ABSTRACT
Vitamin B12 deficiency has been associated with an increased risk of cognitive decline. This literature review explores the current methods available for measuring vitamin B12 in human blood, serum, and urine, and the need for a globally accepted reference range for vitamin B12. We present optical spectroscopy, including chemiluminescence measurements, absorption and fluorescence spectroscopy, surface plasmon resonance, and Raman spectroscopy, as a promising technique for detection and tracking of vitamin B12. Considerations for future research are highlighted, including enhancing the sensitivity of optical spectroscopy and prospective pathways to improve the reproducibility, selectivity, and speed of vitamin B12 detection.
Introduction
Vitamin B12 (cobalamin and its derivatives, as shown in ) Citation(1) deficiency has been associated with an increased risk of poor cognitive health Citation(2, 3). Increased levels of vitamin B12 have been shown to reduce the likelihood of older adults transitioning from mild cognitive impairment to dementia Citation(4) and in at least one case may help reverse the symptoms of frontotemporal dementia, as previously shown in a B12 recovery treatment program Citation(5). The overall effects of vitamin B12 deficiency have been previously discussed in the literature Citation(6, 7). It has been found that vitamin B12 plays an important role in 2 metabolic cycles that can affect the health of the nervous system Citation(8). First, vitamin B12 is crucial in transferring a methyl group from 5-methyltetrahydrofolate to homocysteine (Hcy), thereby generating tetrahydrofolate (THF) – important in DNA synthesis – particularly the DNA synthesis of red blood cells and intestinal wall cells. When this process is impaired, tetrahydrofolate levels are reduced and Hcy levels are increased; increased Hcy levels can be detrimental to cognitive health Citation(9). Secondly, vitamin B12 partakes in a reaction that converts methylmalonyl-coenzyme A (coenzyme-A linked to methylmalonic acid - MMA) to succinyl-coenzyme A (coenzyme-A linked to succinic acid), an important step in the extraction of energy from proteins and fats required for the synthesis of myelin, a material surrounding the axons of neurons that is essential for a functioning nervous system. Increased MMA levels are an indication of impaired myelin synthesis that affects the function of neurons, thereby contributing to impaired cognition Citation(10).
Figure 1. Chemical structure of the basic form of vitamin B12 (cobalamin). Some of its most common derivatives consist of R = –CN (cyanocobalamin), R = –OH (hydroxycobalamin) and R = –CH3 (methylcobalamin).
The growing number of studies indicating the significance of the relationship between vitamin B12 and cognitive health cannot be ignored Citation(11). In order to establish the mechanism that underlies this relationship, it is essential to accurately and reliably measure vitamin B12 Citation(12, 13).
Reference ranges are essential in order to guide the interpretation of data Citation(14), but there is no globally accepted standardized reference range or measurement technique Citation(15). Physiological ranges of vitamin B12 in human blood serum are 200–900 pg/mL in the United States Citation(16), where it is also noted the levels of <500 pg/mL may result in symptoms of vitamin B12 deficiency in older adults. In Australia, the reference range is 200–900 pg/mL with the limit for subclinical deficiency noted as 300 pg/mL Citation(17). The reference range for vitamin B12 in blood serum, however, is dependent on the measurement method Citation(18).
Governments and health organizations therefore usually focus on dietary intake guidelines for vitamin B12 Citation(19, 20) instead of blood ranges. However, these guidelines do not account for conditions like pernicious anaemia (also known as Biermer's disease), a macrocytic anaemia that can prevent the absorption of vitamin B12 Citation(21) and therefore result in anaemia-like symptoms, such as weakness and asthenia, even though the dietary intake of vitamin B12 is within the recommended range. Increasing age also decreases the absorption of the protein-bound form of vitamin B12 Citation(22, 23); in Australian aged care facilities, approximately 14% of residents are reported to have undetected vitamin B12 deficiency Citation(24). An additional tool for establishing vitamin B12 deficiency has been to measure associated biomarkers such as methylmalonic acid (MMA) and homocysteine (Hcy) for diagnostic purposes Citation(25); but the levels of these biomarkers may not reliably indicate cobalamin deficiency when interpreted in isolation, making measurements of vitamin B12 essential for diagnosis Citation(26).
Current methods of detecting and measuring vitamins are mostly based on microbiological and chemical techniques Citation(27, 28) and we briefly cover those in this literature review. In recent years, there has been great progress in developing optical detection techniques that can provide rapid and precise answers across a wide range of chemical and biological target species. These detection methods include fluorescence spectroscopy Citation(29), Raman spectroscopy Citation(30) and surface plasmon resonance (SPR) effects Citation(31). It is in view of these developments that we review the current status of detection techniques for vitamin B12 as they promise rapid, cost-effective and efficient detection at the physiologically-relevant range of concentrations.
Established methods
Microbiological detection
Historically, the first technique for detecting vitamin B12 has been based on microbial cultures, in which the growth of certain microorganism is monitored when exposed to different samples and compared against a calibrated growth curve for specific compounds Citation(32). Lactobacillus leichmannii, microorganisms that require corrinoids as a growth factor, have been used for vitamin B12 measurements as their growth depends on externally supplied vitamin B12 Citation(32–34). In the work by Skeggs et al. Citation(35), which formed the basis of microbiological determination of vitamin B12 levels for decades, a culture of Lactobacillus leichmannii was grown for 24 h inside a liquid skim-milk-based medium with a carefully regulated pH before being added to a refined serum assay and autoclaved for 15 min at 120°C. The results, showing the growth rate of the microbes in the sample, could be read after 24-h incubation at 37°C and were compared against the growth rate curve of these microorganisms exposed to known amounts of cobalamin.
Microbiological techniques have since evolved for better precision and lower limits of detection (LOD) down to 20 pg/mL Citation(36), although certain drawbacks for serum vitamin B12 measurements remain as additional factors like the presence of antibiotics in the blood serum affect the growth rate of the of the microorganisms and therefore the resulting estimates of vitamin B12 concentration Citation(37).
Immunoassays
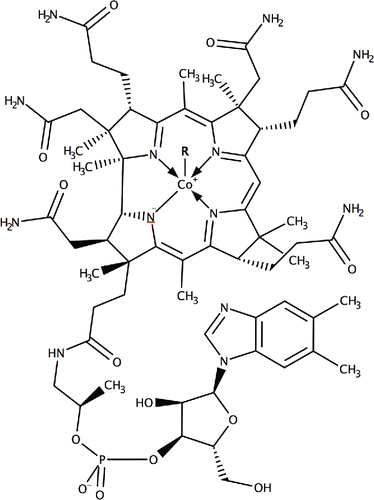
Immunoassays are biological detection techniques for the presence of specific molecules in a sample that use a specific antibody to bind the target molecule onto a substrate for further detection Citation(38). The first example of an immunoassay targeting vitamin B12 in human serum appeared in 1982 Citation(39), while further studies exist that use competitive immunoassays to measure and analyze vitamin B12 Citation(40, 41). Direct competitive enzyme-linked immunosorbent assays (ELISAs) for vitamin B12 have been developed, where immobilized rabbit antibodies that capture cobalamin were immobilized on a cover slip and incubated for 20 h at 4°C before a biotinylated detection antibody is introduced that reacts with horseradish peroxidase-avidin and produces a color reaction that increases with increasing concentration of vitamin B12 Citation(42), as shown in , for example, for a standard response curve of this measurement Citation(43). This method has a LOD of 2.2 pg/mL, although it requires lengthy preparation of the antibody plates and numerous processing steps.
Figure 2. Standard ELISA curve for derivatized vitamin B12 versus vitamin B12. Each point represents the mean of 20 determinations. Vertical bars indicate error bars with 5% value (taken from Citation(43)).
Combining immunoassays with radioactive forms of vitamin B12 has also been shown to be a sensitive tool for measuring vitamin B12 by competitive radioassays Citation(44), where vitamin B12 in a sample competes for binding onto specific antigens against a known amount of the radioactive (57Co) vitamin B12 Citation(45). While this method has been shown to be reliable Citation(46, 47) and the LOD of 288 pg/mL is within the physiological range Citation(48), the requirement for highly trained operators and the cost of radioisotopes and measuring equipment has limited its adoption Citation(49).
High-performance liquid chromatography (HPLC)
HPLC is a chemical technique that separates different compounds contained within a liquid sample by passing a mixture of the sample substance along with one or more liquid solvents through a microporous column Citation(50). Different chemical species show different retention times in passing through the HPLC column under pressure, resulting in different flow rates through the column that can be used to identify them. Vitamin B12 was first isolated using HPLC in 1997 Citation(51) and since then there have been various studies focusing on simultaneously identifying multiple members of the B-vitamin family through HPLC Citation(52–54). It must be noted that, due to the LOD for this technique being in the order of 80 ng/mL Citation(52), HPLC work has primarily been used for identifying vitamin B12 in pre-made samples such as food supplements and dietary supplements Citation(22).
Capillary electrophoresis (CE)
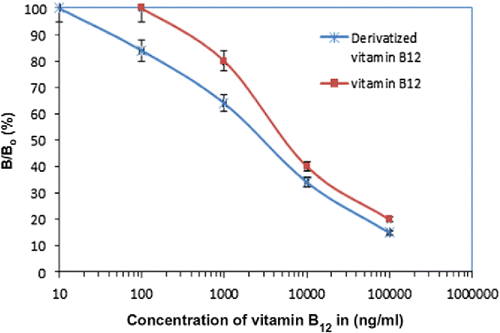
This is a technique in which a capillary is filled with a liquid electrolyte into which the sample under investigation has been mixed before an electric field is applied across it that separates the ions in the sample Citation(55, 56). In the work by Lambert et al. Citation(57), for example, high voltage (15 kV) is applied across the length of a glass capillary filled with a mixture of cobalamin derivatives capillary and electro-osmosis causes the ions in the solution to travel along the length of the capillary. The different cobalamin derivatives separate as they travel along the capillary and are detected near its end ultraviolet light (266 nm) absorption through the sample, a process that takes 25 min and results in the signatures of the compounds in the sample, as shown in (taken from Citation(57)).
Figure 3. A signature of two different cobalamin compounds in a liquid sample as analyzed by capillary electrophoresis (taken from Citation(57)).
The signal detected was then compared against results for individual derivatives using the same measurement to calibrate the process. The LOD for detecting vitamin B12 by CE were 20 μg/mL, as described by Lambert et al. CE is often used in a complimentary fashion to HPLC to cover different concentration and complex size regimes Citation(57).
Radioisotope and mass spectrometry
A significant body of work exists in the literature whereby a radioactive isotope of vitamin B12 is used to enable detection based on radioactivity measurements. Initial studies consist of subjects being given a radioactive isotope of vitamin B12, usually in the form of (57Co) vitamin B12 Citation(58, 59). The radioactivity of blood plasma Citation(60, 61) or even of the whole body Citation(62–65) is then measured and correlated to the amount of vitamin B12, with the ultimate aim of using this technique as a diagnostic tool Citation(66, 67). More recently, a form of vitamin B12 containing 14C has been used in conjunction with mass spectrometry (MS) to measure human absorption of vitamin B12 Citation(68). MS relies on ionizing a sample to create charged fragments of a chemical species that are then separated by accelerating them through an electric or magnetic field. The field deflects them based on their charge-to-mass ratio, resulting in different components arriving at different points of the detection mechanism Citation(69). The actual chemical species are then identified by correlating the mass of detected fragments to known mass profiles of known molecules, with very low detection limits for biological samples that have been modified to include the (14C) isotope Citation(70). This technique has been used for vitamin B12 identification down to a LOD of 100 fg/mL Citation(68), although this limit refers to detecting the modified version of cobalamin that requires a complicated and costly synthesis and a complex experimental setup, rather than the naturally-occurring form of vitamin B12.
Overall the established detection techniques have their basis in microbiology and chemistry, and some of them have indeed shown very low detection limits and selectivity. They do not, however, answer all challenges in measuring vitamin B12 that ideally requires rapid identification of vitamin B12 at a reasonable cost. It is due to these limitations that we turn our attention to a different set of detection techniques based on optical spectroscopy.
Optical detection techniques
Optical detection techniques for chemical species have established themselves as a reliable and sensitive technology for biological molecules Citation(71) such as DNA and proteins Citation(72) and various vitamins Citation(73, 74). Optical detection of chemical species revolves around seeing how light interacts either directly with the target molecules or with intermediate compounds that change their behavior in the presence of the target molecules, and using this change in light properties such as the color of light they emit to identify and quantify the target species Citation(71, 75–77). Some common optical detection techniques for vitamin detection are fluorescence detection Citation(78, 79) and Raman scattering Citation(80–82), where light incident on a sample changes color depending on the chemical bonds present in the sample molecules, but other optical sensing techniques have also shown great promise Citation(83).
Chemiluminescence (CL)
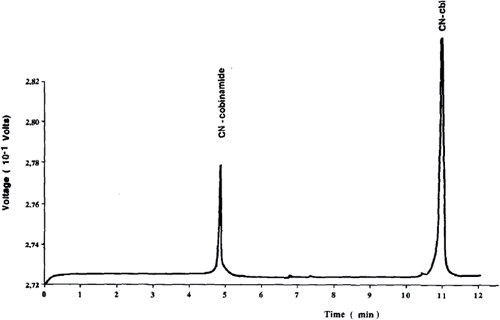
Most vitamin complexes do not spontaneously emit light, with the notable exception of vitamin A Citation(84). The same applies for vitamin B12 that is not known to emit light under optical excitation. Fluorescence detection can be deployed, however, through the interactions of vitamins with light-emitting molecules in the process of chemiluminescence. Cobalt has been shown to enhance the CL reaction between luminol and dissolved oxygen Citation(85), or between luminol and hydrogen peroxide with a LOD of 890 pg/mL Citation(86), as shown in . Similar detection limits were achieved when the CL technique based on luminol was deployed on a lab-on-a-chip system, whereby flow channels are inscribed on a single substrate to enable chemical and biological measurements Citation(87).
Figure 4. Concentration series for detection of vitamin B12 based on chemiluminescence in two different sensing modes (with pre-acidification of vitamin B12 taking place within and outside the microfluidic system, respectively) for a lab-on-a-chip device (taken from Citation(88)).
This CL lab-on-a-chip method promises faster readout times and a more compact geometry than conventional techniques Citation(88). A similar technique was employed by Kamruzzaman et al. Citation(89), who developed a microfluidic chip detector based on the reaction of luminol and silver nitrate in the presence of gold nanoparticles. This method demonstrated a LOD of 40 pg/mL.
Another method based on luminol was demonstrated using the cobalt (II) ion liberated from B12 as the catalyst for a luminol-percarbonate CL reaction, in this case resulting in an increase in the chemiluminescence signal with increasing B12 concentration under UV illumination Citation(90). This technique, however, showed LOD of 9.3 ng/mL, which was improved on in subsequent work to 420 pg/mL Citation(91).
Zhang et al. showed that vitamin B12 could also be detected using CL with dodecylbenzene sulfonate (DBS)–layered double hydroxides (LDHs) used to measure liberated cobalt (II) ions Citation(92). This technique again showed an increasing CL signal with increasing B12 concentrations, with a LOD of 570 pg/mL. This work was of particular interest due to the increased specificity of the DBS LDHs to B12, with significantly reduced cross-sensitivity to other metal ions compared to other work on luminol-based CL detection.
Absorption and fluorescence
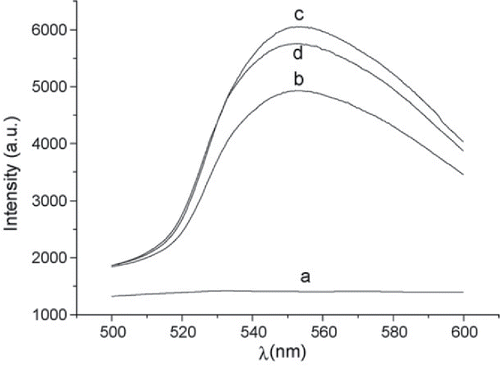
While vitamin B12 is a poor light emitter itself, and its intrinsic absorption at biologically relevant concentrations is too small for direct detection, its presence can affect the efficiency of other light-emitting species. Rhodamine 6G, for example, has been used to indirectly measure vitamin B12 in solution by studying the effect B12 has on the fluorescence resonance energy transfer between aridine orange (AO) and Rhodamine 6G Citation(93), illustrated by the collected spectra shown in for different mixtures of AO, Rhodamine 6G and vitamin B12. Work based on this technique was able to show a LOD of 2 μg/mL Citation(94).
Figure 5. Fluorescence spectra using 454 nm argon laser (10 mW) as the excitation source. (a) aridine orange (AO); (b) Rhodamine 6G (R6G); (c) R6G–AO; (d) Mixture of R6G–AO and vitamin B12 (VB12) (concentrations of AO, R6G and VB12 are 1 × 10−5, 4 × 10−5 and 4 × 10−6 mol/L, respectively for (a–d)) (taken from Citation(94)).
Work by Shang et al. demonstrated that a fluorescent probe 4-N,N-di(2-hydroxyethyl)imino-7-nitrobenzo-2-oxa-1,3-diazole (HINBD) can be used for detection of B12, with the fluorescence quenched by B12 allowing for measurements to be performed by examining the intensity of the fluorophore's signal Citation(95). This technique demonstrated a LOD of 0.1 μg/mL in water.
Vitamin B12 has also been shown to directly quench the fluorescence of CdTe quantum dots, where energy absorbed by the quantum dots is resonantly transferred to the B12 molecules Citation(96). Carbon compounds also experience fluorescence quenching in the presence of vitamin B12 molecules, for example, when using a graphene oxide layer Citation(97) or thermally-reduced carbon dots Citation(98). Using these thermally reduced carbon dots Wang et al. was able to demonstrate a LOD as low as 0.1 μg/mL in an aqueous solution.
Surface plasmon resonance (SPR)
Surface plasmon resonance (SPR) sensing consists of monitoring changes in the color scattered off a metallic surface in the presence of a target molecule Citation(99, 100). Surface plasmons are electron oscillations along the interface of a metal and a dielectric surface. The frequency of these oscillations is very sensitive to the refractive index of the environment, so in a sensor configuration the resonance frequency of surface plasmons will change depending on the refractive index of the sample medium Citation(101). This technique usually requires an antibody that selectively binds target species on the surface of the sensor, where the resonance is strongest. SPR has been shown to be useful for determining vitamin B12 levels in various dietary supplements Citation(102). SPR has also been used to indirectly determine the presence of vitamin B12 by monitoring the interactions between vitamin B12 and its binding proteins, with a LOD of about 1 μg/mL Citation(103).
Raman spectroscopy
Although the optical detection methods discussed above all allow for the detection of B12 in solution, they present various issues for real-world samples or point-of-care applications. Some techniques, luminol-based CL, for example, are susceptible to cross-sensitivity from many of the common metal ions present in biological samples. In addition, these techniques require extensive sample preparation and complex chemical reactions, greatly limiting the scope for real-world deployment.
One technique that can potentially address these shortcomings is Raman spectroscopy, an optical detection technique that directly identifies the chemical bonds that make up individual molecules in a sample Citation(104). Raman scattering, which lends its name to the spectroscopic technique, is a phenomenon by which a small part of the light delivered onto a sample from a laser source changes its wavelength (color) by a small fraction corresponding to the vibrational energy of the chemical bonds in the sample Citation(105). As molecules consist of multiple chemical bonds, this process results in a “Raman fingerprint,” which is representative of all the bonds in a given molecule Citation(106). By using an optical spectrometer, a device that decomposes light into its wavelength components, an identifiable signature for that particular molecule is generated Citation(107). Most biologically relevant molecules consist of similar elements (carbon, oxygen, nitrogen etcetera); the similarity of these chemical bonds makes discriminating between them using Raman spectroscopy challenging Citation(108). Vitamin B12 is unique in that it contains a cobalt ion linked to an organic corrin ring – a structure not found across other molecules in the human body that gives the most prominent peaks in the Raman spectrum of vitamin B12 Citation(109, 110). This makes Raman spectroscopy a particularly attractive technique for measuring vitamin B12 because its unique Raman signature can provide direct molecule identification Citation(111).
The first measurements of vitamin B12 using Raman spectroscopy appeared in 1973 Citation(112, 113), where the Raman spectrum of vitamin B12 (shown in ) was identified and the technique is used to identify vitamin B12 and its derivatives in aqueous solutions Citation(114–116). This is followed by a long hiatus until 1989, where more detailed Raman studies of vitamin B12 start emerging Citation(117, 118) as laser sources and detectors improved.
Figure 6. Raman spectrum of vitamin B12 powder (taken from Citation(125)).
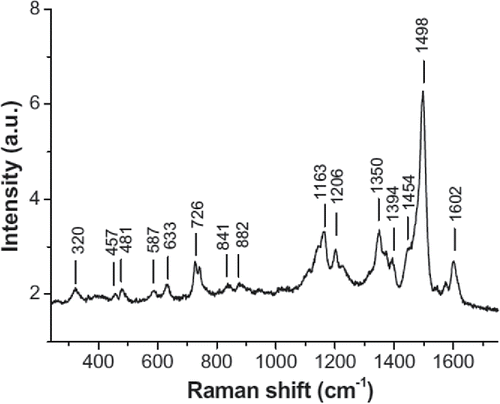
Most studies of vitamin B12 using Raman spectroscopy have concentrated on understanding the molecular structure of the vitamin B12 molecule, for example, in identifying the different vibrational modes of cobalamin Citation(118) and its coenzymes Citation(119). This line of research has given considerable insight into the fine details of the molecular structure of vitamin B12, for example, in characterizing and modeling of the different vibrational modes in the cobalamin molecule Citation(111, 120, 121), and the chemical and conformational changes it undergoes in the body, for example, during its binding process to the coenzymes it helps to metabolize Citation(122–124).
There is a notable lack of systematic studies of the LOD in Raman detection of vitamin B12 which represents a promising pathway for future research. Raman spectroscopy inherently has a low signal intensity in comparison to other optical processes such as fluorescence, with reported LOD down to 250 ng/mL Citation(114), but enhancement techniques like surface-enhanced Raman spectroscopy (SERS) Citation(126–128) may increase signal intensity due to interactions between cobalamin molecules and metallic substrates Citation(125). Raman measurements of vitamin B12, therefore, have the potential to yield both useful and innovative results. A strong case can be provided by other vitamins; SERS measurements of vitamin C show a detection behavior that closely matches the performance of HPLC measurements by using a structured silver substrate to enhance the Raman signal Citation(129), and SERS has also been identified as a reliable technique to build up libraries of spectral signature for vitamin Citation(130). Recent approaches such as producing SERS-active biotags for the characterization of biological samples have shown that the intensity of the SERS signal can be as high as that of fluorescence – but with the added advantage of unique identification of the compound Citation(131) which represents an exciting step towards detecting physiologically-relevant concentration of vitamin B12.
Overall these emerging optical techniques offer potential selectivity, speed and low detection limits for measurements of vitamin B12. The current detection limits for these techniques are summarized in , along with their key advantages and disadvantages.
Table 1. Limit of detection (LOD) and advantages and disadvantages of optical spectroscopy techniques used for measurements of vitamin B12.
Conclusions
Precise measurements of vitamin B12 concentration in humans to aid in diagnosis of vitamin B12 deficiency are still a field of intense research and scrutiny. Established methods are currently approaching their limits in terms of reproducibility, selectivity and speed; for these reasons determining vitamin B12 deficiency remains a time consuming and costly process. With recent advances in technology and our understanding of the interactions between light and cobalamin, some of the new optical detection techniques may develop into field-deployable devices for measuring vitamin B12 and aiding in the diagnosis of its deficiency. Rapid, reliable and reproducible measurements of vitamin B12 levels could further our understanding of the role of vitamin B12 in conditions like dementia and Alzheimer's disease and ultimately contribute to early diagnosis and guide prophylactic actions for at-risk populations.
References
- Clarke, R. (2007) Homocysteine, B vitamins, and the risk of dementia. Am. J. Clin. Nutr. 85: 329–330.
- Morris, M. C., Schneider, J. A., and Tangney, C. C. (2006) Thoughts on B-vitamins and dementia. J. Alzheimer's Dis.: JAD 9: 429–433.
- Smith, A. D. (2008) The worldwide challenge of the dementias: A role for B vitamins and homocysteine?. Food Nutr. Bull. 29: 143–172.
- Blasko, I., Hinterberger, M., Kemmler, G., Jungwirth, S., Krampla, W., Leitha, T., Heinz Tragl, K., and Fischer, P. (2013) Conversion from mild cognitive impairment to dementia: Influence of folic acid and vitamin B12 use in the vita cohort. J. Nutr., Health Aging 16: 687–694.
- Blundo, C., Marin, D., and Ricci, M. (2011) Vitamin B12 deficiency associated with symptoms of frontotemporal dementia. Neurol Sci. 32: 101–105.
- Hunt, A., Harrington, D., and Robinson, S. (2014) Vitamin B12 deficiency. BMJ 349: g5226.
- Golding, P. H. (2016) Experimental vitamin B12 deficiency in a human subject: a longitudinal investigation of the performance of the holotranscobalamin (HoloTC, Active-B12) immunoassay. SpringerPlus 5: 1–17.
- Banerjee, R. (1997) The Yin-Yang of cobalamin biochemistry. Chem. Biol. 4: 175–186.
- Kivipelto, M., Annerbo, S., Hultdin, J., Bäckman, L., Viitanen, M., Fratiglioni, L., and Lökk, J. (2009) Homocysteine and holo-transcobalamin and the risk of dementia and Alzheimers disease: A prospective study. Eur. J. Neurol. 16: 808–813.
- Lewis, M. S., Miller, L. S., Johnson, M. A., Dolce, E. B., Allen, R. H., and Stabler, S. P. (2005) Elevated methylmalonic acid is related to cognitive impairment in older adults enrolled in an elderly nutrition program. J. Nutr. Elder. 24: 47–65.
- Quadri, P., Fragiacomo, C., Pezzati, R., Zanda, E., Tettamanti, M., and Lucca, U. (2005) Homocysteine and B vitamins in mild cognitive impairment and dementia. Clin. Chem. Lab. Med. 43: 1096–1100.
- Engelborghs, S., Vloeberghs, E., Maertens, K., Marien, P., Somers, N., Symons, A., Clement, F., Ketels, V., Saerens, J., Goeman, J., Pickut, B. A., Vandevivere, J., and De Deyn, P. P. (2004) Correlations between cognitive, behavioural and psychological findings and levels of vitamin B12 and folate in patients with dementia. Int. J. Geriatr. Psychiat. 19: 365–370.
- Ariogul, S., Cankurtaran, M., Dagli, N., Khalil, M., and Yavuz, B. (2005) Vitamin B12, folate, homocysteine and dementia: are they really related?. Arch. Gerontol. Geriatr. 40: 139–146.
- Bates, C. J. (1999) Diagnosis and detection of vitamin deficiencies. Br. Med. Bull. 55: 643–657.
- Rothenberg, S. P., and Quadros, E. V. (1997) Quantitative methods for measurement of transcobalamin II. Methods Enzymol 281: 261–268.
- United States National Library of Medicine. (2014) Vitamin B12 level. https://www.nlm.nih.gov/medlineplus/ency/article/003705.htm.
- Australian Government Department of Health and Ageing. (2013) MBS review: Vitamin B12 testing protocol. http://www.health.gov.au/internet/main/publishing.nsf/Content/VitaminB12testing.
- Raven, J. L., Robson, M. B., Morgan, J. O., and Hoffbrand, A. V. (1972) Comparison of three methods for measuring vitamin B12 in serum: Radioisotopic, euglena gracilis and lactobacillus leichmannii. Br. J. Haematol. 22: 21–31.
- National Health and Medical Research Council New Zealand Ministry of Health. (2006) Nutrient reference values for Australia and New Zealand including recommended dietary intakes. https://www.nrv.gov.au/nutrients/vitamin-b12.
- Devalia, V., Hamilton, M. S., Molloy, A. M., and British Committee for Standards in Haematology. (2014) Guidelines for the diagnosis and treatment of cobalamin and folate disorders. Br. J. Haematol. 166: 496–513.
- Lahner, E., and Annibale, B. (2009) Pernicious anemia: New insights from a gastroenterological point of view. World J. Gastroentero. 15: 5121–5128.
- Baik, H. W., and Russell, R. M. (1999) Vitamin B12 deficiency in the elderly. Annu. Rev. Nutr. 19: 357–377.
- Risch, M., Meier, D. W., Sakem, B., Medina Escobar, P., Risch, C., Nydegger, U., and Risch, L. (2015) Vitamin B12 and folate levels in healthy Swiss senior citizens: A prospective study evaluating reference intervals and decision limits. BMC Geriatr. 15: 82.
- Mirkazemi, C., Peterson, G. M., Tenni, P. C., and Jackson, S. L. (2012) Vitamin B12 deficiency in Australian residential aged care facilities. J. Nutr., Health Aging 16: 277–280.
- Stabler, S. P. (1995) Screening the older population for cobalamin (Vitamin B12) deficiency. J. Am. Geriatr. Soc. 43: 1290–1297.
- Hvas, A. M., Ellegaard, J., and Nexo, E. (2001) Increased plasma methylmalonic acid level does not predict clinical manifestations of vitamin B12 deficiency. Arch Intern Med 161: 1534–1541.
- Lee, D. S. C., and Griffiths, B. W. (1985) Human serum vitamin B12 assay methods – A review. Clin. Biochem. 18: 261–266.
- Karmi, O., Zayed, A., Baraghethi, S., Qadi, M., and Ghanem, R. (2011) Measurement of vitamin B12 concentration: A review on available methods. IIOAB J. 2: 23–32.
- Irving, J. B., and Judith, R. M. (1997) Ultraviolet and visible spectroscopies for tissue diagnostics: Fluorescence spectroscopy and elastic-scattering spectroscopy. Phys. Med. Biol. 42: 803.
- Carey, P. R. (1978) Resonance Raman spectroscopy in biochemistry and biology. Q. Rev. Biophys. 11: 309–370.
- Liao, H., Nehl, C. L., and Hafner, J. H. (2006) Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine 1: 201–208.
- Capps, B. F., Hobbs, N. L., and Fox, S. H. (1949) A method for the microbiological assay of vitamin B12. J. Bio. Chem. 178: 517.
- Hoffmann, C. E., and Stokstad, E. L. (1949) The microbiological assay of vitamin B12 with Lactobacillus leichmannii. J. Biol. Chem. 181: 635–644.
- Ford, J. E. (1952) The microbiological assay of vitamin B12. Brit. J. Nutr. 6: 324–330.
- Skeggs, H. R., Nepple, H. M., Valentik, K. A., Huff, J. W., and Wright, L. D. (1950) Observations on the use of Lactobacillus leichmannii 4797 in the microbiological assay of vitamin B12. J. Biol. Chem. 184: 211–221.
- Kelleher, B. P., and Broin, S. D. O. (1991) Microbiological assay for vitamin B12 performed in 96-well microtitre plates. J. Clin. Pathol. 44: 592–595.
- Shojania, A. M. (1980) Problems in the diagnosis and investigation of megaloblastic anemia. Can. Med. Assoc. J. 122: 999–1004.
- Engvall, E., and Perlmann, P. (1971) Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry 8: 871–874.
- Frater-Schroder, M., Kierat, L., Andres, R. Y., and Römer, J. (1982) Solid-phase immunoassay for the vitamin B12-binding protein transcobalamin II in human serum. Anal. Biochem. 124: 92–101.
- Greibe, E., and Nexo, E. (2011) Vitamin B12 absorption judged by measurement of holotranscobalamin, active vitamin B12: Evaluation of a commercially available EIA kit. Clin. Chem. Lab. Med. 49: 1883–1885.
- Hampel, D., Shahab-Ferdows, S., Domek, J. M., Siddiqua, T., Raqib, R., and Allen, L. H. (2014) Competitive chemiluminescent enzyme immunoassay for vitamin B12 analysis in human milk. Food Chem. 153: 60–65.
- Nexo, E., Christensen, A.-L., Petersen, T. E., and Fedosov, S. N. (2000) Measurement of transcobalamin by ELISA. Clin. Chem. 46: 1643–1649.
- Kumar, L. S. S., and Thakur, M. (2011) Competitive immunoassay for analysis of vitamin B 12. Anal. Biochem. 418: 238–246.
- Lau, K. S., Gottlieb, C., Wasserman, L. R., and Herbert, V. (1965) Measurement of serum vitamin B12 level using radioisotope dilution and coated charcoal. Blood 26: 202–214.
- Green, R., Newmark, P. A., Musso, A. M., and Mollin, D. L. (1974) The use of chicken serum for measurement of serum vitamin B12 concentration by radioisotope dilution: description of method and comparison with microbiological assay results. Br. J. Haematol. 27: 507–526.
- Kihara, K., Nakamura, H., Sakata, H., Sugimura, H., Sato, K., Tsunoda, S., Demura, H., Horikawa, N., and Tanaka, A. (1985) Basic and clinical studies on the simultaneous measurement of serum and red blood cell folate and serum vitamin B12 concentrations using Corning vitamin B12 (57Co)/folate (125I) radioassay kit. Kakuigaku 22: 233–244.
- Sakata, H., Iino, Y., Kihara, K., Sugimura, H., Sato, K., Demura, R., and Demura, H. (1989) Basic and clinical studies on the simultaneous measurement of serum and red blood cell folate and vitamin B12 concentrations using Baxter travenol (57Co)vitamin B12/(125I)folate radioassay kit. Kakuigaku 26: 263–270.
- Wide, L. and Killander, A. (1971) A radiosorbent technique for the assay of serum vitamin b12. Scand. J. Clin. Lab. Invest. 27: 151–159.
- Kumar, S. S., Chouhan, R. S., and Thakur, M. S. (2010) Trends in analysis of vitamin B12. Anal. Biochem. 398: 139–149.
- Snyder, L. R., Dolan, J. W., and Gant, J. R. (1979) Gradient elution in high-performance liquid chromatography. I. Theoretical basis for reversed-phase systems. J. Chromatogr. A 165: 3–30.
- Stefova, M., Stafilov, T., Stojanoski, K., and Cepreganova-Krstic, B. (1997) Determination of vitamin B12 in multivitamin tablets by high performance liquid chromatography. Anal. Lett. 30: 2723–2731.
- Chatzimichalakis, P. F., Samanidou, V. F., Verpoorte, R., and Papadoyannis, I. N. (2004) Development of a validated HPLC method for the determination of B-complex vitamins in pharmaceuticals and biological fluids after solid phase extraction. J. Sep. Sci. 27: 1181–1188.
- Papadoyannis, I. N., Tsioni, G. K., and Samanidou, V. F. (1997) Simultaneous determination of nine water and fat soluble vitamins after SPE separation and RP-HPLC analysis in pharmaceutical preparations and biological fluids. J. Liq. Chromatogr. R. T. 20: 3203–3231.
- Sami, R., Li, Y., Qi, B., Wang, S., Zhang, Q., Han, F., Ma, Y., Jing, J., and Jiang, L. (2014) HPLC analysis of water-soluble vitamins (B2, B3, B6, B12, and C) and fat-soluble vitamins (E, K, D, A, and β-Carotene) of Okra (Abelmoschus esculentus). J. Chem. 2014: 6.
- Ewing, A. G., Wallingford, R. A., and Olefirowicz, T. M. (1989) Capillary electrophoresis. Anal. Chem. 61: 292A–303A.
- Karger, B. L., Cohen, A. S., and Guttman, A. (1989) High-performance capillary electrophoresis in the biological sciences. J. Chromatogr. B: Biomed. Sci. Appl. 492: 585–614.
- Lambert, D., Adjalla, C., Felden, F., Benhayoun, S., Nicolas, J. P., and Guéant, J. L. (1992) Identification of vitamin B12 and analogues by high- performance capillary electrophoresis and comparison with high-performance liquid chromatography. J. Chromatogr. A 608: 311–315.
- Glass, G. B. J., Boyd, L. J., Gellin, G. A., and Stephanson, L. (1954) Uptake of radioactive vitamin B12 by the liver in humans: Test for measurement of intestinal absorption of vitamin B12 and intrinsic factor activity. Arch. Biochem. Biophys. 51: 251–257.
- Glass, G. B., Boyd, L. J., and Gellin, G. A. (1955) Surface scintillation measurements in humans of the uptake of parenterally administered radioactive vitamin B12. Blood 10: 95–114.
- Doscherholmen, A., and Hagen, P. S. (1957) Radioactive vitamin B12 absorption studies: results of direct measurement of radioactivity in the blood. Blood 12: 336–346.
- Goldberg, S. R., Trivedi, B. K., and Oliner, L. (1957) Radioactive vitamin B12 studies. Experience with the urinary excretion test and the measurement of absorbed plasma radioactivity. J. Lab. Clin. Med. 49: 583–589.
- Reizenstein, P. G., Cronkite, E. P., and Cohn, S. H. (1961) Measurement of absorption of vitamin B12 by whole-body gamma spectrometry. Blood 18: 95–101.
- Tait, C. E., and Hesp, R. (1976) Measurement of 57Co vitamin B12 uptake using a static whole body counter. Brit. J. Radiol. 49: 948–950.
- Smith, T., and Hesp, R. (1979) Measurement of 57Co-labelled vitamin B12 using a liquid-scintillator whole-body counter. Brit. J. Radiol. 52: 832–835.
- Cardarelli, J. A., Slingerland, D. W., Burrows, B. A., and Miller, A. (1985) Measurement of total-body cobalt-57 vitamin B12 absorption with a gamma camera. J. Nucl. Med. 26: 941–943.
- Kristensen, H. P., and Hald, T. (1962) Measurement of plasma radioactivity following oral administration of 57Co-labelled B12. A simple diagnostic test. Dan. Med. Bull. 9: 167–170.
- Nelp, W. B., McAfee, J. G., and Wagner Jr, H. N. (1963) Single measurement of plasma radioactive vitamin B12 as a test for pernicious anemia. J. Lab. Clin. Med. 61: 158–165.
- Carkeet, C., Dueker, S. R., Lango, J., Buchholz, B. A., Miller, J. W., Green, R., Hammock, B. D., Roth, J. R., and Anderson, P. J. (2006) Human vitamin B12 absorption measurement by accelerator mass spectrometry using specifically labeled14C-cobalamin. PNAS 103: 5694–5699.
- Vogel, J. S., Turteltaub, K. W., Finkel, R., and Nelson, D. E. (1995) Accelerator mass spectrometry. Anal. Chem. 67: 353A–359A.
- Vogel, J. S. and Turteltaub, K. W. (1998) Accelerator mass spectrometry as a bioanalytical tool for nutritional research. In Mathematical modeling in experimental nutrition, A., Clifford, H.-G., Müller, Eds., Springer, New York, pp. 397–410.
- Nie, S., and Zare, R. N. (1997) Optical detection of single molecules. Annu. Rev. Biophys. Biomol. Struct. 26: 567–596.
- Uzunbajakava, N., Lenferink, A., Kraan, Y., Volokhina, E., Vrensen, G., Greve, J., and Otto, C. (2003) Nonresonant confocal Raman imaging of DNA and protein distribution in apoptotic cells. Biophys. J. 84: 3968–3981.
- Tiantian, C., Huaimin, G., Xiaojuan, Y., and Fangfang, L. (2011) Normal raman and SERS spectroscopy of the vitamin E. J. Phys. Conf. Ser. 277: 012010.
- Hancewicz, T. M., and Petty, C. (1995) Quantitative analysis of vitamin a using Fourier transform Raman spectroscopy. Spectrochim. Acta Part A: Mol. Spectrosc. 51: 2193–2198.
- Lee, B. (2003) Review of the present status of optical fiber sensors. Opt. Fiber Technol. 9: 57–79.
- Velasco-Garcia, M. N. (2009) Optical biosensors for probing at the cellular level: A review of recent progress and future prospects. Semin. Cell Dev. Biol. 20: 27–33.
- Schartner, E. P., Tsiminis, G., François, A., Kostecki, R., Warren-Smith, S. C., Nguyen, L. V., Heng, S., Reynolds, T., Klantsataya, E., Rowland, K. J., Abell, A. D., Ebendorff-Heidepriem, H., and Monro, T. M. (2015) Taming the light in microstructured optical fibers for sensing. Int. J. Appl. Glass Sci. 6: 229–239.
- Valeur, B., and Brochon, J.-C., New Trends in Fluorescence Spectroscopy: Applications to Chemical and Life Sciences. Springer Berlin Heidelberg, New York, 2001.
- Chen, J., Li, B. Q., Cui, Y. Q., Yu, E., and Zhai, H. L. (2015) A fast and effective method of quantitative analysis of VB1, VB2 and VB6 in B-vitamins complex tablets based on three-dimensional fluorescence spectra. J. Food Compos. Anal. 41: 122–128.
- Ferraro, J. R., Nakamoto, K., and Brown, C. W. (2003) Introductory Raman Spectroscopy. Academic Press, San Diego.
- Naumann, D. (2001) FT-infrared and FT-Raman spectroscopy in biomedical research. Appl. Spectrosc. Rev. 36: 239–298.
- Movasaghi, Z., Rehman, S., and Rehman, I. U. (2007) Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 42: 493–541.
- Marazuela, D., and Moreno-Bondi, M. C. (2002) Fiber-optic biosensors–an overview. Anal. Bioanal. Chem. 372: 664–682.
- Vanexan, R. J., and Hardy, M. H. Localization of vitamin a by autofluorescence during induced metaplastic changes in cultures of skin. In Vitro 15: 631–640.
- Song, Z., and Hou, S. (2003) Sub-picogram determination of Vitamin B12 in pharmaceuticals and human serum using flow injection with chemiluminescence detection. Anal. Chim. Acta 488: 71–79.
- Akbay, N., and Gök, E. (2008) Determination of vitamin B12 using a chemiluminescence flow system. J. Anal. Chem. 63: 1073–1077.
- Mark, D., Haeberle, S., Roth, G., Von Stetten, F., and Zengerle, R. (2010) Microfluidic lab-on-a-chip platforms: Requirements, characteristics and applications. Chem. Soc. Rev. 39: 1153–1182.
- Lok, K. S., Muttalib, S. Z. b. A., Lee, P. P. F., Kwok, Y. C., and Nguyen, N.-T. (2012) Rapid determination of vitamin B12 concentration with a chemiluminescence lab on a chip. Lab. Chip. 12: 2353–2361.
- Kamruzzaman, M., Alam, A.-M., Kim, K. M., Lee, S. H., Kim, Y. H., Kabir, A. N. M. H., Kim, G.-M., and Dang, T. D. (2012) Chemiluminescence microfluidic system of gold nanoparticles enhanced luminol-silver nitrate for the determination of vitamin B12. Biomed. Microdevices 15: 195–202.
- Murillo Pulgarin, J. A., Garcia Bermejo, L. F., and Sanchez Garcia, M. N. (2011) Flow injection chemiluminescence determination of vitamin B12 using on-line UV-persulfate photooxidation and charge coupled device detection. Luminescence 26: 536–542.
- Murillo Pulgarín, J. A., García Bermejo, L. F., and Sánchez García, M. N. (2011) Chemiluminescent determination of vitamin b12 using charge coupled device (CCD). Anal. Lett. 44: 2593–2605.
- Zhang, L., Rong, W., Lu, C., and Zhao, L. (2014) Organo-modified layered double hydroxide-catalyzed Fenton-like ultra-weak chemiluminescence for specific sensing of vitamin B12 in egg yolks. Talanta 129: 126–131.
- Liu, B. S., Gao, J., and Yang, G. L. (2005) Determination of vitamin B12 concentration by fluorescence quenching with acridine orange-rhodamine 6G energy transfer system. Guang Pu Xue Yu Guang Pu Fen Xi/Spect.Spectral Anal. 25: 1080–1082.
- Xu, H., Li, Y., Liu, C., Wu, Q., Zhao, Y., Lu, L., and Tang, H. (2008) Fluorescence resonance energy transfer between acridine orange and rhodamine 6G and its analytical application for vitamin B12 with flow-injection laser-induced fluorescence detection. Talanta 77: 176–181.
- Shang, Z. B., Wen, Y. J., Yan, X. Q., Sun, H. H., Wang, Y., and Jin, W. J. (2014) Synthesis of a novel fluorescent probe based on 7-nitrobenzo-2-oxa-1,3-diazole skeleton for the rapid determination of vitamin B12 in pharmaceuticals. Luminescence 29: 598–602.
- Vaishnavi, E. and Renganathan, R. (2013) CdTe quantum dot as a fluorescence probe for vitamin B12 in dosage form. Spectrochim. Acta—Part A: Mol. Biom. Spectrosc. 115: 603–609.
- Gholami, J., Manteghian, M., Badiei, A., Javanbakht, M. and Ueda, H. (2015) Label free detection of vitamin B12 based on fluorescence quenching of graphene oxide nanolayer. Fullerenes, Nanotubes Carbon Nanostructures 23: 878–884.
- Wang, J., Wei, J., Su, S., and Qiu, J. (2015) Novel fluorescence resonance energy transfer optical sensors for vitamin B12 detection using thermally reduced carbon dots. New J. Chem. 39: 501–507.
- Oh, Y., Kim, K., Hwang, S., Ahn, H., Oh, J. W., and Choi, J. R. (2016) Recent advances of nanostructure implemented spectroscopic sensors—A brief overview. Appl. Spectrosc. Rev. 51: 656–668.
- Wang, J., Zhang, H. Z., Li, R. S., and Huang, C. Z. (2016) Localized surface plasmon resonance of gold nanorods and assemblies in the view of biomedical analysis. TRAC—Trend. Anal. Chem. 80: 429–443.
- Strobbia, P., Languirand, E., and Cullum, B. M. (2015) Recent advances in plasmonic nanostructures for sensing: A review. Opt. Eng. 54: 100902.
- Vyas, P., and O'Kane, A. A. (2011) Determination of vitamin B12 in fortified bovine milk-based infant formula powder, fortified soya-based infant formula powder, vitamin premix, and dietary supplements by surface plasmon resonance: Collaborative study. J. AOAC Int. 94: 1217–1226.
- Cannon, M. J., Myszka, D. G., Bagnato, J. D., Alpers, D. H., West, F. G., and Grissom, C. B. (2002) Equilibrium and kinetic analyses of the interactions between vitamin B12 binding proteins and cobalamins by surface plasmon resonance. Anal. Biochem. 305: 1–9.
- Kneipp, K., Kneipp, H., Itzkan, I., Dasari, R. R., and Feld, M. S. (1999) Ultrasensitive chemical analysis by raman spectroscopy. Chem. Rev. 99: 2957–2975.
- Krafft, C., and Sergo, V. (2006) Biomedical applications of Raman and infrared spectroscopy to diagnose tissues. Spectroscopy 20: 195–218.
- Ferraro, J. R., Nakamoto, K., and Brown, C. W. (2003) Introductory Raman Spectroscopy: Second Edition. Academic Press, New York.
- Tsiminis, G., Chu, F., Warren-Smith, S. C., Spooner, N. A., and Monro, T. M. (2013) Identification and quantification of explosives in nanolitre solution volumes by Raman spectroscopy in suspended core optical fibers. Sensors (Basel, Switzerland) 13: 13163–13177.
- Kneipp, K., Kneipp, H., Itzkan, I., Dasari, R. R., and Feld, M. S. (2002) Surface-enhanced Raman scattering and biophysics. J. Phys. Con. Mat. 14: R597–R624.
- Day, P. (1967) A theory of the optical properties of vitamin B 12 and its derivatives. Theor. Chim. Acta 7: 328–341.
- Mayer, E., Gardiner, D. J., and Hester, R. E. (1973) Resonance Raman spectra of vitamin B12 and some cobalt corrinoid derivatives. J. Chem. Soc., Faraday Transactions 2: Mol. Chem. Phys. 69: 1350–1358.
- Andruniow, T., Zgierski, M. Z., and Kozlowski, P. M. (2002) Vibrational analysis of methylcobalamin. J. Phys. Chem. A 106: 1365–1373.
- Mayer, E., Gardiner, D. J., and Hester, R. E. (1973) Resonance Raman spectra of vitamin B12 and dicyanocobalamin. Biochim. et Biophys. Acta (BBA)— Gen. Subjects 297: 568–570.
- Wozniak, W. T., and Spiro, T. G. (1973) Resonance Raman spectra of vitamin B12 derivatives (16). J. Am. Chem. Soc. 95: 3402–3404.
- Tsai, C. W., and Morris, M. D. (1975) Application of resonance Raman spectrometry to the determination of vitamin b12. Anal. Chim. Acta 76: 193–198.
- Nestor, J., Spiro, T. G., and Klauminzer, G. (1976) Coherent anti-Stokes Raman scattering (CARS) spectra, with resonance enhancement, of cytochrome c and vitamin B12 in dilute aqueous solution. Proc. National Acad. Sci. 73: 3329–3332.
- Rajoria, D. S., and Nath, A. (1977) IR spectroscopic studies of some cobalamins. J. Inorg. Nucl. Chem. 39: 1291–1294.
- Nie, S., Marzilli, L. G., and Yu, N. T. (1989) Near-infrared Fourier transform Raman spectroscopy of photolabile organocobalt B12 and model compounds. 1. Detection of the cobalt-carbon stretching mode in the solid state and in solution. J. Am. Chem. Soc. 111: 9256–9258.
- Nie, S., Marzilli, P. A., Marzilli, L. G., and Yu, N.-T. (1990) Near-IR Fourier transform Raman spectroscopy of photolabile organocobalt B12 and model compounds. Identification of the Co-C bond stretch in cobalamins. J. Chem. Soc., Chem. Comm. 770–771.
- Dong, S., Padmakumar, R., Banerjee, R., and Spiro, T. G. (1999) Co-C bond activation in B12-dependent enzymes: Cryogenic resonance Raman studies of methylmalonyl-coenzyme a mutase. J. Am. Chem. Soc. 121: 7063–7070.
- Stich, T. A., Brooks, A. J., Buan, N. R., and Brunold, T. C. (2003) Spectroscopic and computational studies of Co3+-corrinoids: Spectral and electronic properties of the B12 cofactors and biologically relevant precursors. J. Am. Chem. Soc. 125: 5897–5914.
- Park, K., and Brunold, T. C. (2013) Combined spectroscopic and computational analysis of the vibrational properties of vitamin B12 in its Co3+, Co2+, and Co1+ oxidation states. J. Phys. Chem. B 117: 5397–5410.
- Dong, S., Padmakumar, R., Maiti, N., Banerjee, R. and Spiro, T. G. (1998) Resonance Raman spectra show that coenzyme B12 binding to methylmalonyl-coenzyme a mutase changes the Corrin ring conformation but leaves the Co-C bond essentially unaffected (4). J. Am. Chem. Soc. 120: 9947–9948.
- Huhta, M. S., Chen, H. P., Hemann, C., Hille, C. R., and Marsh, E. N. G. (2001) Protein-coenzyme interactions in adenosylcobalamin-dependent glutamate mutase. Biochem. J. 355: 131–137.
- Kozlowski, P. M., Andruniow, T., Jarzecki, A. A., Zgierski, M. Z., and Spiro, T. G. (2006) DFT analysis of Co−alkyl and Co−adenosyl vibrational modes in B12-cofactors. Inorg. Chem. 45: 5585–5590.
- Zhang, Z., Wang, B., Yin, Y., and Mo, Y. (2009) Surface-enhanced Raman spectroscopy of Vitamin B12 on silver particles in colloid and in atmosphere. J. Mol. Struct. 927: 88–90.
- Ru, E. C. L., and Etchegoin, P. G. (2009) Principles of surface-enhanced Raman spectroscopy. Elsevier B.V., New York.
- Negri, P., and Dluhy, R. A. (2013) Ag nanorod based surface-enhanced Raman spectroscopy applied to bioanalytical sensing. J. Biophotonics 6: 20–35.
- Zeng, Z., Liu, Y., and Wei, J. (2016) Recent advances in surface-enhanced Raman spectroscopy (SERS): Finite-difference time-domain (FDTD) method for SERS and sensing applications. TRAC—Trend. Anal. Chem. 75: 162–173.
- Dongming, L., Shuhai, J., Jun, W., and Yang, J. (2013) Ordered silver nanoparticle arrays as surface-enhanced Raman spectroscopy substrates for label-free detection of vitamin C in serum. Sens. Actuators, A 201: 416–420.
- Kokaislová, A. and Matějka, P. (2012) Surface-enhanced vibrational spectroscopy of B vitamins: What is the effect of sers-active metals used? Anal. Bioanal. Chem. 403: 985–993.
- Pallaoro, A., Braun, G. B., and Moskovits, M. (2015) Biotags based on surface-enhanced Raman can be as bright as fluorescence tags. Nano Lett. 15: 6745–6750.
