr et al. Citation1966; Avens et al. Citation2012; Bjorndal et al. Citation2013). Larger individuals of both species, apparently of mature size, are occasionally encountered during diurnal scuba dives on deeper reef sites adjacent to the current study sites (Henderson, pers. obs.), and individuals of mature size have been reported from TCI waters previously (Richardson et al. Citation2009). Therefore, although mature turtles occur nearby, they do not appear to utilise the current study sites for nocturnal resting. Bresette et al. (Citation2010) reported size-class partitioning in foraging green turtles, with mature and large subadult individuals preferring deeper waters than juveniles. The current results suggest that this might also hold true in the selection of resting areas, for both greens and hawksbills. Hays et al. (Citation2004) determined that adult green turtles preferentially rest at 10 to 15 m depth, which is linked to buoyancy regulation after diving with a full lung volume. This might also explain the selection of shallower depths by juvenile turtles, and it would have important implications regarding the availability of suitable habitat at these depths.
Green turtles during the present study ranged in CCL from 30.2 to 74.0 cm, which translates to a range from 28.4 to 69.7 cm in straight carapace length (SCL) using the conversion factor provided by Goshe et al. (Citation2010). This is in agreement with other western Atlantic studies, which have found that juvenile green turtles settle inshore from their initial pelagic existence at a size commonly exceeding 25 cm SCL (Hirth Citation1997). However, Avens et al. (Citation2012) recorded an unexpectedly high proportion of smaller individuals from an inshore mass cold stunning event in the Gulf of Mexico, leading them to suggest a possible trend towards inshore recruitment at smaller sizes. The results of the present study do not support the existence of such a trend in the TCI. Using the growth model provided by the same authors (Avens et al. Citation2012), the green turtles from the present study ranged in age from approximately 3 to 17 years, which supports the belief that the pelagic phase of life lasts for as few as three years. However, the relatively low abundance of turtles at this end of the size range may indicate that green turtles recruiting to the TCI generally remain in the pelagic phase for a period exceeding three years.
Hawksbill turtles during the present study ranged in size from 21.0 to 61.1 cm CCL, which equates from 19.3 to 51.3 cm SCL using the conversion factor provided by Snover et al. (Citation2013). This lower limit is similar to the minimum size reported from elsewhere in the tropical western Atlantic (Boulon Citation1994; van Dam and Diez Citation1998; Diez and van Dam Citation2003; Hart et al. Citation2013; Hawkes et al. Citation2014), showing greatest similarity with the 19.5 cm SCL reported from the Dominican Republic (León and Diez Citation1999). Although a number of studies have investigated hawksbill growth rates within size classes, growth across all sizes has been modelled for turtles from Hawaii only (Snover et al. Citation2013). As hawksbill growth rates have been highly variable across study locations (Chaloupka and Limpus Citation1997; Diez and van Dam Citation2002; Snover et al. Citation2013), the aforementioned growth model is unlikely to be a good descriptor of growth in turtles from the western Atlantic and, consequently, it is not possible to estimate the duration of the pelagic phase for hawksbills recruiting to the TCI.
Although turtle size did not differ between study sites during the present study, relative abundance was quite variable. Both species displayed their lowest abundance at Shark Alley, which is the deepest of the three sites and is immediately adjacent to the primary channel connecting the shallow Caicos Bank to the deeper reefs and open ocean. Large sharks commonly undertake nocturnal movements through this channel (Henderson, unpublished data), so the lower abundance at this site may be due to increased predation pressure, or learned avoidance by the turtles (Burkholder et al. Citation2013). Differences in abundance between Admirals’s Aquarium and Tucker’s Reef are less easily explained, given their broadly similar characteristics. However, Stadler et al. (Citation2014) found that a combination of physical and biotic characteristics can make certain reefs more attractive to turtles than others.
Studies on the movement patterns of juvenile green turtles have yielded complex and often contrasting results. While a number of studies have reported limited home ranges (Bjorndal Citation1980; Ogden et al. Citation1983; Lamont et al. Citation2015), other investigations have found that juvenile green turtles can undertake wide-ranging movements, across multiple habitat types, over relatively short timescales (Seminoff et al. Citation2002; Godley et al. Citation2003; Senko et al. Citation2010). It was suggested by Seminoff et al. (Citation2002) that wider-ranging movements might be indicative of patchy food and resting resources, while Godley et al. (Citation2003) suggested the possibility of an ontogenetic factor. Where a limited home range has been observed, juvenile green turtles have been noted to display a high level of fidelity to nocturnal resting sites – even when the home range extends to a number of square kilometres (Makowski et al. Citation2006). However, individual turtles have also been observed to utilise more than one nocturnal resting site. In the present study, 47% of green turtles were recaptured on at least one occasion, and all recaptures occurred at the same site as the original capture, so a degree of resting site fidelity was observed. However, these results suggest that 53% of the turtles were transient visitors to the study sites. Furthermore, although some turtles were recaptured multiple times during the study, single recaptures were most common and most recaptured turtles were encountered over periods of a few months, in a study that extended across 10 years. Such observations could be explained by individual turtles utilising these resting sites for relatively short periods and/or infrequently over longer periods, which could be due to the study sites falling within a much larger activity space, within which the turtles utilise a number of resting sites.
Only 20% of hawksbill turtles were recaptured during the present study, which is considerably lower than the situation with green turtles. Home ranges as small as 0.7 to 0.14 km2 have been reported for juvenile hawksbills from Puerto Rico (van Dam and Diez Citation1998) and the Gulf of Mexico (Cuevas et al. Citation2007), while areas up to 1 km2 have been reported from Japan (Okuyama et al. Citation2005) and Honduras (Berube et al. Citation2012). However, studies from the British Virgin Islands (Witt et al. Citation2010) and Belize (Scales et al. Citation2011) have reported considerably larger areas, indicating geographic variability. The low recapture rate observed during the present study is not consistent with the extremely small home ranges reported by van Dam and Diez (Citation1998) and Cuevas et al. (Citation2007), particularly given the nocturnal reduction in area reported by the latter, nor is it consistent with a high degree of resting site fidelity. Acoustic tracking technology is now being employed to further investigate the spatial ecology of hawksbill turtles within the current study area.
In conclusion, the three reef areas surveyed during the present study were utilised nocturnally by juvenile green and hawksbill turtles; however, individual turtles did not appear to utilise these sites continuously over prolonged periods, as has been reported elsewhere. Despite this transient individual relationship, turtles were regularly encountered over the course of the study, indicating that these reefs are an important nocturnal refugium for local juvenile turtles in general.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
The authors grateful acknowledge the logistical and financial support of the School for Field Studies, particularly the support provided by H. Hertler, Director of the Center for Marine Resource Studies. We are also extremely grateful for the help provided by SFS students and staff during this study.
References
- Arthur KE, Boyle MC, Limpus CJ. 2008. Ontogenetic changes in diet and habitat use in green sea turtle (Chelonia mydas) life history. Marine Ecology Progress Series. 362:303–311.10.3354/meps07440
- Avens L, Goshe LR, Harms CA, Anderson ET, Hall AG, Cluse WM, Godfrey MH, Braun-McNeill J, Stacy B, Bailey R. 2012. Population characteristics, age structure, and growth dynamics of neritic juvenile green turtles in the northeastern Gulf of Mexico. Marine Ecology Progress Series. 458:213–229.10.3354/meps09720
- Bell I. 2013. Algivory in hawksbill turtles: Eretmochelys imbricata food selection within a foraging area on the Northern Great Barrier Reef. Marine Ecology. 34:43–55.10.1111/maec.2013.34.issue-1
- Berube MD, Dunbar SG, Rützler K, Hayes WK. 2012. Home range and foraging ecology of juvenile hawksbill sea turtles (Eretmochelys imbricata) on inshore reefs of Honduras. Chelonian Conservation and Biology. 11:33–43.10.2744/CCB-0898.1
- Bjorndal KA. 1980. Nutrition and grazing behavior of the green turtle Chelonia mydas. Marine Biology. 56:147–154.10.1007/BF00397131
- Bjorndal KA. 1997. Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA, editors. The biology of sea turtles. I vol. Boca Raton: CRC Press; p. 199–231.
- Bjorndal KA, Parsons J, Mustin W, Bolten AB. 2013. Threshold to maturity in a long-lived reptile: interactions of age, size, and growth. Marine Biology. 160:607–616.10.1007/s00227-012-2116-1
- Blumenthal JM, Austin TJ, Bell CDL, Bothwell JB, Broderick AC, Ebanks-Petrie G, Gibb JA, Luke KE, Olynik JR, Orr MF, et al. 2009. Ecology of hawksbill turtles, Eretmochelys imbricata, on a Western Caribbean foraging ground. Chelonian Conservation & Biology. 8:1–10.10.2744/CCB-0758.1
- Blumenthal JM, Austin TJ, Bothwell JB, Broderick AC, Ebanks-Petrie G, Olynik JR, Orr MF, Solomon JL, Witt MJ, Godley BJ. 2009. Diving behavior and movements of juvenile hawksbill turtles Eretmochelys imbricata on a Caribbean coral reef. Coral Reefs. 28:55–65.10.1007/s00338-008-0416-1
- Blumenthal JM, Austin TJ, Bothwell JB, Broderick AC, Ebanks-Petrie G, Olynik JR, Orr MF, Solomon JL, Witt MJ, Godley BJ. 2010. Life in (and out of) the lagoon: fine-scale movements of green turtles tracked using time-depth recorders. Aquatic Biology. 9:113–121.10.3354/ab00222
- Bolten AB. 2003. Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. In: Lutz PL, Musick JA, Wyneken J, editors. The biology of sea turtles. II vol. Boca Raton: CRC Press; p. 243–257.
- Boulon RH. 1994. Growth rates of wild juvenile hawksbill turtles, Eretmochelys imbricata, in St. Thomas, United States Virgin Islands. Copeia. 1994:811–814.
- Bresette MJ, Witherington BE, Herren RM, Bagley DA, Gorham JC, Traxler SL, Crady CK, Hardy R. 2010. Size-class partitioning and herding in a foraging group of green turtles Chelonia mydas. Endangered Species Research. 9:105–116.10.3354/esr00245
- Burkholder DA, Heithaus MR, Fourqurean JW, Wirsing A, Dill LM. 2013. Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? Journal of Animal Ecology. 82:1192–1202.10.1111/1365-2656.12097
- Carman VG, Botto F, Gaitán E, Albareda D, Campagna C, Mianzan H. 2014. A jellyfish diet for the herbivorous green turtle Chelonia mydas in the temperate SW Atlantic. Marine Biology. 161:339–349.10.1007/s00227-013-2339-9
- Carr A, Ogren L. 1960. The ecology and migrations of sea turtles, 4. The green turtle in the Caribbean Sea. Bulletin of the American Museum of Natural History. 121:1–48.
- Carr A, Hirth H, Ogren L. 1966. The ecology and migrations of sea turtles, 6. The hawksbill turtle in the Caribbean Sea. American Museum Novitates. 2248:1–29.
- Carrión-Cortez JA, Zárate P, Seminoff JA. 2010. Feeding ecology of the green sea turtle (Chelonia mydas) in the Galapagos Islands. Journal of the Marine Biological Association of the United Kingdom. 90:1005–1013.10.1017/S0025315410000226
- Chaloupka MY, Limpus CJ. 1997. Robust statistical modelling of hawksbill sea turtle growth rates (southern Great Barrier Reef). Marine Ecology Progress Series. 146:1–8.10.3354/meps146001
- Cuevas E, de los Ángeles Liceaga-Correa M, Garduño-Andrade M. 2007. Spatial characterization of a foraging area for immature hawksbill turtles (Eretmochelys imbricata) in Yucatan, Mexico. Amphibia-Reptilia. 28:337–346.10.1163/156853807781374683
- Diez CE, van Dam RP. 2002. Habitat effect on hawksbill turtle growth rates on feeding grounds at Mona and Monito Islands, Puerto Rico. Marine Ecology Progress Series. 234:301–309.10.3354/meps234301
- Diez CE, van Dam RP. 2003. Sex ratio of an immature hawksbill seaturtle aggregation at Mona Island, Puerto Rico. Journal of Herpetology. 37:533–537.10.1670/56-02N
- Fuentes MMPB, Lawler IR, Gyuris E. 2007. Dietary preferences of juvenile green turtles (Chelonia mydas) on a tropical reef flat. Wildlife Research. 33:671–678.
- Godley BJ, Lima E, Akesson S, Broderick AC, Glen F, Godfrey MH, Luschi P, Hays GC. 2003. Movement patterns of green turtles in Brazilian coastal waters described by satellite tracking and flipper tagging. Marine Ecology Progress Series. 253:279–288.10.3354/meps253279
- Goshe LR, Avens L, Scharf FS, Southwood AL. 2010. Estimation of age at maturation and growth of Atlantic green turtles (Chelonia mydas) using skeletochronology. Marine Biology. 157:1725–1740.10.1007/s00227-010-1446-0
- Hart KM, Sartain AR, Hillis-Starr Z-M, Phillips B, Mayor PA, Roberson K, Pemberton RA Jr, Allen JB, Lundgren I, Musick S. 2013. Ecology of juvenile hawksbills (Eretmochelys imbricata) at Buck Island Reef National Monument. US Virgin Islands Marine Biology. 160:2567–2580.10.1007/s00227-013-2249-x
- Hawkes LA, McGowan A, Broderick AC, Gore S, Wheatley D, White J, Witt MJ, Godley BJ. 2014. High rates of growth recorded for hawksbill sea turtles in Anegada. British Virgin Islands Ecology and Evolution. 4:1255–1266.10.1002/ece3.2014.4.issue-8
- Hays GC, Metcalf JD, Walne AW. 2004. The implications of lung-regulated buoyancy control for dive depth and duration. Ecology. 85:1137–1145.10.1890/03-0251
- Hazel J, Gyuris E. 2006. Vessel-related mortality of sea turtles in Queensland. Australia Wildlife Research. 33:149–154.10.1071/WR04097
- Hazel J, Lawler IR, Marsh H, Robson S. 2007. Vessel speed increases collision risk for the green turtle Chelonia mydas. Endangered Species Research. 3:105–113.10.3354/esr003105
- Hazel J, Hamann M, Lawler IR. 2013. Home range of immature green turtles tracked at an offshore tropical reef using automated passive acoustic technology. Marine Biology. 160:617–627.10.1007/s00227-012-2117-0
- Heithaus MR, McLash JJ, Frid A, Dill LM, Marshall GJ. 2002. Novel insights into green sea turtle behaviour using animal-borne video cameras. Journal of the Marine Biological Association of the United Kingdom. 82:1049–1050.10.1017/S0025315402006689
- Hirth HF. 1997. Synopsis of biological data on the green turtle Chelonia mydas (Linnaeus) 1758. U.S. Fish and Wildlife Services Biological Report. 97:1–120.
- Houghton JDR, Callow MJ, Hays GC. 2003. Habitat utilization by juvenile hawksbill turtles (Eretmochelys imbricata Linnaeus, 1766) around a shallow water coral reef. Journal of Natural History. 37:1269–1280.10.1080/00222930110104276
- IUCN. 2016. The IUCN red list of threatened species[Internet]. Version 2015.4 [cited 2016 Mar 22]. Available from: www.iucnredlist.org
- Lamont MM, Fujisaki I, Stephens BS, Hackett C. 2015. Home range and habitat use of juvenile green turtles (Chelonia mydas) in the northern Gulf of Mexico. Animal Biotelemetry. 3:1–12.
- León YM, Diez CE. 1999. Population structure of hawksbill turtles on a foraging ground in the Dominican Republic. Chelonian Conservation and Biology. 3:230–236.
- Limpus CJ, Limpus DJ. 2000. Mangroves in the diet of Chelonia mydas in Queensland. Australia Marine Turtle Newsletter. 89:13–15.
- Makowski C, Seminoff JA, Salmon M. 2006. Home range and habitat use of juvenile Atlantic green turtles (Chelonia mydas L.) on shallow reef habitats in Palm Beach, Florida, USA. Marine Biology. 148:1167–1179.10.1007/s00227-005-0150-y
- Márquez MR. 1990. FAO species catalogue vol 11: sea turtles of the world an annotated and illustrated catalogue of sea turtle species known to date. Rome: FAO.
- McClenachan L, Jackson JBC, Newman MJH. 2006. Conservation implications of historic sea turtle nesting beach loss. Frontiers in Ecology and the Environment. 4:290–296.10.1890/1540-9295(2006)4[290:CIOHST]2.0.CO;2
- Mendonça MT. 1983. Movements and feeding ecology of immature green turtles (Chelonia mydas) in a Florida Lagoon. Copeia. 1983:1013–1023.10.2307/1445104
- Musick JA, Limpus CJ. 1997. Habitat utilization and migration in juvenile sea turtles. In: Lutz PL, Musick JA, editors. The biology of sea turtles. I vol. Boca Raton: CRC Press; p. 137–164.
- Ogden JC, Robinson L, Whitlock K, Daganhardt H, Cebula R. 1983. Diel foraging patterns in juvenile green turtles (Chelonia mydas L.) in St. Croix United States virgin islands. Journal of Experimental Marine Biology and Ecology. 66:199–205.10.1016/0022-0981(83)90160-0
- Okuyama J, Shimizu T, Abe O, Yoseda K, Arai N. 2005. Dispersal processes of head-started hawksbill turtles (Eretmochelys imbricata) in the Yaeyama Islands waters, Okinawa, Japan. Proceedings of the 2nd International symposium on SEASTAR2000 and Asian Bio-logging Science (The 6th SEASTAR2000 Workshop); 2005 Dec 13–15; Bangkok. p. 63–68.
- Richardson PB, Broderick AC, Campbell LM, Godley BJ, Ranger S. 2006. Marine turtle fisheries in the UK overseas territories of the Caribbean: domestic legislation and the requirements of multilateral agreements. Journal of International Wildlife Law and Policy. 9:223–246.10.1080/13880290600764935
- Richardson PB, Bruford MW, Calosso M, Campbell LM, Clerveaux W, Formia A, Godley BJ, Henderson AC, McClellan K, Newman S, et al. 2009. Marine turtles in the Turks and Caicos Islands: remnant rookeries, regionally significant foraging stocks, and a major Turtle fishery. Chelonian Conservation and Biology. 8:192–207.10.2744/CCB-0871.1
- Scales KL, Lewis JA, Lewis JP, Castellanos D, Godley BJ, Graham RT. 2011. Insights into habitat utilisation of the hawksbill turtle, Eretmochelys imbricata (Linnaeus, 1766), using acoustic telemetry. Journal of Experimental Marine Biology and Ecology. 407:122–129.10.1016/j.jembe.2011.07.008
- Seminoff JA, Resendiz A, Nichols WJ. 2002. Home range of green turtles Chelonia mydas at a coastal foraging area in the Gulf of California. Mexico Marine Ecology Progress Series. 242:253–265.10.3354/meps242253
- Senko J, Koch V, Megill WM, Carthy RR, Templeton RP, Nichols WJ. 2010. Fine scale daily movements and habitat use of East Pacific green turtles at a shallow coastal lagoon in Baja California Sur, Mexico. Journal of Experimental Marine Biology and Ecology. 391:92–100.10.1016/j.jembe.2010.06.017
- Snover ML, Balazs GH, Murakawa SKK, Hargrove SK, Rice MR, Seitz WA. 2013. Age and growth rates of Hawaiian hawksbill turtles (Eretmochelys imbricata) using skeletochronology. Marine Biology. 160:37–46.10.1007/s00227-012-2058-7
- Stadler M, Salmon M, Roberts CM. 2014. Ecological correlates of green turtle (Chelonia mydas) abundance on the nearshore worm reefs of southeastern Florida. Journal of Coastal Research. 31:244–254.
- Taquet C, Taquet M, Dempster T, Soria M, Ciccione S, Roos D, Dagorn L. 2006. Foraging of the green sea turtle Chelonia mydas on seagrass beds at Mayotte Island (Indian Ocean), determined by acoustic transmitters. Marine Ecology Progress Series. 306:295–302.10.3354/meps306295
- van Dam RP, Diez CE. 1997. Diving behavior of immature hawksbill turtles (Eretmochelys imbricata) in a Caribbean reef habitat. Coral Reefs. 16:133–138.10.1007/s003380050067
- van Dam RP, Diez CE. 1998. Home range of immature hawksbill turtles (Eretmochelys imbricate (Linnaeus)) at two Caribbean islands. Journal of Experimental Marine Biology and Ecology. 220:15–24.10.1016/S0022-0981(97)00080-4
- Witt MJ, McGowan A, Blumenthal JM, Broderick AC, Gore S, Wheatley D, White J, Godley BJ. 2010. Inferring vertical and horizontal movements of juvenile marine turtles from time-depth recorders. Aquatic Biology. 8:169–177.10.3354/ab00221