Abstract
Palladium was rubbed against aluminum oxide in an atmosphere of carbon monoxide and oxygen. The synthesis of carbon dioxide was enhanced during the rubbing. The activation energy of this reaction was 2.49 kJ/mol. As the sliding commenced, the desorption of carbon dioxide overshot its equilibrium value before stabilizing. The overshoot correlated with the time interval between the friction tests. The desorption mechanism of carbon dioxide is discussed in terms of the NIRAM (negative-ion-radical action mechanism) approach; exoelectron emission was enhanced by the friction of palladium. The electrons reacted with the adsorbed species to make negative-ion-radicals. These radicals further reacted on the surface to form carbon dioxide, which then desorbed.
INTRODUCTION
On the surface of a catalyst, the chemisorbed gas reacts with another kind of chemisorbed gas. The authors have found that this gas-gas reaction was enhanced by friction and called it “friction catalysis” or “tribo-catalysis” (Hiratsuka, et al. (Citation1), (Citation2)). For example, the synthesis reactions listed below were enhanced by the rubbing of platinum or palladium:
| 1. | water synthesis from hydrogen and oxygen (Hiratsuka, et al. (Citation1)), | ||||
| 2. | carbon dioxide synthesis from carbon monoxide and oxygen (Hiratsuka, et al. (Citation2)). | ||||
Especially in the second reaction, more carbon dioxide was produced during rubbing between aluminum oxide and palladium than between palladium and itself. In these experiments, almost no wear occurred during rubbing between aluminum oxide and palladium as compared to the rubbing of the self-mated palladium pair. The reaction mechanism of this process is Langmuir-Hinshelwood in type, where the surface reaction is the rate-determining step. However, how the reaction is enhanced has not been clarified yet.
In this article, the synthesis reaction of carbon dioxide is studied in detail. The effect of specimen temperature, the relationship between gas desorption and coefficient of friction, and the effect of exposure time on desorption is discussed. Then using the NIRAM (negative-ion-radical action mechanism) approach, the authors discuss in detail the reaction mechanism enhanced by friction of the catalyst.
EXPERIMENTAL PROCEDURE
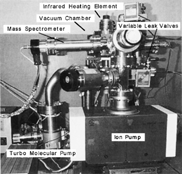
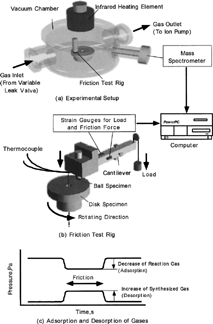
shows a photograph of the test apparatus. The friction test rig was installed in a vacuum chamber. The partial pressures inside the chamber were detected by a quadrupole mass spectrometer. The gases of carbon monoxide (purity 99.95%) and oxygen (purity 99.99%) were introduced through variable leak valves. The chamber was evacuated by a turbo molecular pump and finally by an ion pump down to 10−6 Pa. An infrared heating element above the chamber heated both pin and disk specimens up to 110°C.
shows the experimental procedure, and shows the schematic of the experimental setup. A pin-on-disk type friction test rig was installed in the chamber. shows the detail of the rig. Both load and friction forces were measured by the strain gauges attached on the sides of the arm. The load was applied from outside by dead weights. A thermocouple was used to monitor the temperature of the pin holder, which was related to the temperature of the ball. The inlet and outlet flow rates of gas were adjusted to maintain the equilibrium pressure in the chamber in which the pin-on-disk test rig was installed. When sliding commenced, the partial pressure of the chemisorbed gas decreased, while that of the desorbed gas increased (Mori, et al. (Citation3)). The chemisorption and desorption rates were calculated from the pressure change (Mori, et al. (Citation3), Sasada, et al. (Citation4)). Aluminum oxide and palladium (purity 99.99%) were used as ball (3mm in diameter) and disk specimens, respectively. The ball was attached onto the edge of the pin specimen. The load was 3.0 N, and the sliding velocity was 31 mm/s except in some experiments. The specimens were heated by the lamp from the outside of the vacuum chamber through a glass.
RESULTS
Temperature Effect
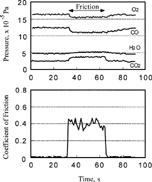
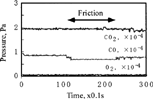
shows the variation of partial pressure of oxygen, carbon monoxide, carbon dioxide, and water as well as the coefficient of friction at 27°C. It is clear that when the sliding commenced, the pressure of carbon monoxide and oxygen decreased, whereas that of carbon dioxide and water increased.
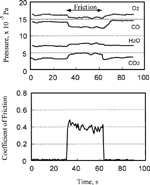
shows the pressure variation of the four gases during sliding at 110°C. The desorption of carbon dioxide slightly increased as compared to the case of 27°C.
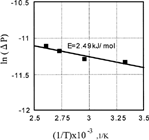
The logarithm function of pressure decrease is plotted against the temperature of palladium in . It is calculated that the activation energy of desorption of carbon dioxide is 2.49 kJ/mol.
Relationship Between Desorption of Carbon Dioxide and Coefficient of Friction
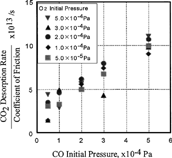
As shown in , both the partial pressures and friction coefficient vary with time. The relationship between them is plotted in . When the pressure of oxygen and carbon monoxide was 1.0 × 10− 4 Pa, their chemisorption rate and the desorption rate of carbon dioxide remained constant and independent of the coefficient of friction as shown in . On the other hand, if the partial pressure of both oxygen and carbon monoxide increased, the desorption rate of carbon dioxide increased with the coefficient of friction, as shown in .
FIG. 6 Relationship between coefficient of friction and chemisorption or desorption rate of gases, O2, CO; 1.0 × 10−4 Pa.
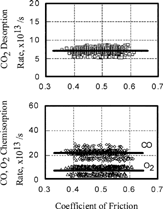
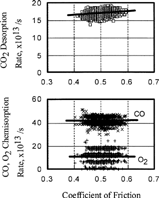
FIG. 7 Relationship between coefficient of friction and chemisorption or desorption rate of gases, O2, CO; 5.0 × 10−4 Pa.
In the ratio between the oxygen chemisorption rate and the coefficient of friction is plotted against oxygen initial pressure. It is clear that the oxygen chemisorption rate over the coefficient of friction was almost zero, which meant that the oxygen chemisorption rate was not affected by the oxygen initial pressure. As shown in , the ratio of the chemisorption rate of carbon monoxide and the coefficient of friction was not affected by the initial pressure of carbon monoxide either.
FIG. 8 Effect of CO and O2 pressure on the ratio of O2 chemisorption rate over coefficient of friction.
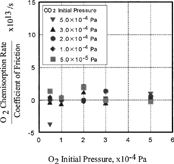
FIG. 9 Effect of CO and O2 pressure on the ratio of CO chemisorption rate over coefficient of friction.
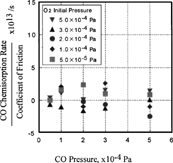
On the other hand, it is noteworthy that the ratio of the desorption rate of carbon dioxide and the coefficient of friction increased with increasing initial pressure of carbon monoxide, as shown in . At 5 × 10− 4 Pa of carbon monoxide, the ratio of the desorption rate of carbon dioxide and the coefficient of friction is 10. This means that the desorption rate is very sensitive to the coefficient of friction when the pressure of carbon monoxide is high. It is also to be noted that oxygen pressure change had a smaller effect than carbon monoxide.
Effect of Exposure Time
shows an example of the pressure change of three gases at the start and end of sliding. At the start of sliding, carbon dioxide showed an abrupt increase, then gradually decreased to the equilibrium pressure.
FIG. 11 Pressure change of carbon monoxide, oxygen, and carbon dioxide at the start and the end of sliding.
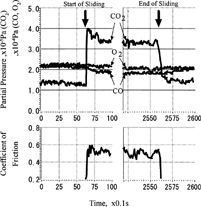
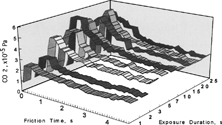
This phenomenon depends on how long the specimens were exposed to the environment of carbon monoxide and oxygen before sliding. shows the pressure change of carbon dioxide as a function of friction time and exposure duration before sliding. Except for one second of exposure, the pressure increased more than the equilibrium. This overshoot was more when the exposure time was long. However, the overshoot pressure did not increase above a saturation at an exposure duration of 20 seconds; after longer exposure than 20 seconds, the initial increase of pressure was the same.
FIG. 12 Effect of exposure duration before friction on CO2 pressure (CO and O2; 5.0 × 10−5 Pa), sliding velocity = 93 mm/s.

By increasing the concentration of the reaction gases (carbon monoxide and oxygen), the pressure increase of carbon dioxide saturated at a shorter exposure duration of seven seconds, as shown in . There is an overshoot of pressure increase even at one second of exposure.
Chemisorption of Carbon Dioxide on Palladium Surface
The pressure change of carbon dioxide (purity 99.99%) was also measured after it was introduced into the chamber in almost the same amount as carbon monoxide. As shown in , when the sliding commenced, the pressure of carbon dioxide did not change. This means that carbon dioxide does not adsorb on the worn surface of palladium during sliding. It reflects that carbon dioxide desorbs as soon as carbon monoxide and oxygen react on the surface.
DISCUSSION
NIRAM Approach
Kajdas developed the concept of NIRAM (negative-ion-radical action mechanism) to account for many chemical phenomena associated with friction processes. The concept of NIRAM is summarized as follows (Kajdas (Citation5), (Citation6)):
Low-energy electrons (1-4 eV) emitted from the surface react with the molecules to become negative-ion-radicals. Some molecules dissociate into anion and radical. They then chemisorb onto the positively charged spots where the electrons had been emitted. The radicals are so active that various kinds of reactions can take place on the surface. NIRAM accounts for tribochemical phenomena from the most simple boundary lubrication processes to several complex tribochemical reactions, e.g.:
| 1. | Triboreaction products of carboxylic acids in boundary lubrication regime (Majzner and Kajdas (Citation7)), | ||||
| 2. | Wear reduction mechanisms by addition-type polymerization (Kajdas, et al. (Citation8), Furey, et al. (Citation9)), | ||||
| 3. | Degradation mechanism of perfluoropolyethers with a diamond-like coating (DLC) in thin-film magnetic rigid disks (Bhushan and Kajdas (Citation10)), and | ||||
| 4. | Fretting corrosion of steel under conditions of fretting contact (Ghasemi, et al. (Citation11)). | ||||
This concept will also encompass the mechanism of chemical wear such as mild wear formation.
The crucial factor of the NIRAM approach is that it utilizes the low-energy electrons emitted during friction (exoelectrons). It is widely recognized that when the two materials are in sliding contact, charged particles such as electrons, anions, and cations are emitted, which is called triboemission (Molina, et al. (Citation12)). Among those particles, low-energy electrons (1-5 eV) are of particular importance, because they react with molecules, which then dissociate, forming an anion and a radical (dissociative electron attachment) (Marienfeld, et al. (Citation13)). When the electrons do not have enough energy, they just react with the molecules to become negative-ion-radicals. Those molecules then induce many of the chemical reactions listed before.
Tribochemical and Tribocatalytic Reactions
Chemical reactions such as oxidation of metals are enhanced by heating. On the other hand, the surface can easily be oxidized by repeated rubbing in ambient average temperature and pressure under operating conditions not generating a sufficient amount of heat necessary to overcome the activation energy. For example, to produce iron oxide from iron and oxygen (O2) the activation energy (Ea) for the thermochemical reaction is 54 kJ/mol (Heinicke (Citation14)). On the other hand, for the oxidation process of iron with oxygen (O2) under mechanical treatment, the activation energy amounts only to 0.7 kJ/mol (Heinicke (Citation14)). Thus, in this case the friction temperature does not increase to a certain value to initiate the static (thermochemical) oxidation process. This is called the tribochemical effect. In a tribochemical reaction, the low-energy electrons (exoelectrons) emitted during a friction process can initiate the reaction process.
The tribocatalytic reaction at elevated temperatures should be regarded as the normal catalytic reaction initiated by heat and enhanced by the action of emitted triboelectrons. It is assumed/hypothesized that in the case of pure tribocatalytic process the reaction is initiated only by electrons (Kajdas, et al. (Citation15)).
Linking tribochemistry with catalysis and tribocatalysis (Kajdas (Citation16)), it has been also assumed that flash temperature, as the maximum computed friction temperature, can be expressed in the form of the thermionic emission. That assumption was clearly confirmed by examining thermionic emission due to frictionally generated temperatures (Vick (Citation17)).
Accordingly, flash temperatures might also be expressed in the form of electronic energy. In this context the flash temperature effect as the factor initiating tribochemical reactions and possible interaction results of UV photons, present in micro-triboplasma (Nakayama and Nevshupa (Citation18)), with negatively charged particles for triggering tribochemical reactions has also been recently discussed (Kajdas (Citation19)). Thus, it is possible to conclude that low-energy electrons might be considered as the governing factor of both tribochemical reaction initiation processes and initiating and/or enhancing selected catalytic reactions; for example, water synthesis from molecular oxygen and hydrogen on platinum catalyst.
Mechanism of Oxidation of Carbon Monoxide
The characteristics of the desorption of carbon dioxide are summarized as follows:
| 1. | Desorption of carbon dioxide overshoots before being stable at the equilibrium pressure. | ||||
| 2. | The amount of overshoot depends on the exposure time and the pressure of reaction gases. | ||||
| 3. | Desorption of carbon dioxide correlates positively with the coefficient of friction. | ||||
| 4. | On the other hand, the chemisorption of carbon monoxide and oxygen does not overshoot and does not correlate with the friction value. | ||||
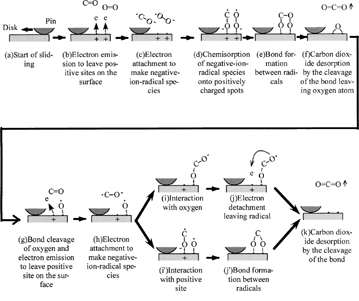
Using NIRAM, the experimental results are explained as follows ():
| 1. | Electrons are emitted to leave positive sites on the surface. | ||||
| 2. | Electrons react with carbon monoxide and oxygen to produce negative-ion-radical species. | ||||
| 3. | Negative-ion-radical species chemisorb onto positively charged spots. | ||||
| 4. | A bond is formed between radicals. | ||||
| 5. | Carbon dioxide desorbs by the cleavage of the bond leaving the oxygen atom. | ||||
| 6. | The oxygen bond is cleaved and an electron is emitted to leave the positive site on the surface. | ||||
| 7. | An electron is attached to make negative-ion-radical species. | ||||
| 8. | Negative-ion-radical of carbon monoxide interacts with oxygen or with a positive site. | ||||
| 9. | An electron is detached, leaving a radical or oxygen-oxygen bond formed between radicals. | ||||
| 10. | Carbon dioxide desorbs by the cleavage of the bond. | ||||
The key factor of a series of reactions is the exoelectron emission process and weakness of the chemical bond between adsorbent and adsorbate. Aluminum oxide is known to emit electrons more than metals. When palladium is rubbed against aluminum oxide, it should generate more electrons than when rubbed against itself. This is the reason why the aluminum oxide/palladium rubbing system produced more carbon dioxide than the palladium/palladium rubbing system.
On the other hand, when aluminum oxide is rubbed against itself, the chemical bonds of aluminum oxide to carbon monoxide and oxygen are too strong to break. This is the reason why aluminum oxide/aluminum oxide does not produce carbon dioxide, even if it emits a lot of exoemission (actually friction between aluminum oxide and itself has been studied as the promoter of the tribo-polymerization of model lubricants, Furey, et al. (Citation9)). Next we will discuss the mechanism of the overshoot of carbon dioxide desorption.
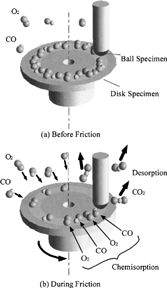
schematically shows the reaction mechanism. Before friction, carbon monoxide and oxygen chemisorb on the surface, which makes domains (Conrad, et al. (Citation20)). If the exposure time is long, chemisorbed atoms are saturated and there are many atoms “waiting for the reaction” on the surface. When rubbing starts, exoelectrons are emitted from the surface to promote the NIRAM process. Then carbon dioxide desorbs immediately because it does not chemisorb on the palladium surface. Following the initial period, carbon monoxide and oxygen do not chemisorb on all the worn surface. So after the initial overshoot, the pressure goes down to the equilibrium value during sliding. Stages of the reaction process are schematically shown in .
FIG. 17 Reaction process of the carbon monoxide oxidation by the exoelectron emission and negative-ion-radical formation.
Low activation energy is also explained by the exoelectron emission and NIRAM. The activation energy for the synthesis reaction of carbon dioxide on various palladium surfaces has been studied. It is 121 kJ/mol on (100) surface (Graham, et al. (Citation21)), 105 kJ/mol on (110) surface (Adamson and Gast (Citation22)), and 134 kJ/mol on (111) surface (Gasser (Citation23)). shows, on the other hand, that the apparent activation energy with friction is extremely low. It might be explained as follows: The friction causes the high temperature in a limited duration of time known as flash temperature. The temperature rise associated with friction can equalize the effect of ambient temperature, which results in the low activation energy. However, we need to remember that in high temperature, thermionic electrons are emitted (Vick, et al. (Citation17)). From this context, the high temperature associated with flash temperature is interpreted as a high rate of electron emission. In addition to the thermionic electrons that are emitted during flash temperature, exoelectrons are emitted during the sliding process. These two kinds of electrons equalized the effect of ambient temperature.
The relationship between coefficient of friction and desorption rate of carbon dioxide is also explained by the exoelectron and NIRAM. It is to be noted that although the variation of the coefficient of friction is very fast, the desorption increases or decreases in accordance with the coefficient of friction. This also means that the carbon monoxide and oxygen were ready to react before sliding commenced, and that the exoelectron emission associated with friction triggered the NIRAM process. The friction force originates from the shear fracture at the real area of contact. High friction means larger contact area. As a result, exoelectrons are emitted more when the friction is high. Thus the desorption of carbon dioxide correlates positively with the coefficient of friction.
In contrast, the chemisorption occurs not only just after the contact but also during the whole exposure period. This is the reason why chemisorption does not overshoot during the initial period of sliding. No correlation between coefficient of friction and adsorption of carbon monoxide and oxygen clarifies the reaction processes; the chemisorption of those molecules is not affected by the exoelectron emission.
CONCLUSIONS
The synthesis reaction of carbon dioxide from carbon monoxide and oxygen is enhanced by friction between aluminum oxide and palladium. The experimental results are summarized as follows:
| 1. | The activation energy of this reaction associated with friction was 2.49 kJ/mol. | ||||
| 2. | The desorption rate of carbon dioxide correlated with the coefficient of friction, whereas the chemisorption rate of carbon monoxide and oxygen had no correlation with it. | ||||
| 3. | As the sliding commenced, the desorption of carbon dioxide overshot before stabilizing at the equilibrium value. The overshoot correlated with the time interval between the friction tests. It was higher with increasing interval time between the friction tests. However, the overshoot did not increase beyond the saturation value. | ||||
The experimental findings mentioned above are well explained by exoelectron emission associated with friction and the NIRAM (negative-ion-radical action mechanism) approach. Exoelectron emission is enhanced by the friction of palladium. The exoelectrons attach onto the chemisorbed species to make negative-ion-radicals. Then they react with each other on the surface to be desorbed as carbon dioxide.
Acknowledgments
Presented at the STLE/ASME Tribology Conference in Ponte Vedra Beach, Florida October 27-29, 2003 Review led by Ronald Reich
REFERENCES
- Hiratsuka , K. , Kuzuya , M. and Sasada , T. 1990 . “Friction Catalysis in the Synthesis Reaction of H2O during Adhesive Wear” . Proc. 33rd Japan Congress on Materials Research , 33 : pp 191 – 195 .
- Hiratsuka , K. , Kuzuya , M. and Sasada , T. 1991 . “Friction Catalysis in the Synthesis Reaction of CO2 during Adhesive Wear” . Proc. 34th Japan Congress on Materials Research , 34 : pp 119 – 123 .
- Mori , S. , Suginoya , M. and Tamai , Y. 1982 . “Chemisorption of Organic Compounds on a Clean Aluminum Surface Prepared by Cutting under High Vacuum” . ASLE Trans. , 25 ( 2 ) : pp 261 – 266 .
- Sasada , T. , Hiratsuka , K. and Saito , H. 1990 . “Adsorption of Surrounding Gas Molecules on Pure Metal Surfaces during Wear Processes” . Wear , 135 : pp 251 – 264 .
- Kajdas , C. 1994 . “Importance of Anionic Reactive Intermediates for Lubrication Component Reactions with Friction Surfaces” . Lubr. Sci. , 6 ( 3 ) : pp 203 – 228 .
- Kajdas , C. 1987 . “About an Anionic-Radical Concept of the Lubrication Mechanism of Alcohols” . Wear , 116 : pp 167 – 180 .
- Majzner , M. and Kajdas , C. 2003 . “Reactions of Carboxylic Acids under Boundary Friction Conditions” . Tribologia , 34 ( 1 ) : pp 63 – 80 .
- Kajdas , C. , Furey , M. J. and Kempinski , R. 1997 . “Tribopolymerization: 2. NIRAM Applications to the Antiwear Action of Addition-Type Monomers” . Proc. 2nd Symposium on Tribochemistry , : pp 127 – 139 .
- Furey , M. J. , Kajdas , C. , Keminski , R. and Tripathy , B. S. 1997 . “Action Mechanism of Selected Vinyl Monomers under Boundary Lubrication of an Alumina-on-Alumina System” . Lubr. Sci. , 10 ( 1 ) : pp 3 – 25 .
- Bhushan , B. and Kajdas , C. 2001 . “The Present State of the Art on Degradation Models of Perfluoropolyethers with DLC Coatings in Thin-Film Magnetic Rigid Disks” . In Fundamentals of Tribology and Bridging the Gap between the Macro- and Micro/Nanoscales , 735 – 746 . Boston : Kluwer Academic Publishers .
- Ghasemi , H. M. , Furey , M. J. and Kajdas , C. 1993 . “Surface Temperatures and Fretting Corrosion of Steel under Conditions of Fretting Contact” . Wear , 162–164 : pp 357 – 369 .
- Molina , G. J. , Furey , M. J. , Ritter , A. L. and Kajdas , C. 2001 . “Triboemission from Alumina, Single Crystal Sapphire, and Aluminum” . Wear , 249 ( 3–4 ) : pp 214 – 219 .
- Marienfeld , S. , Barsotti , S. , Leber , E. , Schramm , A. , Ruf , M.-W. , Hotop , H. , Rosa , A. , Nandi , D. , Kumar , S. V. K. , Krishnakumar , E. and Illenberger , E. 2001 . “On the Temperature Dependence of SF6 − and SF5 − Formation in Collisions of Low Energy Electrons with SF6 Molecules” . XXII International Conference on Photonic, Electronic and Atomic Collisions (XXII ICPEAC) Abstract , MO-050
- Heinicke , G. 1984 . Tribochemistry , p 122 Berlin : Akademie Verlag .
- Kajdas , C. , Hiratsuka , K. and Hashimoto , M. 2004 . “Mechanism of Water Synthesis under Pt/Pt Tribological System in Vacuum” . In Tribology: Science and Applications , Edited by: Herman , M. A. Warsaw : Polish Academy of Sciences . published by
- Kajdas , C. 2001 . “Tribochemistry” . In Tribology 2001: Scientific Achievements, Industrial Applications, Future Challenges , Edited by: Franek , F. , Bartz , W. J. and Pauschitz , A. 39 – 46 . Vienna : Austrian Tribology Society .
- Vick , B. , Furey , M. J. and Kajdas , C. 2002 . “An Examination of Thermionic Emission Due to Frictionally Generated Temperatures” . Tribol. Lett. , 13 ( 2 ) : pp 147 – 153 .
- Nakayama , K. and Nevshupa , R. A. 2002 . “Plasma Generation in a Gap around a Sliding Contact” . J. Phys., D: Appl. Phys. , 35 : pp 1 – 4 .
- Kajdas , C. 2002 . “An Attempt to Model the Initiation Process of Tribochemical Reactions by the Flash Temperature Effect” . 1st ESF-Nanotribology Workshop in Portovenere, Abstracts , : p 19
- Conrad , H. , Ertl , G. and Kueppers , J. 1978 . “Interactions between Oxygen and Carbon Monoxide on a Pd(111) Surface” . Surf. Sci. , 76 : pp 323 – 342 .
- Graham , G. W. , Logan , A. D. and Shelef , M. 1993 . “Oxidation of CO by O2, NO, and Mixtures of O2 and NO over Pd(100)” . J. Phys. Chem. , 97 ( 21 ) : p 5445
- Adamson , A. W. and Gast , A. P. 1997 . Physical Chemistry of Surfaces , 6th ed. , p 736 New York : John Wiley .
- Gasser , R. P. H. 1985 . An Introduction to Chemisorption and Catalysis by Metals , p 215 Oxford : Clarendon Press .