Abstract
During mate choice, three-spined stickleback (Gasterosteus aculeatus) females make use of male olfactory cues to determine the suitability of potential mates in a species- and habitat-dependent manner. This signal contains peptides reflecting the individual male’s MHC profile and a so-called male validation factor (MVF) indicating the species identity of the sender. In a two-armed flow channel, we exposed gravid females to male odour. The odours from males that produced only the MVF but no MHC signal yet were used to examine whether the MVF of distant stickleback populations, one from Canada and one from Germany – genetically isolated since approximately 31–59 thousand years – has changed to such a degree that present-day females only react to the MVF produced by males from their population of origin. The fish used in the experiments were bred from in vitro-fertilized eggs in either country; those from Canada were shipped for analysis to the Plön laboratory to minimize experimental variability. Given the choice between the two MVFs, females did not significantly prefer the MVF of males of their own origin over the other. However, compared to water, both types of females preferred their own MVF significantly and the foreign MVF almost significantly, respectively, suggesting that they could smell MVFs from both origins. These results suggest that, despite long-term genetic isolation between Canadian and German populations, the three-spined stickleback male validation factor has remained constant and thus represents an evolutionarily conserved signal.
INTRODUCTION
Females choose their mates to attain potential paternal care and good genes for their offspring (Andersson & Simmons Citation2006). The first crucial step towards this goal is correct species recognition, ensuring both genetic compatibility and fertile offspring as well as avoiding wasted investment. The second step is individual mate choice. Whereas the mechanism underlying the species recognition mechanism is often unknown, the olfactory immunogenetic Major Histocompatibility Complex (MHC) signal appears to play a major role in mate choice decisions in vertebrates [reviews: Zelano & Edwards Citation2002 (birds); Havlicek & Roberts Citation2008 (humans); Milinski Citation2003 (fish)], in addition to behavioural and visual cues.
The MHC is a crucial component of the vertebrate immune system involved in the detection of antigens (peptides) from infectious diseases and parasites (Janeway et al. Citation2001). The genes of the MHC are highly polymorphic (e.g. > 1,000 alleles in humans, Mack et al. Citation2012). This polymorphism is maintained by parasite-mediated selection (Takahata & Nei Citation1990; Eizaguirre et al. Citation2012) and amplified by sexual selection (Hamilton & Zuk Citation1982; Eizaguirre et al. Citation2009). Upon liberation from the peptide-MHC complex at cell surfaces, the MHC peptide ligands are secreted through bodily fluids and can be evaluated by chemosensory receptors (i.e., olfaction) (Boehm & Zufall Citation2006). Their function as behavioural modulators, including mate choice, has been demonstrated in different species, e.g. mice (Leinders-Zufall et al. Citation2004), fish (Milinski et al. Citation2005) and humans (Milinski et al. Citation2013). Each individual carries only a small, optimal number of MHC alleles (Wegner et al. Citation2003; Forsberg et al. Citation2007; Rekdal et al. Citation2019). This optimum is a tradeoff between having many alleles for maximizing disease detection and having few alleles to reduce negative selection of T-cells, which are necessary for fighting the detected disease (Janeway et al. Citation2001; Wölfing et al. Citation2008).
In sticklebacks, females choose their mate in a habitat-dependent manner, to locally optimize their offspring’s immunity (Andreou et al. Citation2017). By comparing the combined MHC profiles of herself and a male, the female aims at approaching optimality of the MHC allele count for her offspring with regard to her population of origin (Andreou et al. Citation2017; Gahr et al. Citation2018). Parasite communities differ between habitat types to a certain degree; lake parasite species are usually more diverse and abundant than parasites found in river fish communities (Kalbe & Kurtz Citation2006; Eizaguirre et al. Citation2011; Feulner et al. Citation2015). The optimal individual number of MHC alleles is therefore higher in lake than in river sticklebacks (Wegner et al. Citation2003; Milinski Citation2006; Andreou et al. Citation2017; Gahr et al. Citation2018). Habitat-dependent mate choice thus contributes to the divergence and maintenance of sticklebacks as distinct river and lake ecotypes across their range of distribution (Chain et al. Citation2014; Feulner et al. Citation2015). Finally, when selecting among suitable males the one displaying the brightest red colouration increases the probability that the male carries MHC alleles providing resistance against the current immunological challenges (Milinski & Bakker Citation1990).
Freshwater populations in Germany and Canada both date back to the end of the last glaciation period (Mäkinen & Merilä Citation2008; Johnson & Taylor Citation2004; respectively), after which marine sticklebacks have repeatedly colonized freshwater habitats, subsequently differentiating into lake and river ecotypes. By favouring males with locally adapted MHC genes, population-dependent mate choice would thus effectively select against migrants (Stutz & Bolnick Citation2017), facilitating ecotype differentiation and potentially leading to speciation through genetic isolation (Gavrilets Citation2004; Ingley & Rosenthal Citation2016). However, since the MHC is a conserved feature of all jawed vertebrate species, three-spined sticklebacks must make use of an additional species-specific odour signal to validate the perceived olfactory cue and information gathered from MHC peptides (Milinski et al. Citation2010).
Experiments by Milinski et al. (Citation2010) demonstrated the requirement for this additional signal in olfactory mate choice of female three-spined sticklebacks, referred to as male validation factor (MVF). In its absence, females could not distinguish plain water from water containing MHC peptides. Indeed, without a species-specific recognition signal, a female might follow an olfactory signal of a male of the wrong species and potential predator (McLennan Citation2003).
Andreou et al. (Citation2017) demonstrated that females do not distinguish between the MVF from river and lake three-spined stickleback populations originating from adjacent habitats in Northern Germany. Hence, since the MVF does not contribute to population-dependent mate choice, it seems to merely aid in species identification, which helps limit inadvertent inter-species hybridizations, which should be avoided (McLennan Citation2003; Rafferty & Boughman Citation2006).
An important but so far unresolved question concerns the evolutionary stability of the MVF. Here, we set out to address this question by studying geographically isolated populations. Following colonization events of the Atlantic basin, the European/Atlantic stickleback populations became genetically isolated from those of the Pacific Ocean approximately 31–59 thousand years ago (Fang et al. Citation2018, Fst = 0.1813 from microsatellites, C.L. Gahr unpublished data), potentially leading to divergence of the MVF signal through genetic drift. We, therefore, considered the possibility that female three-spined sticklebacks from geographically highly distinct populations, i.e. from the west coast of Canada and Europe, might no longer be able to recognize male validation factors of three-spined stickleback males originating from another continent and consequently genetic clade (i.e. Pacific vs Atlantic ancestry). To experimentally test this hypothesis, we examined whether female three-spined sticklebacks originating from either a Canadian or a German lake population have a preference for their own continental MVF in an olfactory mate choice assay. We made use of the fact that males do not emit MHC signals before completion of their nest, a physiological state that can be determined both through nest appearance and male final nest-building behaviour (Milinski et al. Citation2010). By contrast, starting in spring, males produce MVF independently of the nest´s status (Sommerfeld et al. Citation2008; Milinski et al. Citation2010). Gravid females produce neither an MHC signal nor a validation factor (functionally proven by Milinski et al. Citation2010), probably because male three-spined sticklebacks are not choosy. To the best of our knowledge, Rowland (Citation1989) conducted the only experiment addressing the possibility of male mate choice in sticklebacks; using dummy females, he found that when presented individually, males courted the more distended dummy more extensively. However, presenting the same two dummies sequentially did not result in any difference in male courtship.
In three-spined sticklebacks (Gasterosteus aculeatus L.) males build a nest with algae to which they try to attract as many gravid females as possible; up to 30 clutches have been found in a single nest (Kraak et al. Citation1999), although an average of seven clutches appears to be more common (Mori Citation1987). Thereafter, the male guards and fans the eggs until the fry hatch and leave (Wootton Citation1976). Females are attracted to males with bright-red breeding colouration, which is a costly signal that reveals strength and health, e.g. resistance to parasites (Milinski & Bakker Citation1990). Females respond to the male’s ’zig-zag’ courtship dance that finally leads them to the nest entrance. A decisive moment comes when the female puts her nose into the nest entrance, a behaviour referred to as “nosing” (van der Assem Citation1967; Wootton Citation1976). Thereafter, she either enters the nest and spawns or leaves without spawning. It has been proposed that this “nosing” behaviour helps the female to evaluate the odour signal emanating from the nest owner’s MHC profile (Reusch et al. Citation2001; Aeschlimann et al. Citation2003). The male likely deposits his MHC signal within the nest during the so-called creeping through behaviour (‘t Hart Citation1985; B. Wölfing unpublished data). Through the male’s fanning activity, the odour leaves the nest at the opposite end as a cloud (suggested by ‘t Hart; demonstrated by B. Wölfing unpublished data). The odour likely contains peptide ligands originally bound to and subsequently released from molecules of the MHC. These peptides directly reflect an individual’s genetic MHC profile which is transmitted in his body odour (Boehm & Zufall Citation2006; Milinski Citation2006).
Using tank water from males with unfinished nests (i.e., a situation where the male probably does not send an MHC signal and is not ready to receive mates), female sticklebacks could be exposed to the male’s MVF without his MHC signal (Milinski et al. Citation2010). It is important to note that our previously described flow channel design (Reusch et al. Citation2001; Aeschlimann et al. Citation2003; Milinski et al. Citation2005, Citation2010; Eizaguirre et al. Citation2011; Andreou et al. Citation2017; Gahr et al. Citation2018) excludes male visual and behavioural signals from the female´s mate choice decision. When the MVF odour of a male is supplemented by the addition of chemically synthesized MHC peptides, it represents a functionally complete olfactory mimic of a reproductive male (Gahr et al. Citation2018).
MATERIALS AND METHODS
Animal origin and housing
All fish used in this study were first-generation laboratory-reared in-vitro offspring from wild-caught three-spined sticklebacks (Gasterosteus aculeatus) originating from the Grosser Plöner See lake (n = 30, 54°14ʹ61”N, 10°40ʹ86.9”E) in northern Germany and the McCreight lake (n = 31, 50°16ʹ46.5”N, 125°39ʹ03.1”W) from Vancouver Island, BC, Canada. Parental fish were caught during the 2015 breeding season, and after in vitro fertilization, eggs were shipped to our laboratory in Germany. To increase survivability, the eggs were cleaned using Acriflavine and Methylene Blue (SigmaAldrich) before being transported at 4 °C. This treatment was replicated with the German in-vitro clutches to minimize potential effects of differential handling. To initiate breeding conditions, seasonal changes were simulated in the laboratory by cycling the fish through winter (6 °C, 12:12 L:D), spring (12 °C, 12:12 L:D) and finally summer (18 °C 18:6 L:D) conditions. Fish were housed individually upon transfer to the summer conditions, fed ad libitum with live Chironomidae sp. larvae and were spine-clipped for sex-typing and MHC-allele analysis. Once individually housed, males were provided each with standardized nesting material consisting of green polyester threads (cut to a length of ~ 10 cm) and a sand-filled petri dish. Nest progression was monitored daily and nest status was determined based on nest appearance and male behaviour (see Wootton Citation1976 for details), using only males with an incomplete nest for the experiment.
All animal experiments described were approved by the Ministry of Nature, Environment and Country Development, Schleswig Holstein, Germany. Moreover, permits were granted by Canadian and German governmental institutions for all steps from catching fish in Canada and bringing them to Germany.
Experimental design
Gravid female sticklebacks were placed in a flow chamber fed by two columns with constant laminar water flow of filtered lake water (Reusch et al. Citation2001; Aeschlimann et al. Citation2003; Milinski et al. Citation2005; Eizaguirre et al. Citation2011; Andreou et al. Citation2017; Gahr et al. Citation2018). Females were able to freely investigate the water composition in the chamber for two periods of 300 sec each, with spatial reversal of the water source after the first 300 sec period. Determining odour preference in this setup has been shown to reliably predict mate choice (“supporting text” of Milinski et al. Citation2005), which provides the opportunity to test the effect of male-derived MVF signalling on female mate choice. To this end, water was taken from the tank of both a single Canadian and a German male [containing male validation factor, but no MHC-associated component (Milinski et al. Citation2010)] per trial, and presented for choice to either a German or a Canadian gravid female in sequential fashion. Females were tested for their preference for either of the two male validation factors in a direct comparison. In consecutive control trials, each female underwent three different preference tests with 1 hr breaks in between trials, presented with the MVF from either the sympatric or the allopatric male to be compared to plain water, respectively. Finally, the water from the sympatric male was spiked with either synthesized MHC peptides dissolved in phosphate-buffered saline (PBS) or plain PBS, allowing us to functionally validate the absence of the male’s own MHC signal. To do this, the number of MHC alleles of the male and female were matched in such a way that spiking with synthetic peptides would result in a super-optimal MHC signal, if the male’s own MHC signal was also present. Females are known to avoid the odour of super-optimal males, i.e. males with a higher than the optimally complementary number of MHC alleles (Milinski et al. Citation2005), and this would be revealed by preference for, the un-spiked side of the water column; if this was the case, the trial would be eliminated from the analysis. Although this procedure greatly reduced the number of potential test pairs, this MHC matching was necessary to ensure that the previously recorded female preference was solely based on the male’s MVF signal in the absence of male’s MHC. Furthermore, some male sticklebacks will not build a nest in captivity, and some females do not produce clutches. This also occurs under field conditions, where it could be shown that weaker fish become reproductively active later in the season, when the stronger beginners are worn out (Eizaguirre et al. Citation2009). As no individual fish was used more than once, i.e. not in other pairs, the number of suitable male + female combinations decreased over the course of the experiment. In the end, several unused gravid females, and males with unfinished nest remained for which no fitting partner existed. However, mate choice experiments (e.g. Rafferty & Boughman Citation2006; Milinski et al. Citation2010) have shown in a similar setup that even small sample sizes can provide statistically significant mate choice data. All experiments were performed in double-blinded fashion in the Plön laboratory. Each female–male combination was used only once to avoid pseudo replication and thus is a single independent statistical unit.
Run validation
To validate a female’s readiness to spawn during the trial, she had to spontaneously spawn in her home tank in the absence of a male within 24 hr after her final test (Milinski et al. Citation2005). Otherwise, all of her trial results were discarded. Further, females that remained on the same side in the first and second run of a trial, thus demonstrating an unwillingness to explore, were designated as “no choice” and excluded from the analysis. Two males and their associated trial results were also excluded, because the female showed a clear preference for the non-peptide PBS side, thus suggesting the presence of the male’s own MHC peptides in these trials.
MHC-analysis
DNA was extracted from clipped spines using the DNeasy 96 Blood & Tissue Kit (Qiagen), following the manufacturer´s protocol. MHC allele numbers were determined using Reference Strand-mediated Conformation Analysis (RSCA) as described by Lenz et al. (Citation2009). The results were analyzed using the GeneMarker software (Version 2.4.2, Softgenetics). This analysis was conducted to allow for MHC matching of males and females in order to be able to validate the absence of the male’s own MHC peptides as described.
Peptides
The four different MHC-ligand peptides used in this study were: SYIPSAEKI, SFVDTRTLL, ASNENMETM and AAPDNRETF (Milinski et al. Citation2005, Citation2010). Peptides were chemically synthesized, purified, verified by mass spectroscopy (MALDI-TOF), and dissolved in phosphate-buffered saline (PBS), as described by Milinski et al. (Citation2005). Using four peptides allows us to clearly determine whether the male MHC signal was present (see above).
Statistical analysis
All statistical analyses were done in R (Version 3.3.2) using the build-in packages for statistical analysis (Wilcoxen-signed-rank) and the ggplot2 package for graphical representation. Times from the first and second run of each female–male combination were added up and regarded as one variable (controlling for weak side preference). We determined the time each female spent in front of the inlets (front quarter of the test chamber) with one type of signal (sum of seconds during the first and second half of trial) compared with the summed-up time spent in front of the inlets with the other signal. We tested whether this time averaged over all females in one treatment was statistically significantly higher for one signal than the time spent in front of the other inlet (i.e. signal). Except for one experiment, where we had a directional prediction from a previous experiment, we used two-tailed tests.
RESULTS
The sample size in our present experiments was small, owing to the inevitable constraints of MHC matching and the strict exclusion of unripe or non-explorative females (see Supplementary Material for details on excluded females). As a result of these strict criteria, data from five Canadian and three German females were available for analysis. Because the results pointed in the same direction in the two sub-populations (i.e. a condition for the Fisher combined probability test), we performed a Fisher’s combined probability test, resulting in a combined P with n = 8 females. First, we asked whether the females spent more time in front of their own male’s MVF inlet than would be predicted by chance (i.e. whether the time spent in front of that respective inlet significantly differed from 50%). Unexpectedly, this was neither the case for the Canadian (Wilcoxon-signed rank test: z = 0.674, P = 0.5) nor the German (Wilcoxon-signed rank test: z = 0.535, P = 0.59) females (). Using Fisher’s combined probability test we show that the indifference also holds true in an overall analysis (P = 0.66). We performed a power analysis to test, whether there is enough power to accept the Null-hypothesis; for this analysis, we required an effect size similar in magnitude to the effect found in the comparisons between male fish-water and plain water, that is, 0.78. Our analysis resulted in a power of 0.47; although this value does not permit us to accept the Null-hypothesis (Cohen Citation1988), it does support our findings as a trend. Previous work by Milinski et al. (Citation2010) found that females prefer their own population’s MVF to plain water, when investigated with comparably low sample sizes (t = 5.149, n = 7 pairs, P = 0.0021 two-tailed, in Milinski et al. Citation2010). Building on this prediction, we performed one-tailed Wilcoxon-signed rank tests for the comparison between own MVF and plain water. This assumption held true for the Canadian females (z = – 2.032, P = 0.021, one-tailed) but showed only a trend in the German females (z = – 1.604, P = 0.054, one-tailed), in the present case potentially due to the smaller sample size compared to the previous analysis. Of note, following Fisher’s combined probability test, the preference of MVF over water holds true overall (P = 0.009, ).
Fig. 1. Female preference for her own population’s MVF (x-axis), measured in % of time (y-axis) spend in front of the respective inlet. The medians (red) did not significantly differ from 50% for the Canadian (Wilcoxon-signed rank test: z = 0.674, P = 0.5) nor the German (Wilcoxon-signed rank test: z = 0.535, P = 0.59) females. Using Fisher’s combined probability test we show that this also holds true overall (P = 0.66)
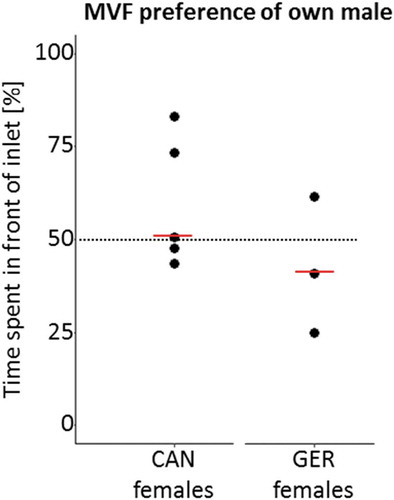
Fig. 2. Females are able to identify both the sympatric (A) and allopatric (B) MVF from water and spend more time (sec, y-axis) in front of the respective inlet. This difference is significant for the Canadian (z = – 2.032, P = 0.021, one-tailed) but only shows a trend in the German (z = – 1.604, P = 0.054, one-tailed) females in case of the sympatric male and only for the Canadian females in case of the allopatric male (z = – 2.023, P = 0.043) but not the German females (z = – 1.604, P = 0.11). In both cases (A and B) Fishers combined probability test shows an overall preference for MVF over plain water in both the sympatric male in A: P = 0.009 and the allopatric male in B: P = 0.03
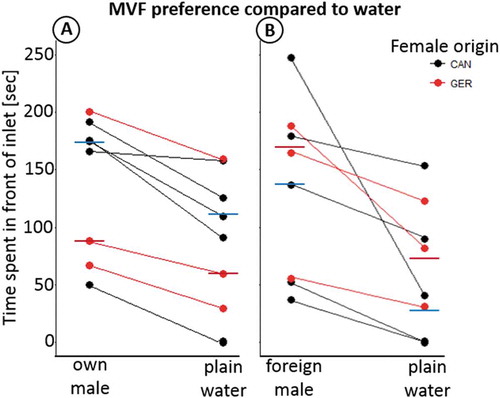
With respect to the preference of the foreign male’s MVF to plain water, we could not exclude the possibility of a repulsive effect on the females. Thus, we performed a two-tailed Wilcoxon-signed-rank test to account for this possibility. Similar to the previous comparisons, we found that the Canadian females preferred the German MVF over plain water (z = – 2.023, P = 0.043) whereas the German females did not spend significantly more time in front of the Canadian MVF inlet (z = – 1.604, P = 0.11). However, Fisher’s combined probability test allows us to state that, overall, the females distinguished and preferred the foreign male’s MVF to plain water (P = 0.03, ).
To exclude the possibility that the results reflect the presence of a complete (MVF plus MHC peptides) male signal, we combined the test males´ water sources with exogenous-synthesized MHC peptides. We have previously shown that through the addition of exogenous peptides to a complete male signal, the MHC peptide complexity is perceived as reflecting a super-optimal MHC genotype, which is invariably rejected by the choosing female (Milinski et al. Citation2005, Citation2010). This control includes all females considered in the analysis of the experiment (Fig. S1 in Supplementary Material). Because we had to discard females that did not prefer the peptide-spiked side, statistical analysis is not applicable.
DISCUSSION
Stickleback populations, from Canada and Germany, have been genetically isolated since approximately 31–59 thousand years. Our present results challenge the hypothesis that the male validation factor (MVF), which presumably serves olfactory species recognition, has changed by genetic drift. Indeed, using populations from Canada and Germany, we found that females recognize the MVF of local and distant origins equally well. Since there has been no contact between sticklebacks from both continents, a potential differentiation in the MVF can only be neutral, but not adaptive.
Since females of either origin were indifferent with respect to their response to MVF odour of either Canadian or German male three-spined sticklebacks, it was necessary to exclude the possibility that this outcome was due to lack of recognition. This explanation could be rejected, because both types of female preferred their own MVF to water, as was already shown for German females (Milinski et al. Citation2010). Moreover, since both MVFs validated a peptide-MHC signal, this recognition is functionally relevant. Our experiments also indicate that cross-recognition of MVFs occurs, since Canadian females clearly preferred the German MVF over water, and, when given Canadian MVF, the German females preferred the Canadian MVF, although the latter result was only weakly supported. However, overall, our results indicate that the male validation factor of three-spined sticklebacks is evolutionarily conserved and has remained functionally equivalent in the two isolated populations for about 40 thousand years.
It is interesting to note that the geographic separation of the two stickleback populations in Germany and Canada is associated with considerable genetic differences (Berner et al. Citation2010; Feulner et al. Citation2015), yet the male validation factor (MVF) that accompanies the male MHC odour signal has remained functionally stable. This result extends our previous finding that females do not distinguish between river and lake MVFs from adjacent populations in Germany (Andreou et al. Citation2017). The evolutionary conservation of the MVF signal makes it an ideal identifier of the species, allowing females to determine whether a perceived MHC signal comes from a male three-spined stickleback. The presence of a species-specific validation factor prevents potential hybridization during the breeding season, since other fish may also engage in MHC-based mate choice (Boehm & Zufall Citation2006; Milinski Citation2006). McLennan (Citation2003) showed that females can olfactorily distinguish between species (guppies and stickleback), followed by Rafferty and Boughman (Citation2006) who showed that females can distinguish between sub-species of three-spined sticklebacks using olfactory cues. We hypothesize that the particular nature of the male validation factor may facilitate these distinctions.
Why is the MVF so stable? We hypothesize that mutation in the gene(s) controlling the production of MVF, would be quickly purged from the population, since such a change would be detrimental to successful mate choice. In analogy, we predict that other aspects of the complex mate choice behaviour re similarly conserved. We note however that our findings should be replicated in other settings.
In conclusion, our results provide evidence that the MVF is evolutionarily conserved and functions in signalling species identity. Our finding that the stickleback MVF appears to lack population-specific features is biologically important, since it suggests that information about populations and/or individuals primarily rests with the polymorphic MHC system, supporting the unique status of MHC genes as magic traits (Gavrilets Citation2004; Andreou et al. Citation2017).
AUTHOR CONTRIBUTIONS
C.L. Gahr, T. Boehm and M. Milinski conceived the study. C.L. Gahr and M. Milinski designed the study. C.L. Gahr collected the data. C.L. Gahr and M. Milinski analysed the data. All authors drafted and revised the article.
excluded_females.xlsx
Download MS Excel (10.3 KB)ACKNOWLEDGEMENTS
We thank D. Martens for help with maintaining the fish, S. Liedtke and T.L. Lenz for help with the RSCA, T. Henrich, M. Kalbe for help with catching the fish and in vitro fertilization and two reviewers for constructive comments.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
SUPPLEMENTARY MATERIAL
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/03949370.2020.1789748
Additional information
Funding
REFERENCES
- Aeschlimann PB, Häberli MA, Reusch TBH, Boehm T, Milinski M. 2003. Female sticklebacks Gasterosteus aculeatus use self-reference to optimize MHC allele number during mate selection. Behav Ecol Sociobiol. 54(2):119–126. doi:10.1007/s00265-003-0611-6
- Andersson M, Simmons LW. 2006. Sexual selection and mate choice. Trends Ecol Evol. 21(6):296–302. doi:10.1016/J.TREE.2006.03.015
- Andreou D, Eizaguirre C, Boehm T, Milinski M. 2017. Mate choice in sticklebacks reveals that immunogenes can drive ecological speciation. Behav Ecol. 28(4):953–961. doi:10.1093/beheco/arx074
- Berner D, Roesti M, Hendry AP, Salzburger W. 2010. Constraints on speciation suggested by comparing lake-stream stickleback divergence across two continents. Mol Ecol. 19(22):4963–4978. doi:10.1111/j.1365-294X.2010.04858.x
- Boehm T, Zufall F. 2006. MHC peptides and the sensory evaluation of genotype. Trends Neurosci. 29(2):100–107. doi:10.1016/j.tins.2005.11.006
- Chain FJJ, Feulner PGD, Panchal M, Eizaguirre C, Samonte IE, Kalbe M, Lenz TL, Stoll M, Bornberg-Bauer E, Milinski M, Reusch TBH. 2014. Extensive copy-number variation of young genes across stickleback populations. PLoS Genet. 10(12):e1004830. doi:10.1371/journal.pgen.1004830
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. Hillsdale (NJ): Lawrence Erlbaum Associates.
- Eizaguirre C, Lenz TL, Kalbe M, Milinski M. 2012. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat. Commun. 3(1):621. doi:10.1038/ncomms1632
- Eizaguirre C, Lenz TL, Sommerfeld RD, Harrod C, Kalbe M, Milinski M. 2011. Parasite diversity, patterns of MHC II variation and olfactory based mate choice in diverging three-spined stickleback ecotypes. Evol Ecol. 25:605–622. doi:10.1007/s10682-010-9424-z
- Eizaguirre C, Yeates SE, Lenz TL, Kalbe M, Milinski M. 2009. MHC-based mate choice combines good genes and maintenance of MHC polymorphism. Mol Ecol. 18(15):15:3316–3329. doi:10.1111/j.1365-294X.2009.04243.x
- Fang B, Merilä J, Ribeiro F, Alexandre CM, Momigliano P. 2018. Worldwide phylogeny of three-spined sticklebacks. Mol Phylogenet Evol. 127:613–625. doi:10.1016/j.ympev.2018.06.008
- Feulner PGD, Chain FJJ, Panchal M, Huang Y, Eizaguirre C, Kalbe M, Lenz TL, Samonte IE, Stoll M, Bornberg-Bauer E, et al. 2015. Genomics of divergence along a continuum of parapatric population differentiation. PLoS Genet. 11(7):e1005414. doi:10.1371/journal.pgen.1005414
- Forsberg LA, Dannewitz J, Petersson E, Grahn M. 2007. Influence of genetic dissimilarity in the reproductive success and mate choice of brown trout – females fishing for optimal MHC dissimilarity. J Evol Biol. 20(5):1859–1869. doi:10.1111/j.1420-9101.2007.01380.x
- Gahr CL, Boehm T, Milinski M. 2018. Female assortative mate choice functionally validates synthesized male odors of evolving stickleback river–lake ecotypes. Biol Lett. 14(12):20180730. doi:10.1098/rsbl.2018.0730
- Gavrilets S. 2004. Fitness landscapes and the origin of species (MPB-41). Princeton (NJ) (Oxford): Princeton University Press. doi:10.2307/j.ctv39x541
- Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites? Science. 218(4570):384–387. doi:10.1126/science.7123238
- Havlicek J, Roberts SC. 2008. MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology. 34(4):497–512. doi:10.1016.j.psychneuen.2008.10.007
- Ingley SJ, Rosenthal GG. 2016. Digest: mechanisms of assortative mating and ecological speciation. Evolution. 71(1):185–186. doi:10.1111/evo.13132
- Janeway CA, Travers P, Walport M, Shlomchik M. 2001. Immunobiology: the immune system in health and disease, 5th ed. New York: Garland Science.
- Johnson LS, Taylor EB. 2004. The distribution of divergent mitochondrial DNA lineages of threespine stickleback (Gasterosteus aculeatus) in the northeastern Pacific Basin: post-glacial dispersal and lake accessibility. J Biogeogr. 31(7):1073–1083. doi:10.1111/j.1365-2699.2004.01078.x
- Kalbe M, Kurtz J. 2006. Local differences in immunocompetence reflect resistance of sticklebacks against the eye fluke Diplostomum pseudospathaceum. J. Parasitol. 132(1):105–16. doi:10.1017/S0031182005008681
- Kraak SBM, Bakker TCM, Mundwiler B. 1999. Sexual selection in sticklebacks in the field: correlates of reproductive, mating, and paternal success. Behav Ecol. 10(6):696–706. doi:10.1093/beheco/10.6.696
- Leinders-Zufall T, Brennan P, Widmayer P, Chandrasani SP, Maul-Pavicic A, Jäger M, Xiao-Hong L, Breer H, Zufall F, Boehm T. 2004. MHC class I peptides as chemosensory signals in the vomeronasal organ. Science. 306(5698):1033–1037. doi:10.1126/science.1102818
- Lenz TL, Eizaguirre C, Becker S, Reusch TBH. 2009. RSCA genotyping of MHC for high-throughput evolutionary studies in the model organism three-spined stickleback Gasterosteus aculeatus. BMC Evol Biol. 9:57. doi:10.1186/1471-2148-9-57
- Mack SJ, Cano P, Hollenbach JA, He J, Hurley CK, Middleton D, Moraes ME, Pereira SE, Kempenich JH, Reed EF, et al. 2012. Common and well documented HLA alleles: 2012 update to the CWD catalogue. Tissue Antigens. 81(4):194–203. doi:10.1111/tan.12093
- Mäkinen HS, Merilä J. 2008. Mitochondrial DNA phylogeography of the three-spined stickleback (Gasterosteus aculeatus) in Europe-evidence for multiple glacial refugia. Mol Phylogenet Evol. 46(1):167–182. doi:10.1016/j.ympev.2007.06.011
- McLennan DA. 2003. The importance of olfactory signals in the gasterosteid mating system: sticklebacks go multimodal. Biol J Linn Soc. 80(4):555–572. doi:10.1111/j.1095-8312.2003.00254.x
- Milinski M. 2003. The function of mate choice in sticklebacks: optimizing MHC genetics. J Fish Biol. 63(s1):1–16. doi:10.1111/j.1095-8649.2003.00215.x
- Milinski M. 2006. The major histocompatibility complex, sexual selection, and mate choice. Annu Rev Ecol Evol Syst. 37(1):159–186. doi:10.1146/annurev.ecolsys.37.091305.110242
- Milinski M, Bakker TCM. 1990. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 344(6264):330–333. doi:10.1038/344330a0
- Milinski M, Croy I, Hummel T, Boehm T. 2013. Major histocompatibility complex peptide ligands as olfactory cues in human body odour assessment. Proc R Soc Lond B. 280:20122889. doi:10.1098/rspb.2012.2889
- Milinski M, Griffiths SW, Reusch TBH, Boehm T. 2010. Costly major histocompatibility complex signals produced only by reproductively active males, but not females, must be validated by a “maleness signal” in three-spined sticklebacks. Proc R Soc Lond B. 277:391–398. doi:10.1098/rspb.2009.1501
- Milinski M, Griffiths S, Wegner KM, Reusch TBH, Haas-Assenbaum A, Boehm T. 2005. Mate choice decisions of stickleback females predictably modified by MHC peptide ligands. Proc Natl Acad Sci USA. 102(12):4414–4418. doi:10.1073/pnas.0408264102
- Mori S. 1987. Divergence in reproductive ecology of the three-spined stickleback, Gasterosteus aculeatus. Jpn J Ichtyhol. 34(2):165–175. doi:10.11369/jji1950.34.165
- Rafferty NE, Boughman JW. 2006. Olfactory mate recognition in a sympatric species pair of three-spined sticklebacks. Behav Ecol. 17:965–970. doi:10.1093/beheco/arl030
- Rekdal SL, Anmarkrud JA, Lifjeld JT, Johnsen A. 2019. Extra‐pair mating in a passerine bird with highly duplicated major histocompatibility complex class II: preference for the golden mean. Mol Ecol. 28(23):5133–5144. doi:10.1111/mec.15273
- Reusch TBH, Häberli MA, Aeschlimann PB, Milinski M. 2001. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature. 414(6861):300–302. doi:10.1038/35104547
- Rowland WJ. 1989. The ethological basis of mate choice in male threespine sticklebacks, Gasterosteus aculeatus. Anim Behav. 38(1):1:112–120. doi:10.1016/S0003-3472(89)80070-3
- Sommerfeld RD, Boehm T, Milinski M. 2008. Desynchronizing male and female reproductive seasonality: dynamics of male MHC-independent olfactory attractiveness in sticklebacks. Ethol Ecol Evol. 20(4):325–336. doi:10.1080/08927014.2008.9522515
- Stutz WE, Bolnick DI. 2017. Natural selection on MHC IIβ in parapatric lake and stream stickleback: balancing, divergent, both or neither? Mol Ecol. 26(18):4772–4786. doi:10.1111/mec.14158
- ‘t Hart M. 1985. The influence of fertilization on the creeping through cycle of the three-spined stickleback. Behaviour. 93(1–4):194–202. https://www.jstor.org/stable/4534441
- Takahata N, Nei M. 1990. Allelic genealogy under over dominant and frequency-dependent selection and polymorphism of major histocompatibility complex loci. Genetics. 124(4):967–978. PMCID:PMC1203987.
- van der Assem J. 1967. Territory in the three-spined stickleback Gasterosteus aculeatus L. An experimental study in intraspecific competition. Behaviour (Suppl). 16:1–164.
- Wegner KM, Kalbe M, Kurtz J, Reusch TBH, Milinski M. 2003. Parasite selection for immunogenetic optimality. Science. 301(5638):1343. doi:10.1126/science.1088293
- Wölfing B, Traulsen A, Milinski M, Boehm T. 2008. Does intra-individual major histocompatibility complex diversity keep a golden mean? Proc R Soc Lond B. 364:1513. doi:10.1098/rstb.2008.0174
- Wootton RJ. 1976. The biology of the sticklebacks. London (UK): Academic Press Inc.
- Zelano B, Edwards SV. 2002. An MHC component to kin recognition and mate choice in birds: predictions, progress, and prospects. Am Nat (Suppl). 160(6):S225–S237. doi:10/1086/342897
