Abstract
Fish are significant predators of amphibian larvae in streams and larvae can detect these predators through both visual and olfactory cues. The ability to effectively recognize these cues may depend on the evolutionary history of predator and prey such that recently introduced predators may not be recognized as readily as those that have consistently coexisted with the prey species. As such, the relatively recent introduction of rainbow trout (Oncorhynchus mykiss) into Southern Appalachian headwater streams where the black-bellied salamander (Desmognathus quadramaculatus) and brook trout (Salvelinus fontinalis) naturally coexist has raised concern. The objective of this study was to determine whether black-bellied salamander larvae respond to cues from introduced rainbow trout. To evaluate this, salamander activity metrics (general activity and number of movements) were recorded before and after exposure to either native trout predator cue (brook), introduced trout predator cue (rainbow), or conditioned tap water (control). Larvae were collected from different stream reaches based on their cooccurrence with brook trout only, rainbow trout only, rainbow and brook trout, or no trout predators. Larvae that co-occur with trout reduced their activity when exposed to brook trout predator cue, but their response to rainbow trout predator cue depended on their previous co-occurrence. A follow-up test to determine the influence of alarm cue on predator response indicated that the alarm cue enhanced the response to the rainbow trout predator.
INTRODUCTION
Introduced fish species represent one of the most widespread predators of amphibians (Rahel & Smith Citation2018). In many cases, fish have been introduced into aquatic habitats to augment or establish a recreational fishery. For example, both brown trout (Salmo trutta) and rainbow trout (Oncorhynchus mykiss) are cold-water species that have been introduced into the tailwaters of many impounded rivers due to the suitable habitat created by the release of cool hypolimnetic water (Dibble et al. Citation2015). However, the introduction of these species has been associated with negative impacts on native fauna. A recent study by Miro et al. (Citation2018) found that non-native trout negatively impacted four out of six amphibian species they studied, with effects that included increased population fragmentation and extirpation. Additionally, a review of the role alien predators has played in amphibian decline by Kats and Ferrer (Citation2003) found that out of 19 different native amphibians, 10 of them were impacted negatively by introduced rainbow trout. Some of the effects included decreased larval survivorship, reduced metamorphic size, and habitat use alteration. This reduced survivorship or significant population decline may result from an inability to recognize and appropriately respond to novel predators (Sih et al. Citation2010).
At the proximate level, predator–prey interactions are a battle of the senses where predator and prey each have an advantage when they gain information about the other (Ferrari et al. Citation2010a). For prey to persist with predators, they must be able to recognize and effectively respond to predatory threats (Epp & Gabor Citation2008), whether that is through avoiding predation through early detection or employing antipredator behavior to increase the chance of survival during an attack. Prey exhibit a trade-off between exposure to predation risk and energy intake through foraging (Lima & Bednekoff Citation1999; Higginson et al. Citation2012).
Aquatic prey often detect predators through visual and olfactory cues (Ferrari et al. Citation2010a). Common chemical cues that aquatic prey species respond to have been reviewed by Ferrari et al. (Citation2010a) and include kairomones such as predator cues or the “signature odor” of the predator; disturbance cues which usually result from the release of ammonia or urea by distressed prey; alarm cues which are released by injured prey tissue and provide no other context to prey except there is an actively foraging predator in the vicinity; and dietary cues that derive from the predator’s excretory products after consumption of prey. Larval amphibians have been found to respond to kairomones more effectively than visual cues, even in areas with high water clarity (Hickman et al. Citation2004; Bronmark & Hansson Citation2012).
Responses to chemical cues can be influenced through learning, diet effects, or can be innate (Ferrari & Chivers Citation2009). Innate predator recognition by prey species is facilitated through predator cues (Ferrari et al. Citation2010b). Innate predator recognition derives from consistent predator-prey regimes (Wisenden Citation2003; Gall & Mathis Citation2010). Naïve prey lack an innate predator recognition that leaves them susceptible to a predation event, and predator naivety is considered an important factor in the failure of native prey to respond to nonnative predators (Sih et al. Citation2010). This was demonstrated by Martin (Citation2014), who exposed both farm-raised and wild-caught red swamp crayfish (Procambarus clarii) to chemical cues from largemouth bass (Micropterus salmoides). Compared to wild-caught individuals, the naïve farm-raised P. clarii lacked the appropriate predator-avoidance responses, both in terms of movement and use of structural refuge, when exposed to chemical cues from bass. However, naïve prey may lack innate predator recognition and still detect nonnative predators. For example, Epp and Gabor (Citation2008) found that captive-reared San Marcos salamanders (Eurycea nana) showed innate recognition of cues from the nonnative redbreast sunfish (Lepomis auratus) and attributed this to the fish being closely related to the natural predator (Lepomis cyanellus) of the salamander since closely related species should share a similar chemical signal (Ferrari et al. Citation2007, Citation2008). This ability to display anti-predator responses upon a first encounter with a predator for which no innate response exists is known as generalization of predator recognition (Ferrari & Chivers Citation2009).
Prey can also learn to recognize an introduced predator through the association of alarm cues from both injured conspecific or heterospecific individuals within their prey-guild, paired with visual or predator cues (Brown Citation2003; Manassa et al. Citation2013). Iberian green frog tadpoles (Rana perezi) learn to associate a natricine snake (Natrix maura), a common predator of adult frogs, and a nonpredatory zebra danio fish (Brachydanio rerio) as dangerous after a single exposure to paired alarm and predator cues (Gonzalo et al. Citation2007).
Lastly, predator diet can also affect recognition of introduced and native predators by prey. Diet effects are closely related to alarm cues and are considered a post-digestion alarm cue (Ferrari et al. Citation2010a). For example, a study by Nunes et al. (Citation2013) found that nine different species of larval anurans significantly decreased their activity when exposed to diet cues from a native predator (Aeshma sp.) and a nonnative (Procambarus clarkii) predator that had both been fed conspecifics.
The Southern Appalachian brook trout (Salvelinus fontinalis) is the only native salmonid in the Southern Appalachians. Logging in the early 1900s decimated brook trout populations and an urgency to re-establish recreational fishing led to the stocking of nonnative rainbow trout (Kelly et al. Citation1980). However, their competitive impact on native brook trout or how they would affect the native amphibian fauna does not appear to have been considered.
The black-bellied salamander (Desmognathus quadramaculatus) is a stream-dwelling endemic of the Southern Appalachian region and inhabits high order, cool, large cobble mountain streams from 375 to 1,725 m (Huheey Citation1966; Mills Citation1996; Petranka Citation1998). The black-bellied salamander possesses the longest larval stage in the genus Desmognathus, lasting up to 3 years in some instances (Petranka Citation1998). The introduction of rainbow trout into headwater mountain streams has likely increased the encounters between larval black-bellied salamanders and rainbow trout. The degree to which the salamander can recognize the nonnative predator is unclear, but due to their short coevolutionary time spent as predator–preys it is likely they lack an innate response. Rainbow trout were originally introduced into Southern Appalachia in the early 1900s to augment a recreational fishery and were later stocked in headwater streams in the 1960s (Galbreath et al. Citation2001).
In this study, we aimed to determine if larval black-bellied salamanders can recognize and respond to rainbow trout predator cue. Also, with the movement of rainbow trout into brook trout areas, we aimed to test predator-avoidance responses from larvae inhabiting stream reaches with either one trout predator or both trout predators together. Lastly, we aimed to identify if larval black-bellied salamanders from a brook trout reach can enhance and maintain their response to rainbow trout when exposed to alarm cue and predator cue simultaneously.
MATERIALS AND METHODS
Larval collection and maintenance
Larval collection, maintenance, and testing was carried out under permits from the Tennessee Wildlife Resource Agency (TWRA) (Permit #1947), Tennessee Department of Environment and Conservation (TDEC) (Permit #2019-031), and the East Tennessee State University IACUC (#P190102). Larval black-bellied salamanders were collected via dip-netting/hand-collection from both Hampton Creek Cove (HCC), Carter County, TN and Lamar Alexander Rocky Fork State Park (LARFSP), Unicoi County, TN. Trout population densities in Hampton Creek have been monitored by the TWRA for over 2 decades (Habera et al. Citation2016) and based on these data, three sites along the creek were selected to collect larval salamanders based on the type of trout predators present; the middle sections of Left Prong of Hampton Creek (both rainbow and brook trout) (36.149993, – 82.050581), the upper sections of Left Prong Hampton Creek (brook trout only, upstream movement of rainbow trout is inhibited by a 3 m high dam and rainbows were removed in 2008) (36.141499, – 82.047049), and the lower sections of Hampton Creek (rainbow trout only) (36.153891, – 82.056997). Larval collection sites were greater than 200 m in distance. Larvae were also collected from the upper reaches of Long Branch Creek in LARFSP which has no trout present (36.061401, – 82.571452). A total of 150 larvae were collected, 90 from HCC (30 from each site) and 30 from LARFSP during July–August 2019. Later, 30 larvae were collected from HCC (brook trout site) during March–April 2020. In the laboratory, larvae from each site were held separately in 75.7 L tanks filled with moderately hard water (USEPA Citation2002) and fitted with aerators. The tanks were housed in a temperature-controlled environmental chamber at 15–16 °C with a 12:12 light:dark cycle. Larvae from the same collection site were further grouped based on snout-vent-length (SVL), to the nearest mm, to avoid the risk of cannibalism. Individuals between 18 and 30 mm were used as the target size since metamorphosis in streams of the Southern Appalachians can occur at SVLs between 35 and 42 mm (Petranka Citation1998). Larvae were fed a mixture of Daphnia magna and Lumbriculus variegatus (Carolina Biological Supply Company, Burlington, NC) 4 days per week and every 2 days 50% water changes occurred to control the accumulation of ammonia. Lastly, larvae were housed and allowed to acclimate to testing conditions for 10–14 days before behavioral testing.
Fish collection
Wild rainbow and brook trout were obtained by electroshocking from Briar Creek, Washington County, TN (36.228182, – 82.385848) with the assistance of fisheries biologists from the TWRA. Once collected, fish were transported back to the laboratory, separated by species, and monitored for 48-hr for signs of injury, disease, or distress in a 378 L aquarium maintained at 16 °C by using a Cyclone Drop-in Chiller (Aqua Logic Inc. San Diego, CA) and filled with dechlorinated tap water. Fish were then separated by species and placed in their own 94.6 L aquaria with an aerator. Water was maintained at 16 °C using the chiller mentioned above. Fish acclimated for 5–6 days before cue collection and were fed blood worms and brine shrimp ad libitum to avoid diet effects. Ammonia, pH, and chlorine was monitored bi-weekly using the Hach Model HA-62 water test kit (Hach Inc. Loveland, CO) with 25% water changes every 10 days. Fish were all housed in a temperature controlled 12:12 light:dark room.
Cue collection
After acclimation to laboratory conditions, three individuals of each fish species were weighed to the nearest tenth of a gram using a P-603D Precision Balance (Denver Instrument Company, Bohemia, NY) and placed into their own 5.67 L aquaria with an aerator and dechlorinated tap water at a ratio of 50 mL water per 1.0 g of fish for 24 hr. The predator cue was then pooled by species and filtered through 1.5 µm polyester fiber filter (Acurel Filter Fiber, Cranbury, NJ) to remove solid particles and then separated into 30 mL aliquots. All stimuli were held at – 6 °C in Falcon 50 mL Conical Centrifuge Tubes (Fisher Scientific, Waltham, MA) and used within 8 weeks. Control cue (dechlorinated tap water) was also generated using the same methods.
Alarm cue from D. quadramaculatus was prepared following Mirza et al. (Citation2006) by sacrificing 3–5 larvae to get a concentration of roughly 1.0 g of salamander mass per 100 mL of dechlorinated tap water. Larvae were chilled for 15 min to induce a hypothermic state and then killed by cranial compression (AVMA Citation2013). Whole larvae were then macerated with a mortar and pestle using enough dechlorinated tap water to maintain a concentration of 1 g of salamander mass per 100 mL of dechlorinated tap water. Alarm cue mixture was then filtered to remove solid particles and then immediately frozen at – 6 °C in Falcon 15 mL Conical Centrifuge Tubes (Fisher Scientific, Waltham, MA).
Predator cue recognition
The experimental procedure used to evaluate larval recognition of predator cues was modified from Chapman et al. (Citation2017). The behavioral chamber was a glass 5.7 L aquarium (15.24 × 25.4 cm) containing 1.0 L of moderately hard water. The chamber was surrounded with black plastic to minimize external visual influences. Airline tubing (0.5 cm diameter) served as the port for introducing stimulus into the tank and was glued to the side and bottom of the tank in such extended 10 cm along the bottom. The focal species is nocturnal and has increased activity at night (Peterman et al. Citation2008), therefore testing was done in complete darkness between 21:00 and 02:00 in a 16 °C temperature-controlled room.
Prior to testing, larvae were randomly selected from their holding tanks and randomly placed in the behavioral chamber for 10 min before the start of the experiment. After acclimation, the pre-stimulus phase was initiated by drawing two 25 mL aliquots of test water (via the airline tubing described above) from the behavioral chamber using two separate 30 mL syringes. The first aliquot of water was discarded, while the syringe containing the second aliquot was set aside. A third 30 mL syringe was used to inject 25 mL of dechlorinated tap water into the behavior chamber, immediately after which the contents of the second syringe was injected back into the chamber. After the pre-stimulus period was completed, the post-stimulus phase was initiated by again removing two 25 mL aliquots of water from the behavioral chamber as described above. However, in this phase 25 mL of a random treatment [dechlorinated tap water (control), brook trout cue, or rainbow trout cue] was injected into the chamber, immediately followed by injection of 25 mL of behavioral chamber water back into the tank to disperse the treatment solution.
After both the pre- and post-stimulus injection process, an additional 1 min acclimation period was allowed for complete dispersion of stimulus. Larval behavior during both 8 min phases was observed using 2 Defender 4 K (8MP) Wired Security System infrared cameras (Defender Phoenixm2, Niagara Falls, ON) with the recordings assessed after the experiment by an observer who was blind to the treatment groups. Larval behaviors evaluated included: Time spent active: the total time the larvae spent moving during the testing period and the total number of movements: a movement was defined as a single period of activity; regardless how active the individual was. After completion of an individual behavior trial, the chamber and tubing were cleaned with hot tap water for 2 min, followed by rinsing with cold moderately hard water for 1 min to flush out any residual cue. A total of 90 larvae from HCC (30 from each site) and 30 larvae from LARFSP were tested.
Conditioned response
The objective of this experiment was to determine if larval black-bellied salamanders exhibited an enhanced response to rainbow trout cue when they had previous exposure to an alarm/predator cue combination. The experimental procedure closely followed that described above, including use of the same behavioral chambers, stimulus injection procedure, pre- and post-stimulus periods, use of cameras to record behavior and behavior assessment. The key differences were that this assay was conducted over a 5-day period (3 days of testing separated by 1 day without testing) and larvae used in this experiment were obtained from the brook trout section of HCC and were separated into ‘control’ and ‘experimental’ groups with 15 larvae tested in each group.
The pre-stimulus cue was always 20 mL dechlorinated tap water, but the post-stimulus cue varied by treatment group and day. For the post-stimulus treatment on day 1, individuals in both control and experimental groups were exposed to 20 mL dechlorinated tap water to establish baseline activity. On day 3, individuals in the experimental group were exposed to 10 mL of predator cue and 10 mL of alarm cue, and the control group were exposed to 10 mL of predator cue and 10 mL of dechlorinated tap water. On day 5, both control and experimental groups were exposed 10 mL of predator cue and 10 mL of dechlorinated tap water in the post-stimulus phase. Cleaning of chambers to remove residual cue followed that described above.
Statistical analysis
All behavioral data were evaluated using residual plots and Shapiro–Wilks-Z tests and were found to meet the assumptions of normality and homoscedasticity. For the study of predator cue recognition, a two-way analysis of variance (ANOVA) followed by a Tukey’s HSD post hoc test was employed. One factor was the stream reach the larvae originated from and the other factor was the treatment the larvae received. The post-stimulus period was subtracted from the pre-stimulus period to control for individual animal behavior. This analysis was performed using SAS 9.4M6 (SAS Institute, Cary, NC, USA) software.
For the experiment examining the combination of rainbow trout and alarm cue, values for the post-stimulus behavioral metrics were again subtracted from the pre-stimulus values and a two-way repeated measures ANOVA followed by a Tukey’s HSD post-hoc test was used to observe the change in the behavior metrics between the control and experimental groups over the 3-day testing period. The analysis was performed using SigmaPlot 11. All statistical comparisons were performed at α = 0.05.
RESULTS
Experiment 1: Determining predator cue recognition
In this experiment, we evaluated predator cue recognition based on activity metrics between larval D. quadramaculatus collected from stream reaches with different trout predator assemblages.
There was a significant interaction between cue treatment and the stream reach larvae were collected from for both activity metrics (P < 0.001). Larvae collected from no trout stream reaches and stream reaches with only brook trout exhibited no significant difference in number of movements when exposed to any of the three cue treatments (). For larvae from the brook trout stream reach, time spent active was significantly (P = 0.045) reduced as compared to control when faced with brook trout cue, but not with rainbow trout cue. Additionally, there was no significant difference in time spent active between larvae from the brook trout section when they were exposed to either brook or rainbow trout cue. Larvae from stream reaches that contained rainbow trout or rainbow and brook trout exhibited significant (P < 0.001) reductions in time spent active and number of movements when exposed to both brook and rainbow trout cue as compared to the control larvae.
Fig. 1. Mean ± SE (a) time spent active (sec) and (b) number of movements for larval D. quadramaculatus from varying trout predator sections. Bars with different letters are significantly different at α = 0.05 within a particular stream reach, while bars with different numbers are significantly different across stream reaches but within a given predator cue treatment.
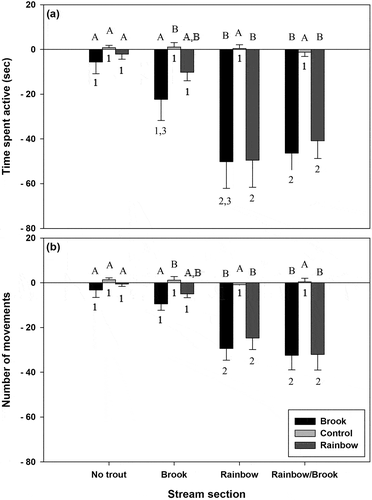
There was no significant difference in time spent active or number of movements between larvae from any of the stream reaches when they were exposed to the dechlorinated water control (). Similarly, there was no significant difference in the two activity metrics for any cue treatments between larvae from the no trout stream reach and the brook trout stream reaches. However, when exposed to either brook trout or rainbow trout cue, larvae from the rainbow trout and rainbow/brook stream reaches both exhibited significant (P = 0.024) decreases in time spent active and number of movements as compared to larvae from the no trout section and, in most cases, from the brook trout section as well. The one exception to this was that time spent active in larvae from the rainbow/brook section was not significant from that in larvae from the brook trout section, although the P-value was close to the significant threshold (P = 0.068).
Experiment 2: Determining conditioned response
The objective of this experiment was to determine if pairing alarm cue and predator cue from rainbow trout enhances the response of larval D. quadramaculatus from brook trout reaches to rainbow trout cue.
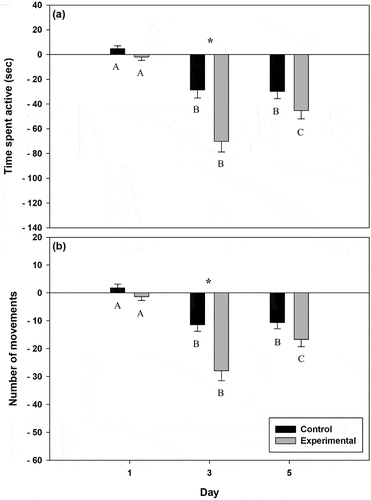
Over the 3 days of testing in both the control and experimental treatment groups, there was a significant interaction between the treatment and day for time spent active (F2,27 = 5.985, P = 0.004) and for number of movements (F2,27 = 5.307, P = 0.008). On day 1, for both activity metrics, there was no significant difference between the treatment groups (). This was expected since all larvae were exposed to only control cue. On day 3, both activity metrics possessed a significant difference within and between the treatment groups as well as a significant difference in relation to day 1. Larvae in the experimental group exhibited a significantly stronger response than those in the control group (), indicating that the paired alarm and predator cue stimulus elicits a stronger predator-avoidance response than predator cue alone. On day 5 there was no significant difference between treatment groups, but within treatment groups, larvae exhibited a significant reduction in relation to day 1 (). However, larvae in the control group did not vary between day 3 and day 5 (). For the experimental group, both time spent moving and number of movements were significantly different from what was recorded for day 3.
Fig. 2. Mean ± SE (a) time spent active (sec) and (b) number of movements for larval D. quadramaculatus from a brook trout reach. Black is the control group and grey is the experimental group. Responses not connected by the same letter are significantly different within treatment groups (α = 0.05) and responses separated by an asterisk (*) are significantly different between groups (α = 0.05).
DISCUSSION
For the present study, a reduction in activity was used to determine if individuals are displaying predator-avoidance behavior when exposed to chemical cues. Freezing or decreasing activity is a common predator-avoidance mechanism that is used in several amphibian species, especially salamanders (Petranka Citation1998; Davis & Gabor Citation2015). Therefore, it was assumed that any individuals which decreased their activity when exposed to kairomones were displaying predator-avoidance behavior. Both time spent moving and number of movements complimented each other and produced almost identical results for each stream reach.
The objective of the first experiment was to determine if larval black-bellied salamanders can recognize and respond to novel rainbow trout predator cue. Also, with the movement of rainbow trout into native brook trout areas, we aimed to test predator-avoidance responses from larvae inhabiting stream reaches with either one trout predator or both. The hypothesis was that larval black-bellied salamanders would lack predator recognition to nonnative rainbow trout and display innate recognition to native brook trout predator cue. The results from the first experiment did not support the hypothesis in that the key driver for larval response was presence of the particular predator in the stream reach, regardless of whether that predator was native or not. The response of larvae from the brook trout reach to the rainbow trout cue appeared heightened over control larvae, but the difference was not significant. This could indicate a lack of capacity to recognize the nonnative trout, or it could be an artefact of low sample size.
Our original hypothesis was based on the idea that behavioral responses to predation risk are strong where predator–prey interactions have co-evolved (Watkins Citation1996; Ferrari et al. Citation2010a), while attacks by novel predators may result in reduced or ineffective responses by native prey due to the lack of prior experience (Schlaepfer et al. Citation2005). Gall and Mathis (Citation2010) found that larval eastern hellbenders (Cryptobranchus alleganiensis) responded to cues from nonnative salmonids when compared to a blank control but not nearly as strongly as they responded to native predatory fish. They attributed this weaker response to the short amount of time that predator and prey coexisted. Alternatively, the difference in response to trout predators observed in the present experiment could be the result of the relative risk associated predation exposure. Studies have shown that inexperienced fish and larval amphibians in low-risk environments do not respond to novel kairomones, while those in high-risk environments do (Brown et al. Citation2013, Citation2015; Chivers et al. Citation2014). The encroachment of rainbow trout into brook trout habitat of Hampton Creek has only recently occurred and rainbow trout were removed via electroshocking in 2008 (Habera et al. Citation2016). As such, larvae in this reach may not encounter rainbow trout regularly and so identify them as a low-risk predator.
Larvae from reaches with rainbow trout and reaches with both trout species strongly reduced their activity to trout predators and exhibited the strongest reduction in activity when compared to larvae from stream reaches with no trout or with brook trout only (). Polo-Cavia et al. (Citation2020) similarly found that larval Iberian frogs (Rana iberica) that coexist with native and nonnative salmonids also exhibited decreased activity to cue from both predators. The stronger response that larvae from stream reaches that contained rainbow trout may again be related to the degree of predatory risk the species poses. Previous studies have indicated that rainbow trout will outcompete brook trout for food resources (Rose Citation1986; Thibault & Dodson Citation2013), suggesting the former may be a more aggressive predator. Additionally, in habitat with both trout predators, rainbow trout have been known to force brook trout towards the periphery of the stream and inhabit middle sections (Lohr & West Citation1992), which also is the habitat preference for larval and juvenile black-bellied salamanders (Petranka Citation1998). Apart from increased predator density, this likely increases interaction between predator and prey, deeming it a high predation risk environment.
Larvae from stream reaches with rainbow trout only exhibited a heightened response to brook trout cue even though the native species was not known to occur in the reach. This could suggest those larvae had previous exposure to brook trout cue since brook trout do occur in the upper reaches in the system. However, the distance between the sections containing the two trout species was roughly 240 m which would have led to significant dilution of any cue derived from brook trout before it reached the rainbow trout section (Large et al. Citation2011). Alternatively, it is possible that predator cue generalization is playing a role. Predator cue generalization can occur when two predatory species are closely related (Davis et al. Citation2012; Ferrari et al. Citation2016). For example, fathead minnows (Pimephales promelas) generalize predator cue when exposed to brown trout and novel rainbow trout, both in the family Salmonidae (Ferrari et al. Citation2010b)
The lack of predator avoidance response in larval salamanders from the reach with no trout predators was expected. Anti-predator behavior is costly in terms of time and access to resources (Sih et al. Citation2010; Anson & Dickman Citation2013) and should only be maintained if it provides net fitness benefits. The loss or removal of a strong selective force imposed by a predator may therefore lead to the relaxation of predator recognition and associated anti-predator behavior in prey (Lahti et al. Citation2009; Wertheim et al. Citation2015). The generational time it takes for relaxation of antipredator behavior is relatively limited and possibly species specific. Jolly et al. (Citation2018) found that wild caught northern quolls (Dasyurus hallucatus) that were isolated from predators for 13 generations, showed no recognition or aversion to nonnative predators. Although the history of salmonids in this reach is unknown, larvae that inhabit this stream do not currently encounter trout predators. Garcia et al. (Citation2012) found that American bullfrog (Lithobates catesbeianus) tadpoles varied in their response to a historical predator, largemouth bass (Micropterus salmoides). They found that tadpoles from populations that co-occur with bass exhibited antipredator behavior (increased refuge use) while individuals that did not co-occur failed to exhibit any antipredator behavior towards chemical cues from the bass or chemical cues with diet cues (largemouth bass fed bullfrog tadpoles). This is also true for predator-naïve agile frog (Rana dalmatina) tadpoles. A study by Hettyey et al. (Citation2016) reported that R. dalmatina tadpoles from fishless habitats displayed a weak antipredator response to predatory fishes when compared to conspecifics from nearby floodplain populations that were exposed to fish predators.
The objective of the second experiment was to determine if larval black-bellied salamanders from the brook trout reach would exhibit an enhanced response to rainbow trout after they had been exposed to rainbow trout predator cue combined with alarm cue. The hypothesis was that larvae would decrease their activity when exposed to alarm cue and predator cue as compared to exposure to predator cue alone and would continue that response once alarm cue was removed. The results from this experiment did not support the hypothesis entirely since the addition of alarm cue did reduce activity in larvae, but that heightened response was not maintained once alarm cue was removed.
On day 1, both treatment groups were exposed to control cue and did not significantly differentiate in activity from one another. For day 3, the addition of alarm cue caused a significant decrease in activity in the experimental group compared to the control group. This was not surprising because alarm cues should invoke stronger behavioral responses to avoid predation since damaged epidermal tissues are released in no other context and advertise the presence of an actively foraging predator (Ferrari et al. Citation2010a). This has been demonstrated by Kenison et al. (Citation2018) in studies exposing rusty crayfish (Orconectes rusticus) to salamander and fish predator cues and conspecific alarm cues. They found that antipredator responses were increased when crayfish were exposed to predator cues in combination with conspecific alarm cues compared to just predator cue. On day 5 of the present study, both treatment groups were subjected to rainbow trout predator cue and there was no significant difference between them. Thus, while alarm cue may have strengthened the response on day 3, this response was not maintained on day 5, indicating the larvae did not “learn” from the single exposure event on day 3. However, it is possible that a single conditioning event was not enough to induce a learned response. Prey must continually update their perception of risk and adjust their behavior in response to predation risk because it can fluctuate widely across space and time (Lucon-Xiccato et al. Citation2016). Exposure to a single predation event does not always inform prey about the risk of being attacked in the future because predation risk may be assessed based on the frequency of events over longer periods of time (background risk) (Lucon-Xiccato et al. Citation2016). As a result, it seems background risk often has a large effect in determining future antipredator decisions (Lima & Bednekoff Citation1999; Brown et al. Citation2015). Lucon-Xiccato et al. (Citation2016) found that wood frog tadpoles (Lithobates sylvaticus) exposed to alarm cues from conspecifics raised in a high-risk environment exhibited a stronger antipredator response and an enhanced learned response to novel predators as compared to tadpoles exposed to alarm cues from conspecifics raised in a low-risk environment. Black-bellied salamanders in rainbow/brook trout and rainbow trout reaches are experiencing a nonnative predator more frequently than larvae from the brook trout reach. This difference in predator frequency and level of risk may be why larvae exhibited a weak predator-avoidance response in the first experiment and did not maintain an enhanced response to rainbow trout predator cue once alarm cue was removed in the second. Additionally, it could also be that more than one exposure event may be required for larvae to maintain a heightened response to nonnative rainbow trout. For example, Vilhunen (Citation2006) found that conditioning Arctic char (Salvelinus alpinus) with predator cue and alarm cue increased their chances of survival, with Arctic char that had experienced multiple conditioning events having a higher chance of survival as compared to Arctic char that had only received one conditioning event. For black-bellied salamanders, further work studying alarm cue and predator cue response in reaches with rainbow trout may be needed to verify this.
CONCLUSION
The results of this study suggest that co-occurrence and predation risk with a salmonid predator was a more important driver of anti-predator behavior for black-bellied salamander larvae than an innate response to a native predator. Predation risk and experience seems to play a role in predator recognition and predator generalization for larval black-bellied salamanders in this system. Larvae from reaches absent of rainbow trout did not maintain predator avoidance behavior once alarm cue was removed, further promoting the idea that predation risk, experience, and predator intensity may play a more important role. However, further work assessing the possibility of learned predator recognition in truly naïve individuals and predator conditioning through exposure to multiple predation events may be needed to rule out learned predator recognition in larval black-bellied salamanders. Studies expanding on the two hypothesis of predator generalization and learning may require using individuals from high-risk and low-risk predator environments, wild caught vs naïve individuals, and generational testing.
HIGHLIGHTS
Larvae responded to rainbow trout predator cue based on their previous co-occurrence with that predator.
Larvae did not show the capacity to learn to identify a novel predator as dangerous, although consecutive exposure events may be required.
Predation risk and experience likely play a role in predator recognition and predator generalization for larval salamanders in this system.
ACKNOWLEDGEMENTS
The authors are thankful to the anonymous reviewers for their valuable comments and feedback on an earlier draft of the manuscript. We also thank Trevor Chapman for help with experimental design and statistical advice. Special thanks to Celeste Gallardo and Anna Grizzard for help with field collections. Additionally, we would like to thank the Tennessee Wildlife Resource Agency (TWRA) and Tennessee Department of Environment and Conservation (TDEC) for permitting the collection of larvae and a special thank you to Jim Habera of the TWRA for all the help he provided in collecting trout. Lastly, we would like to thank East Tennessee State University’s Institutional Animal Care and Use Committee (IACUC) for permitting this study.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the authors.
Additional information
Funding
REFERENCES
- Anson JR, Dickman CR. 2013. Behavioral responses of native prey to disparate predators: naiveté and predator recognition. Oecologia. 171(2):367–377. doi:10.1007/s00442-012-2424-7
- [AVMA] American Veterinary Medical Association. 2013. Guidelines on euthanasia. Available from: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf [Accessed 2020 Oct 20].
- Bronmark C, Hansson LA, editors. 2012. Chemical ecology in aquatic systems. Oxford (UK): Oxford University Press.
- Brown GE. 2003. Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish. 4(3):227–234. doi:10.1046/j.1467-2979.2003.00132.x
- Brown GE, Elvidge CK, Ramnarine I, Ferrari MC, Chivers DP. 2015. Background risk and recent experience influences retention of neophobic responses to predators. Behav Ecol Sociobiol. 69(5):737–745. doi:10.1007/s00265-015-1888-y
- Brown GE, Ferrari MC, Elvidge CK, Ramnarine I, Chivers DP. 2013. Phenotypically plastic neophobia: a response to variable predation risk. Proc Biol Sci. 280(1756):20122712. doi:10.1098/rspb.2012.2712
- Chapman TL, Spivey KL, Lundergan JM, Schmitz AL, Bast DL, Sehr EK, Gall BG. 2017. Only fear the fatal foe: predation risk assessment by eastern newts (Notophthalmus viridescens) in response to common snapping turtles and other potential predators. Ethol Ecol Evol. 29(3):218–228. doi:10.1080/039493702015.1137358
- Chivers DP, McCormick MI, Mitchell MD, Ramasamy RA, Ferrari MC. 2014. Background level of risk determines how prey categorize predators and non- predators. Proc Biol Sci. 281(1787):20140355. doi:10.1098/rspb.2014.0355
- Davis DR, Epp KJ, Gabor CR, Ebensperger L. 2012. Predator generalization decreases the effect of introduced predators in the San Marcos salamander, Eurycea nana. J Ethol. 118:1191–1197. doi:10.1111/eth.12025
- Davis DR, Gabor CR. 2015. Behavioral and physiological antipredator responses of the San Marcos salamander (Eurycea nana). Physiol Behav. 139:145–149. doi:10.1016/j.physbeh.2014.11.013
- Dibble KL, Yackulic CB, Kennedy TA, Budy P. 2015. Flow management and fish density regulate salmonid recruitment and adult size in tailwaters across western North America. Ecol Appl. 25:2168–2179. doi:10.1890/14-2211.1
- Epp KJ, Gabor CR. 2008. Innate and learned predator recognition mediated by chemical signals in Eurycea nana. J Ethol. 114:607–615. doi:10.1111/j.1439-0310.2008.01494.x
- Ferrari MC, Chivers DP. 2009. Temporal variability, threat sensitivity and conflicting information about the nature of risk: understanding the dynamics of tadpole antipredator behavior. Anim Behav. 78:11–16. doi:10.1016/j.anbehav.2009.03.016
- Ferrari MC, Crane AL, Chivers DP. 2016. Certainty and the cognitive ecology of generalization of predator recognition. Anim Behav. 111:207–211. doi:10.1016/j.anbehav.2015.10.026
- Ferrari MC, Gonzalo A, Messier F, Chivers DP. 2007. Generalization of learned predator recognition: an experimental test and framework for future studies. Proc Biol Sci. 274(1620):1853–1859. doi:10.1098/rspb.2007.0297
- Ferrari MC, Lysak KR, Chivers DP. 2010a. Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim Behav. 79:515–519. doi:10.1016/j.anbehav.2009.12.006
- Ferrari MC, Messier F, Chivers DP. 2008. Can prey exhibit threat-sensitive generalization of predator recognition? Extending the predator recognition continuum hypothesis. Proc Biol Sci. 275:1811–1816. doi:10.1098/rspb.2008.0305
- Ferrari MC, Wisenden BD, Chivers DP. 2010b. Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool. 88:698–724. doi:10.1139/Z10-029
- Galbreath PF, Adams ND, Guffey SZ, Moore CJ, West JL. 2001. Persistence of native southern Appalachian brook trout populations in the Pigeon River system, North Carolina. N Am J Fish Manage. 21:927–934. doi:10.1577/1548-8675(2001)021<0927:PONSAB>2.0.CO;2
- Gall BG, Mathis A. 2010. Innate predator recognition and the problem of introduced trout. J Ethol. 116:47–58. doi:10.1111/j.1439-0310.2009.01718.x
- Garcia TS, Thurman LL, Rowe JC, Selego SM. 2012. Antipredator behavior of American bullfrogs (Lithobates catesbeianus) in a novel environment. J Ethol. 118:867–875. doi:10.1111/j.1439-0310.2012.02074.x
- Gonzalo A, López P, Martín J. 2007. Iberian green frog tadpoles may learn to recognize novel predators from chemical alarm cues of conspecifics. Anim Behav. 74:447–453. doi:10.1016/j.anbehav.2006.11.032
- Habera JW, Bivens RD, Carter BD, Williams CE. 2016. Region IV trout fisheries report no. 17-02. Nashville (TN): Tennessee Wildlife Resources Agency.
- Hettyey A, Thonhauser KE, Bókony V, Penn DJ, Hoi H, Griggio M. 2016. Naive tadpoles do not recognize recent invasive predatory fishes as dangerous. Ecology. 97:2975–2985. doi:10.1002/ecy.1532
- Hickman CR, Stone MD, Mathis A. 2004. Priority use of chemical over visual cues for detection of predators by graybelly salamanders (Eurycea multiplicata griseogaster). Herpetologica. 60:203–210. doi:10.1655/03-26
- Higginson AD, Fawcett TW, Trimmer PC, McNamara JM, Houston AI. 2012. Generalized optimal risk allocation: foraging and antipredator behavior in a fluctuating environment. Am Nat. 180:589–603. doi:10.1086/667885
- Huheey JE. 1966. The desmognathine salamanders of the Great Smoky Mountains National Park. J Ohio Herpetol Soc. 5(3):63–72. doi:10.2307/1562609
- Jolly CJ, Webb JK, Phillips BL. 2018. The perils of paradise: an endangered species conserved on an island loses antipredator behaviours within 13 generations. Biol Lett. 14(6):20180222. doi:10.1098/rsbl.2018.0222
- Kats LB, Ferrer RP. 2003. Alien predators and amphibian declines: review of two decades of science and the transition to conservation. Divers Distrib. 9:99–110. doi:10.1046/j.1472-4642.2003.00013.x
- Kelly GA, Griffith JS, Jones RD. 1980. Changes in distribution of trout in Great Smoky Mountains National Park. 1900-1977. Vol. 102. Washington (DC): US Department of the Interior, US Fish and Wildlife Service.
- Kenison EK, Weldy PY, Williams RN. 2018. There must be something in the water: assessing the behavioral responses of rusty crayfish (Orconectes rusticus) to fish and amphibian predator kairomones. J Ethol. 36:77–84. doi:10.1007/s10164-017-0529-5
- Lahti DC, Johnson NA, Ajie BC, Otto SP, Hendry AP, Blumstein DT, Coss RG, Donohue K, Foster SA. 2009. Relaxed selection in the wild. Trends Ecol Evol. 24:487–496. doi:10.1016/j.tree.2009.03.010
- Large SI, Smee DL, Trussell GC. 2011. Environmental conditions influence the frequency of prey responses to predation risk. Mar Ecol Prog Ser. 422:41–49. doi:10.3354/meps08930
- Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat. 153:649–659. doi:10.1086/303202
- Lohr SC, West JL. 1992. Microhabitat selection by brook and rainbow trout in a southern Appalachian stream. Trans Am Fish Soc. 121:729–736. doi:10.1577/1548-8659(1992)121<0729:MSBBAR>2.3.CO;2
- Lucon-Xiccato T, Chivers DP, Mitchell MD, Ferrari MC. 2016. Making the dead talk: alarm cue-mediated antipredator behavior and learning are enhanced when injured conspecifics experience high predation risk. Biol Lett. 12:20160560. doi:10.1098/rsbl.2016.0560
- Manassa RP, Dixson DL, McCormick MI, Chivers DP. 2013. Coral reef fish incorporate multiple sources of visual and chemical information to mediate predation risk. Anim Behav. 86:717–722. doi:10.1016/j.anbehav.2013.07.003
- Martin CW. 2014. Naïve prey exhibit reduced antipredator behavior and survivorship. PeerJ. 2:665. doi:10.7717/peerj.665
- Mills GR. 1996. A study on the life history and seasonal foraging habits of the salamander Desmognathus quadramaculatus (Holbrook) in WV [Thesis]. Huntington (WV): Marshall University.
- Miro A, Sabas I, Ventura M. 2018. Large negative effect of non-native trout and minnows on Pyrenean lake amphibians. Biol Conserv. 218:144–153. doi:10.1016/j.biocon.2017.12.030
- Mirza RS, Ferrari MC, Kiesecker JM, Chivers DP. 2006. Responses of American toad tadpoles to predation cues: behavioural response thresholds, threat-sensitivity and acquired predation recognition. Behaviour. 143:877–889. doi:10.1163/156853906778017926
- Nunes AL, Richter-Boix A, Laurila A, Rebelo R. 2013. Do anuran larvae respond behaviorally to chemical cues from an invasive crayfish predator? A community-wide study. Oecologia. 171:115–127. doi:10.1007/s00442-012-2389-6
- Peterman WE, Crawford JA, Semlitsch RD. 2008. Productivity and significance of headwater streams: population structure and biomass of the black‐bellied salamander (Desmognathus quadramaculatus). Freshw Biol. 53:347–357. doi:10.1111/j.1365-2427.2007.01900.x
- Petranka JW. 1998. Salamanders of the United States and Canada. Washington (DC): Smithsonian.
- Polo-Cavia N, Boyero L, Martín-Beyer B, Navazo T, Bosch J. 2020. Effects of coexistence and predator experience on anti-predatory responses of montane amphibian larvae towards native and introduced salmonids. Biol Invasions. 22:379–390. doi:10.1007/s10530-019-02095-6
- Rahel FJ, Smith MA. 2018. Pathways of unauthorized fish introductions and types of management responses. Hydrobiologia. 817:41–56. doi:10.1007/s10750-018-3596-x
- Rose GA. 1986. Growth decline in subyearling brook trout (Salvelinus fontinalis) after emergence of rainbow trout (Salmo gairdneri). Can J Fish Aquat Sci. 431:187–193. doi:10.1139/f86-021
- Schlaepfer MA, Sherman PW, Blossey B, Runge MC. 2005. Introduced species as evolutionary traps. Ecol Lett. 8:241–246. doi:10.1111/j.1461-0248.2005.00730.x
- Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM, Preisser E, Rehage JS, Vonesh JR. 2010. Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos. 119:610–621. doi:10.1111/j.16000706.2009.18039.x
- Thibault I, Dodson J. 2013. Impacts of exotic rainbow trout on habitat use by native juvenile salmonid species at an early invasive stage. Am Fish Soc. 142:1141–1150. doi:10.1080/00028487.2013.799516
- [USEPA] United States. Environmental Protection Agency. 2002. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, 5th ed. Washington (DC): USEPA.
- Vilhunen S. 2006. Repeated antipredator conditioning: a pathway to habituation or to better avoidance? J Fish Biol. 68:25–43. doi:10.1111/j.0022-1112.2006.00873.x
- Watkins TB. 1996. Predator-mediated selection on burst swimming performance in tadpoles of the Pacific tree frog (Pseudacris regilla). Physiol Zool. 69:154–167. doi:10.1086/physzool.69.1.30164205
- Wertheim JO, Murrell B, Smith MD, Kosakovsky-Pond SL, Scheffler K. 2015. RELAX: detecting relaxed selection in a phylogenetic framework. Mol Biol Evol. 32:820–832. doi:10.1093/molbev/msu400
- Wisenden BD. 2003. Chemically mediated strategies to counter predation. In: Collin SP, Marshall NJ, editors. Sensory processing in aquatic environments. New York (NY): Springer; p. 236–251.
