Abstract
Proper nest site selection can reflect risk avoidance to offspring. We searched for oviposition site selection and features related to this behavior in the basing-digging foam-nesting Leptodactylus labyrinthicus and Leptodactylus syphax, both species have tadpoles that initially develop in terrestrial nests but complete their larval phase in water. To both species we compared environmental features of nest sites with Random Points (RPs). Nests of L. labyrinthicus were most commonly placed next to ponds, while L. syphax nests were restricted to sites bordering seasonal small streams. Discrimination between both species was reasonable (error 17%); L. syphax nests were on harder soil and more elevated points in relation to water level and L. labyrinthicus nests were most often beside water bodies with potential predators. Compared to RPs, L. labyrinthicus nests (error 30%) were more often hidden and closer to water, and L. syphax nests (error 11%) were more often hidden, in flatter terrains, in softer soils and closer to water. Even though L. labyrinthicus and L. syphax build nests in sites differing in hydric features and soil inclination/hardness, both species select hidden points, which seems to be important to avoid predation by dipteran larvae and desiccation.
Introduction
The ecological theory predicts that offspring success is affected by interactions among multiple abiotic and biotic factors and, among frogs, it has been shown that parental choices can reflect risk avoidance to eggs/tadpoles through proper nest site selection (Holomuzki Citation1995; Evans et al. Citation1996; Spieler and Linsenmair Citation1997; Marsh and Borrell Citation2001; Mitchell Citation2002; Murphy Citation2003a; Rudolf and Rödel 2005). Oviposition site selection occurs when parents choose spawning sites favorable for the development/survival of their offspring (Bernardo Citation1996; Kam et al. Citation1996; Resetarits Jr. 1996; Morris Citation2003, Poelman et al. Citation2013; Haramura Citation2008). Consequently, parents select conditions that protect their offspring (more often eggs/tadpoles) from adversities such as desiccation, competition, cannibalism, parasitism, and predation (Resetarits Jr. and Wilbur 1989; Crump Citation1991; Hopey and Petranka Citation1994; Petranka et al. Citation1994; Kiesecker and Skelly Citation2000; Skelly Citation2001; Binckley and Resetarits Jr. 2002, 2003; Rieger et al. Citation2004; Halloy Citation2006; Silva and Giaretta Citation2008a; García et al. Citation2013). However, egg-laying selection is not always effective and substantial offspring losses can occur by causes such as desiccation and predation (Spieler and Linsenmair Citation1997, Murphy Citation2003a, Citationb; Rudolf and Rödel 2005; Silva and Giaretta Citation2008a).
Neotropical foam-nesting frogs (Leiuperinae and Leptodactylinae) are good models for oviposition site selection studies because they: i) vary in details within their reproductive modes, ii) breed in diverse habitats (e.g., forests, swamps, pools, ponds, streams) and iii) have conspicuous egg-clutches (Halloy and Fiaño 2000; Marsh and Borrell Citation2001; Murphy Citation2003a, Citationb; Giaretta and Facure Citation2006; Halloy Citation2006; Silva and Giaretta Citation2008a, Pinto and Menin Citation2017) which make field studies relatively easy.
We first checked how widespread selection is and try to identify underlying factors associated with eventual choices in selection of oviposition sites. Herein, we searched for oviposition site selection and the environmental features related to this behavior in Leptodactylus labyrinthicus (Spix, 1824) and Leptodactylus syphax Bokermann, 1969, two Neotropical basing-digging foam-nesting frogs.
Material and methods
Model species
Major features distinguishing reproductively L. labyrinthicus from L. syphax include the fact that the first most often use lower land swamp areas, where tadpoles grow in ponds/streamlets, while L. syphax is restricted to slope terrains, where tadpoles grow in places subject, at least periodically, to water flowing (Silva et al. Citation2005; Silva and Giaretta Citation2008b, Citation2009). Noteworthy, L. labyrinthicus occurs syntopically with L. syphax in broad areas in southeast and central Brazil in rocky outcrop habitats (Silva and Giaretta Citation2009; de Sá et al. Citation2014; pers. obs. ). Reproductively, L. labyrinthicus (140 mm SVL) and L. syphax (70 mm) () build foam nests (= egg clutch) on land in basins the females excavate at the margins water bodies while in amplexus (Silva and Giaretta Citation2009). The tadpoles of both species initially develop in the nest and complete their larval phase in water. Major reproductive differences between them include that L. labyrinthicus tadpoles’ development in the nest exclusively depends of feeding on unfertilized (trophic) eggs and, after nest abandonment, feed largely on frog’s eggs (even cannibalistically); whereas L. syphax has most ordinary frog behaviors regarding larval development (Eterovick and Sazima Citation2000; Silva et al. Citation2005, Silva and Giaretta Citation2008b, Citation2009) and lacks oophagy (Silva and Giaretta Citation2009).
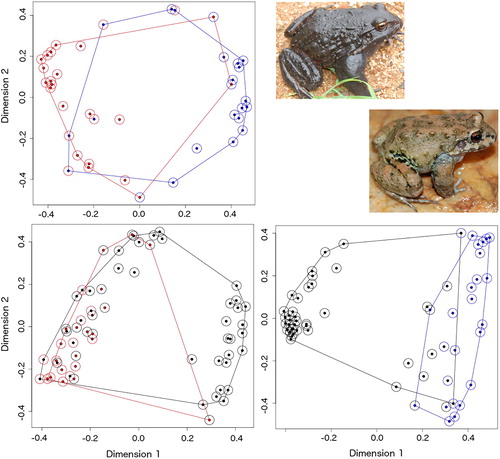
Figure 1 Discrimination according to environmental variables related to oviposition site in two Leptodactylus (Anura) species in South Cerrado (Brazil). Above left, interspecific analysis (Leptodactylus labyrinthicus x Leptodactylus syphax). Below left – Nests and random points to L. labyrinthicus (red, inset above). Below right – Nests and random points to L. syphax (blue, inset below). Random points in black. All plots are displays of a Multidimensional Scale Analysis on Random Forest results. Inset photos not in scale.
Study sites
We collected data in the field (2006–2009) along the reproductive season of both species (essentially from September to December) in the Cerrado Biome at three sites: (1) the Paranaiba River valley at Araguari (MG) (18°29′S, 48°30′W, 550 m altitude, (2) Clube Caça e Pesca, Uberlândia (MG) (18°55′S, 48°17′W, 750 m), and (3) Serra de Caldas Novas, Caldas Novas (GO) (17°43′S, 48°40′W, 900 m). Both species are sympatric and sometimes syntopic in localities 1 and 3, with L. syphax being absent in 2. In all localities, there are natural or human-disturbed swamps or seasonally wet rock outcrops mostly covered with grasses and scattered scrubs/small trees and narrow forests strips along permanent or seasonal rock bottomed streams. Climate is similar among localities having a wet hot season from September to April and a dry mild season from May to August; annual mean precipitation is around 1500 mm (Oliveira and Marquis 2002). For L. labyrinthicus, the data were gathered from swamps (range 30–600 m2), permanent ponds (6–30 m diameter), and temporary pools (<1 m deep); and for L. syphax, from sites around seasonal rocky streamlets (130–480 m length, 0.3–6 m wide, 0.02–1.5 m deep).
Sampling methods
To describe nesting sites and get insights on factors related to nest site selection, we compared environmental features of effective nest sites with surrounding (randomly selected) points (Ficetola et al. Citation2006; Wang et al. Citation2008; Pereyra et al. Citation2011). Within breeding sites, the microhabitat of each reference (real) nest and its comparative surround points (two to each real nest) were described based on measurements of abiotic and biotic features that could potentially influence choice (). Within the breeding sites of L. labyrinthicus (swamps and ponds), random points to each nest were established by sorting direction and distance from the reference nest (0–360°; 0 − 4 m apart). For L. syphax, which reproduces along drainage channels or streamlets, random points to each nest were established (sorted 0 − 5 m) up and downstream from the reference nest and from the water course border (0 − 3 m, according to field observations).
Table 1 Features of nesting sites compared between species and between nests and random points for the two studied Leptodactylus species in South Cerrado (Brazil). Values are presented as medians (range) or frequencies.
Water bodies adjacent to the nests sites or random points were analyzed for maximum depth, turbidity, flow (presence/absence), and occurrence of putative predators (e.g., predatory insects); large ponds were measured one meter from the edge. Stagnant waters around nests (n = 3 sites) were analyzed for conductivity, organic matter, pH, and dissolved oxygen.
Measured microhabitat features of nesting sites and random points were: 1) soil hardness, 2) soil slope, 3) distance from the closest water body, 4) maximum depth of the adjacent water body, 5) distance from water, 6) height in relation to water body (deepest point) and 7) the lower perpendicular distance to the deepest point of stream/drainage channel (just to L. syphax). Soil hardness was estimated by measuring the depth a sharp-pointed iron bar (1 m length, 86 g) reached when released from one-meter height (mean of three points around the nests and the random points). The slope was measured from the lowest point in degrees with a plastic half-disc protractor. We also categorized the following variables: 1) foam nest exposure to sun light: exposed or hidden (beneath grasses, leaf-litter or rock crevices; 90 − 100% surface area covered), 2) water flow: lentic or lotic, 3) water turbidity: clear or turbid (muddy sediments or rotten vegetal matter), 4) nest in terrain sloped towards the nearby water body (yes/no), and 5) presence of potential tadpoles predators (e.g., fishes, naiads, L. labyrinthicus tadpoles) in the adjacent water body (based on direct observation or deep netting) (yes/no).
Data analyses
To assess if species differ in features of nest site micro-habitat and perform site selection we performed comparisons: 1) between species and 2) between actual nests and random points, respectively. We compared microhabitat features of the points using the “Random Forest” (RF) discriminant function (random Forest package, Liaw and Wiener 2002). This algorithm constructs many (set to 500) classification trees using bootstrap samples of the data (each split using the best predictors (out of 3) randomly chosen at that node) then generating classifiers and aggregating results by voting to classes (Liaw and Wiener 2002). RF results include an estimate of the among-object distances subject to Multidimensional Scaling Analysis (MDS), which was performed using the “proximity.plot” function of the rfPermute package (Archer Citation2014), which also allows display the results graphically. The significances of the variable differences in discriminating both species and clutches from random points were accessed by permuting the response variable used in the Random Forest model and according to Mean Decrease Accuracy or Mean Decrease Gini (function “rfPermute” (Archer Citation2014)). The directly or indirectly packages related the application of randomForest were done in R (R Development Core Team Citation2015).
Results
Leptodactylus labyrinthicus males (n ∼ 100) called from and built foam nests (n ∼ 50) beside temporary or permanent pools in natural and human disturbed open area swamps; L. syphax bred (n ∼ 50 calling males, n = 29 nests) along seasonally flowing streams in rock outcrops. Even though both species often co-occur in rock outcrops along L. syphax geographic distribution (AAG unpublished), at the studied sites, syntopic nesting sites (defined as between 0.5–6 m apart) between both species was rare (n = 2 sites). For both species, nest sites were often close (0–250 cm distance) to calling sites. Within reproductive sites, distances between neighboring nests of L. labyrinthicus averaged 106 cm (±138, range 5–390; n = 14 nests) and for L. syphax 143 cm (± 147, 0–375; n = 10). Nests and tadpoles of L. labyrinthicus (n = 6 cases) and L. syphax (n = 14 cases) were sometimes found in malodorous anoxic slightly acid black-watered pools with rotten leaf litter (mean organic matter = 0.24 ± 0.06 mg/L, mean pH = 6.4 ± 0.16; n = 3 samples measured).
Regarding the measured features (), randomForest discrimination resulted in reasonable separation between both species (error 17%), with class error of 0.20 for L. labyrinthicus and 0.14 for L. syphax (). Soil hardness and height from the water (L. syphax nests on harder soil and elevated points) and predator presence (L. labyrinthicus more often beside water bodies with potential predators) were significantly (p < 0.04) related to species discrimination ().
For L. labyrinthicus, the nesting sites and random points were discretely differentiated one each other (error = 30%) (). There were two significant predictors: exposition (nests more often hidden) (p < 0.005) and distance (nests closer to the water) (p < 0.020). For L. syphax discrimination was appreciable (error = 11%) (). Important predictors, in decreasing order, were: exposition (nests more often hidden) (p < 0.005), inclination (nests in flatter terrains) (p < 0.03), hardness (nests in softer soils) (p < 0.015) and water distances (nests closer to the water and to the channel’s deepest point) (p < 0.025).
In summary, ecological conditions of nest microhabitats are less variable and differed from those of random points for both species, this difference being more significant for L. syphax.
Discussion
Historically, L. labyrinthicus and L. syphax were tough to be close relatives, belonging to the same species group, that of Leptodactylus pentadactylus (Heyer, Citation1979), but recent molecular studies placed L. syphax in the Leptodactylus fuscus group (de Sá et al. Citation2014). This phylogenetic placement implies that female basin digging for foam/egg deposition would likely be the result of convergent evolution of this behavior. Functionally, the basin avoids spread of the foam to sides, making it more compact and easier to hide from predatory flies (Silva and Giaretta Citation2009, see below) and reduce desiccation (surface area to volume ratio).
Both species differed in nest site soil harness, reflecting the fact L. syphax breed in rock outcrops while L. labyrinthicus can also breed in soft soiled swamps. This also explains the fact that L. labyrinthicus more often occur side by side with potential aquatic predators, especially when in permanent water bodies. As in a basin-digging tree frog (Luza et al. Citation2015), egg clutches of both L. labyrinthicus and L. syphax were more often hidden than random points. Foam nest desiccation and maggot infestation cause major mortality of eggs and tadpoles of both species in the basin phase (Silva et al. Citation2005; Silva and Giaretta Citation2009). Nests of L. syphax having about 550 eggs can be completely destroyed if infested by 600–800 maggots and, accordingly, those placed beneath grass tuffs, leaf-litter, or in rocky crevices were not infested by maggots (Silva and Giaretta Citation2009). Thus, the behavior of hiding foam nests in both species may allow them to prevent infestation by the visually orientated predatory dipterans and retard desiccation.
Features of the water bodies (depth, turbidity, flow, and predator presence) adjacent to nests and to the random points were quite similar one another and had poor discriminatory power, what reveal some uniformity in the aquatic environments both species use. Other than water distance, important environmental features are related to the exact nest-site placement, not to surrounding water bodies. Accordingly, nests and tadpoles were often associated with black malodorous stagnant waters, which were also anoxic. As eggs and tadpoles (foam phase) are not submerged (not even in direct contact to water), these transitory odd features do not preclude nest deposition. Besides, normally, well-developed tadpoles enter water through nest flooding (typically 9–12 days after egg-laying) after rain showers that drastically modify the adjacent water body conditions (Silva and Giaretta Citation2008b, Citation2009).
The water body proximity was also an important factor for L. labyrinthicus and L. syphax. Obviously, to both, water proximity enhances the chances of proper dispersion to feeding grounds; after all, clutches just have to be a little bit out of water to avoid aquatic predators. Leptodactylus syphax also selected nest sites in flatter terrains and softer soils, features related to the presence of loose earth (in contrast to rock surfaces), which allow basin digging.
The differences found between effective nesting sites and surrounding areas (random points) were more consistent for L. syphax (lower class errors), which may suggest that oviposition site selection in this species is finer than that of L. labyrinthicus. This difference appears to be mainly related to the type of breeding sites, since the ecological context affects the responses and the strategies adopted by the parents (Marsh and Borrell Citation2001; Murphy Citation2003a, Citationb). It is noticeable that the swamps used by L. labyrinthicus are more homogeneous than the rocky habitat of L. syphax; characterized, by diverse levels of soil hardness and slope (Silva et al. Citation2005; Silva and Giaretta Citation2009, present study). Therefore, such variations in the environmental conditions probably require a greater ability of L. syphax to select proper egg-laying microhabitats to avoid dissection, those in flat terrain with loose earth.
Even though not directly tested to the studied species, our data agrees with previous reports that predation and desiccation are key factors in oviposition site selection for several frog species in different ecological contexts (Crump Citation1991; Hopey and Petranka Citation1994; Holomuzki Citation1995; Spieler and Linsenmair Citation1997; Murphy Citation2003a, Citationb; Rudolf and Rödel 2005; Silva and Giaretta Citation2008a, García et al. Citation2013), further experimental studies could better address these possibilities. The significant differences found between effective nest sites and nearby areas in some habitat features suggest that both species do not distribute their clutches randomly, but actively select their oviposition sites; even in a micro environmental scale, since our random points were conservatively established close (<5 m around) to the nests. Our data provide some evidences on nest site selection to L. labyrinthicus and L. syphax which seems to ensure relative protection for eggs and nestling tadpoles against predation (especially by flies), drying, and/or allow to post-basing tadpoles to have access to proper feeding grounds.
Acknowledgements
The directors of the PESCAN allowed access to their facilities, and Mrs. Adir G. Lemos allowed field works at his property. IBAMA permit 134/05. Hugo C. M. Costa, L. Magrini and L. E. Oliveira helped in field works. W. R. Heyer, C. Lomônaco, G. B. Jacobucci, M. Menin and two anonymous reviewers provided helpful comments on the draft.
Additional information
Funding
References
- Archer E. 2014. rfPermute: Estimate Permutation p-values for Random Forest Importance Metrics. R package version 1.6.1. http://CRAN.R-project.org/package=rfPermute.
- Bernardo J. 1996. Maternal effects in animal ecology. American Zoologist. 36:83–105.
- Binckley CA, Resetarits Jr WJ. 2002. Reproductive decisions under threat of predation: squirrel treefrog (Hyla squirella) responses to banded sunfish (Enneacanthus obesus). Oecologia. 130:157–161.
- Binckley CA, Resetarits Jr WJ. 2003. Functional equivalence of non-lethal effects: generalized fish avoidance determines distribution of gray treefrog, Hyla chrysoscelis, larvae. Oikos. 102:623–629.
- Crump ML. 1991. Choice of oviposition site and egg load assessment by a treefrog. Herpetologica. 47:308–315.
- De Sá R, Grant T, Camargo A, Heyer WR, Ponssa ML, Stanley, E. 2014. Systematics of the Neotropical Genus Leptodactylus Fitzinger, 1826 (Anura: Leptodactylidae): Phylogeny, the relevance of non-molecular evidence, and species accounts. South American Journal of Herpetology. 9(Special Issue 1):S1–S128.
- Eterovick PC, Sazima I. 2000. Description of the tadpole of Leptodactylus syphax, with a comparison of morphological and ecological characters of tadpoles and adults of the species in the L. pentadactylus group (Leptodactylidae, Anura). Amphibia-Reptilia. 21:341–350.
- Evans M, Yaber C, Hero J-M. 1996. Factors influencing choice of breeding site by Bufo marinus in its natural habitat. Copeia. 1996:904–912.
- Ficetola G F, Valota M, Bernardi F. 2006. Temporal variability of spawning site selection in the frog Rana dalmatina: consequences for habitat management. Animal Biodiversity and Conservation. 29:157–163.
- García CG, Lescano JN, Leynaud GC. 2013. Oviposition-site selection by Phyllomedusa sauvagii (Anura: Hylidae): An arboreal nester inhabiting arid environments. Acta Oecologica. 51:62–65.
- Giaretta AA, Facure KG. 2006. Terrestrial and communal nesting in Eupemphix nattereri (Anura, Leiuperidae): interactions with predators and pond structure. Journal of Natural History. 40:2577–2587.
- Halloy M. 2006. Choice of oviposition site in Pleurodema borellii (Leptodactylidae): importance of conspecific tadpole size. South American Journal of Herpetology. 1:72–78.
- Halloy M, Fiaño JM. 2000. Oviposition site selection in Pleurodema borellii (Anura, Leptodactylidae) may be influenced by tadpole presence. Copeia. 2000:606–609.
- Haramura T. 2008. Experimental test of spawning site selection by Buergeria japonica (Anura: Rhacophoridae) in response to salinity level. Copeia. 2008:64–67.
- Heyer WR. 1979. Systematics of the pentadactylus species group of the frog genus Leptodactylus (Amphibia, Leptodactylidae). Smithsonian Contributions to Zoology. 301:1–43.
- Holomuzki JR. 1995. Oviposition sites and fish-deterrent mechanisms to two stream anurans. Copeia. 1995:607–613.
- Hopey ME, Petranka JW. 1994. Restriction of wood frogs to fish-free habitats: how important is adult choice? Copeia. 1994:1023–1025.
- Kam YC, Chuang ZS, Yen CF. 1996. Reproduction, oviposition site selection, and tadpole oophagy of an arboreal nester, Chirixalus eiffingeri (Rhacophoridae), from Taiwan. Journal of Herpetology. 30:52–59.
- Kiesecker JM, Skelly DK. 2000. Choice of oviposition site by gray treefrogs: the role of potential parasitic infection. Ecology. 81:2939–2943.
- Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News. 2:18−22.
- Luza AL, Silva ER, Failace DM, Colombo P. 2015. Nest site selection by Hypsiboas faber (Anura, Hylidae) in southern Brazil. Iheringia, Série Zoologia. 105(4):453–460.
- Marsh DM, Borrell BJ. 2001. Flexible oviposition strategies in túngara frogs and their implications for tadpole spatial distributions. Oikos. 93:101–109.
- Mitchell NJ. 2002. Nest-site selection in a terrestrially breeding frog with protracted development. Australian Journal of Zoology. 50:225–235.
- Morris DW. 2003. Toward an ecological synthesis: a case for habitat selection. Oecologia. 136:1–13.
- Murphy PJ. 2003a. Does reproductive site choice in a Neotropical frog mirror variable risks facing offspring? Ecological Monographs. 73:45–67.
- Murphy PJ. 2003b. Context-dependent reproductive site choice in a Neotropical frog. Behavioral Ecology. 14:626–633.
- Oliveira PS, Marquis RJ. 2002. The Cerrados of Brazil: ecology and natural history of a Neotropical savanna. Columbia: Columbia University Press.
- Pereyra LC, Lescano JN, Leynaud GC. 2011. Breeding-site selection by red-belly toads, Melanophryniscus stelzneri (Anura: Bufonidae), in Sierras of Córdoba, Argentina. Amphibia-Reptilia. 32:105−112.
- Petranka JW, Hopey ME, Jennings BT, Baird SD, Boone SJ. 1994. Breeding habitat segregation of wood frogs and American toads: the role of interspecific tadpole predation and adult choice. Copeia. 1994:691–697.
- Pinto RMC, Menin M. 2017. Aspects of the natural history of Leptodactylus knudseni Heyer, 1972 (Anura: Leptodactylidae) in a pristine forest in Central Amazonia, Brazil, with comments on ontogenetic variation of its tadpoles. Journal of Natural History. 51:1−12.
- Poelman EH, Wijngaarden RPA, Raaijmakers CE. 2013. Amazon poison frogs (Ranitomeya amazonica) use different phytotelm characteristics to determine their suitability for egg and tadpole deposition. Evolutionary Ecology. 27:661–674.
- R Development Core Team. 2015. R Foundation for Statistical Computing. Vienna: Austria. Available from http://www.Rproject.org.
- Resetarits Jr WJ. 1996. Oviposition site choice and life history evolution. American Zoologist. 36:205–215.
- Resetarits Jr WJ, Wilbur HM. 1989. Choice of oviposition site by Hyla chrysoscelis: role of predators and competitors. Ecology. 70:220–228.
- Rieger JF, Binckley CA, Resetarits Jr WJ. 2004. Larval performance and oviposition site preference along a predation gradient. Ecology. 85:2094–2099.
- Rudolf VHW, Rödel MO. 2005. Oviposition site selection in a complex and variable environment: the role of habitat quality and conspecific cues. Oecologia. 142:316–325.
- Silva WR, Giaretta AA. 2008a. Seleção de sítios de oviposição em anuros (Lissamphibia). Biota Neotropica. 8:243–248.
- Silva WR, Giaretta AA. 2008b. Further notes on the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Anura, Leptodactylidae). Brazilian Journal of Biology. 68:403–407.
- Silva WR, Giaretta AA. 2009. On the natural history of Leptodactylus syphax with comments on the evolution of reproductive features in the L. pentadactylus species group (Anura, Leptodactylidae). Journal of Natural History. 43:191–203.
- Silva WR, Giaretta AA, Facure KG. 2005. On the natural history of the South American pepper frog, Leptodactylus labyrinthicus (Spix, 1824) (Anura, Leptodactylidae). Journal of Natural History. 39:555–566.
- Skelly, DK. 2001. Distributions of pond-breeding anurans: an overview of mechanisms. Israel Journal of Zoology. 47:313–332.
- Spieler M, Linsenmair KE. 1997. Choice of optimal oviposition sites by Hoplobatrachus occipitalis (Anura, Ranidae) in an unpredictable and patchy environment. Oecologia. 109:184–199.
- Wang Y, Wu Z, Lu P, Zhang F, Li Y. 2008. Breeding ecology and oviposition site selection of black-spotted pond frogs (Rana nigromaculata) in Ningbo, China. Frontiers of Biology. 3:530–535.
