Abstract
The groundwater geochemistry of the fractured rock aquifer system in the Montérégie Est region, southern Quebec, Canada, was studied as part of a regional groundwater resources assessment. The 9218 km² study area included three major watersheds that were divided into five hydrogeological contexts: Northern St. Lawrence Lowlands, Southern St. Lawrence Lowlands, Appalachian Uplands, Appalachian Piedmont and Monteregian Hills. A large part of this study area was invaded by the Champlain Sea from 13,000 to 11,000 years ago. Study objectives were to identify the mechanisms controlling groundwater composition and to support the understanding of the aquifer hydrodynamics. Groundwater from 206 wells drilled into the rock aquifer was sampled and analyzed for conventional parameters and isotopic analyses were also done on selected samples (δ2H, δ18O and 3H of water; δ13C and 14C of dissolved inorganic carbon). The interpretation of geochemical results was based on a multivariate statistical analysis, which led to the definition of eight water groups. The study allowed the delineation of a 2200-km² zone containing brackish groundwater of marine origin in the northwestern part of the study area. This zone is surrounded by sodic and alkaline groundwater originating from Na+-Ca2+ ionic exchange. Young groundwater and therefore recharge zones were only encountered in the southern part of the Lowlands, in the northern part of the Piedmont and in the Appalachian Uplands. In the southern part of Lowlands, recharge is presumed to be slow and water composition shows the influence of the former presence of the Champlain Sea. Relatively deep groundwater circulation was also inferred to occur from the Appalachian Uplands toward mixing zones mainly located to the west at the Appalachian frontal thrust faults and around the Monteregian Hills. The geochemical interpretation provided indications on regional recharge and discharge zones as well as groundwater flow, which could not have been determined otherwise.
L’étude hydrogéochimique du système aquifère rocheux fracturé de la Montérégie Est, sud du Québec, Canada, a fait partie d’une évaluation régionale des ressources en eau souterraine. La région d’étude de 9 218 km² couvrait trois bassins versants qui avaient été divisés en cinq contextes hydrogéologiques : les Basses-terres-du-Saint-Laurent nord, les Basses-terres-du-Saint-Laurent sud, le Piémont appalachien, les Hautes-terres des Appalaches, et les Collines montérégiennes. Une partie importante de cette région a été envahie par la Mer de Champlain il y a environ 13 000 à 11 000 ans. L’étude avait comme objectifs d’identifier les mécanismes contrôlant la composition de l’eau souterraine et de supporter la compréhension de l’hydrodynamique de l’aquifère. L’étude est basée sur les analyses chimiques de 206 échantillons d’eau de puits dans l’aquifère rocheux et d’analyses isotopiques sur une sélection d’échantillons (δ2H, δ18O et 3H de l’eau; δ13C et 14C du carbone inorganique dissous). Huit groupes d’eau ont été définis par des méthodes statistiques multivariées. L’étude a permis de circonscrire une zone de 2 200 km² d’eau saumâtre d’origine marine dans le nord-ouest de la région. Cette zone est entourée d’eaux alcalines et sodiques résultant de l’échange ionique Na+-Ca2+. Des eaux jeunes associées aux zones de recharge sont rencontrées dans le sud des Basses-terres, dans le nord du Piémont ainsi dans les Hautes-terres appalachiennes. La recharge est présumée lente dans le sud des Basses-terres où la chimie des eaux est influencée par l’ancienne Mer de Champlain. Une circulation relativement profonde d’eau souterraine est présumée se produire à partir des Hautes-terres jusqu’à des zones de mélange situées surtout à l’ouest des chevauchements du front appalachien ainsi qu’autour des montérégiennes. L’interprétation géochimique a donné des indications clés sur les zones de recharge et d’émergence de l’eau souterraine et sur le système d’écoulement régional qui n’auraient pas pu être obtenues autrement.
Introduction
Montérégie Est is a 9218 km² region located in southern Quebec, Canada, to the east of Montreal (Figure ). The fractured rock regional aquifer system was studied between 2009 and 2013 as part of a systematic aquifer characterization program (Programme d’acquisition de connaissances sur les eaux souterraines, PACES) launched by the province of Quebec in 2008 (Palmer et al. Citation2011; Ministère du Développement durable, de l’Environnement et de la Lutte contre les changements climatiques [MDDELCC] Citation2017). Besides providing the required PACES outputs, the Montérégie Est study aimed to develop an efficient integrated approach using not only conventional hydrogeological techniques and geochemical data sets, but also other information sources, such as geotechnical soundings, borehole geophysics and surface seismic (Lefebvre et al. Citation2011; Carrier et al. Citation2013). This approach was adapted from a previously developed methodology for the characterization of heterogeneity in shallow granular aquifers to study the migration of contaminant plumes (Paradis et al. Citation2014; Tremblay et al. Citation2014). For the specific groundwater geochemical study reported in this paper, initial available geochemical data were sparse and most data were more than 30 years old. There was thus a need to significantly increase the geochemical data set to provide a regional groundwater quality assessment and to help understand the groundwater flow system.
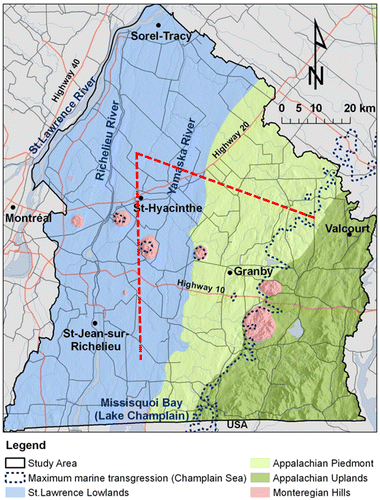
Figure 1. Montérégie Est location and hydrogeological contexts based on physiography. Map also shows topography, main roads, the Champlain Sea maximum marine transgression limit (~13,000 to 11,000 years ago) and the trace of the cross sections shown on the three-dimensional block diagram of Figure (dashed red lines).
Following the example of previous regional aquifer studies (Cloutier et al. Citation2008; Blanchette et al. Citation2010; Montcoudiol et al. Citation2015; Pétré et al. Citation2016; Rey et al. Citation2017), the perspective of this work was to use the indications provided by groundwater geochemistry and isotopes to develop a better understanding of specific aspects of the flow system that could not be determined on the basis of its geological context or by physical hydrogeological data. Although the processes leading to the geochemical and isotopic composition of groundwater are identified and graphical evidence of their occurrence is shown, this work is not meant to explain these processes in detail. Geochemical evidence is rather used to trace the paths of groundwater flow and identify relationships between different parts of the flow system, from recharge to discharge areas. The specific objectives of the groundwater geochemical study were thus to:
| • | Define groundwater groups and identify their origins, their relations, and the main mechanisms controlling their physical and chemical characteristics; | ||||
| • | Use groundwater groups to support the interpretation of groundwater regional circulation patterns and identify recharge and discharge zones. | ||||
Study area
The Montérégie Est region is bordered by the St. Lawrence River to the northwest and by the states of Vermont and New York (USA) to the south (Figure ). It covers three major watersheds: those of the Richelieu River and Missisquoi Bay, which are sub-watersheds of the Lake Champlain watershed, and that of the Yamaska River. The territory includes 108 municipalities and ~790,000 inhabitants. Agriculture is one of the major economic drivers of this region. Five distinct hydrogeological contexts were defined in Montérégie Est based on physiography and bedrock geology: Northern St. Lawrence Lowlands, Southern St. Lawrence Lowlands, Appalachian Uplands, Appalachian Piedmont and Monteregian Hills. Figure shows the physiographic features whereas Figure illustrates subsurface conditions, including bedrock geology and surficial sediments.
Figure 2. Three-dimensional block diagram of subsurface conditions in Montérégie Est (cross-section locations shown on Figure 1). The generally east–west cross section goes from the Lowlands to the Appalachian Uplands and crosses the thrust faults of the Appalachian Front. The generally north–south cross section remains in the Lowlands but crosses a Monteregian Hill. Till (green) covers most of the bedrock, with local accumulations of fluvio-glacial sediments (orange) or old sediments (brown), and is apparent at the surface in the Appalachian Piedmont and Uplands. Lacustrine (purple) and marine (light blue) fine sediments can form large accumulations in the North Lowlands (more than 30 m thick).
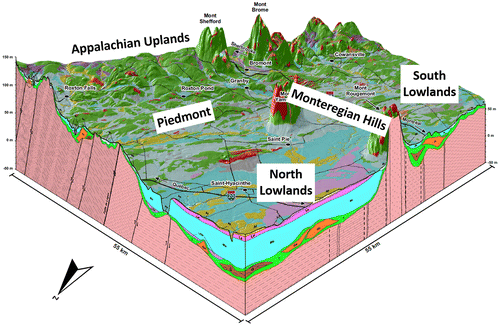
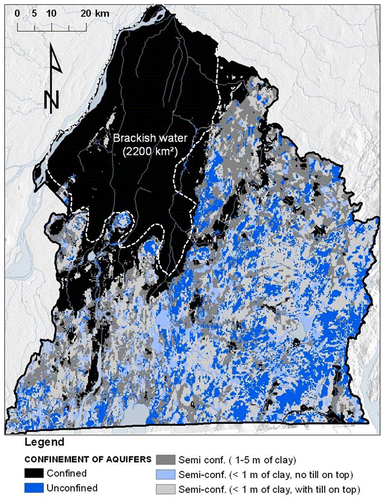
The St. Lawrence Lowlands occupy the sedimentary St. Lawrence Platform, exhibiting a low deformation and made up of Cambrian and Ordovician black and red shales, dolostones and limestones (Clark et al. Citation1979; Globensky Citation1985). The northern part of the Lowlands is covered by thick marine clay and silt deposits left by the Champlain Sea. The maximum extension of the Champlain Sea basin is shown in Figure (Parent and Occhietti Citation1988). Occhietti and Richard (Citation2003) corrected the 14C ages obtained from seashells to establish the span of the Champlain Sea between about 13,000 to 11,000 years before present. Fractured rock aquifers underlying these marine deposits that exceed ~10 m thick contain brackish water. This area of non-potable confined groundwater extends over ~2200 km2 (Beaudry et al. Citation2011; McCormack Citation1980), as shown in Figure . The southern part of the Lowlands is covered by variable thicknesses and discontinuous units of marine clay and till (Gaucher Citation1984; Prichonnet Citation1984) causing discontinuous and variable groundwater confinement contexts (Figure ).
Figure 3. Confinement level of the Montérégie Est fractured rock aquifer system, and extent of brackish groundwater to the north of the region.
The Appalachian Uplands correspond to the Internal Humber Zone of the Appalachians, which are made up of Devonian ridge continental margin rocks that are highly deformed and subjected to low-grade metamorphism (Slivitzky and Saint-Julien Citation1987; Brisebois and Nadeau Citation2003). Maximum elevation is approximately 500 m above sea level. The last glaciations, from up to 16,000 years ago, eroded the summits and left behind glacial valleys having a variable thickness of sediments and discontinuous till layers (Gaucher Citation1984; Prichonnet Citation1984). Groundwater is generally under unconfined conditions at high elevation and semi-confined down the valleys (Figure ). Apart from the northern portion of the Lowlands, the surficial cover is usually less than 10 m thick, except in valleys. The Appalachian Piedmont is located between the Uplands and Lowlands. Its bedrock is part of the Appalachian External Humber Zone, which is similar to that of the Uplands, but surface sediments are similar to those of the southern part of the Lowlands. At the time of the last glacial maximum, the Piedmont corresponded to the Champlain Sea shore as illustrated in Figure . The fifth hydrogeological context corresponds to the Monteregian Hills, which are seven Cretaceous, mainly mafic, intrusives puncturing the other contexts along a northwest–southeast axis. Erosion uncovered these plutons and geophysics revealed the presence of several buried dykes around these hills (Feininger and Goodacre Citation1995; Séjourné et al. Citation2013). The Champlain Sea level did not reach the summit of most of the hills.
Lands in the Lowlands and Piedmont are widely used for agriculture and also include a few cities. The Monteregian Hills and Uplands are less populated, activities are rural, and the forest cover is more important. The average annual total precipitation for Montérégie Est is approximately 1100 mm/year and the average temperature is 5.9°C, based on 16 weather stations, for the period from 1970 to 1999 (Carrier et al. Citation2013). For the Uplands, the average precipitation is generally higher and average temperature is generally lower. Evapotranspiration is relatively constant for the entire region and is equivalent to about half of total precipitation (Carrier et al. Citation2013).
Despite the in-depth interpretation of existing and additionally acquired geological and hydrogeological data (described in Carrier et al. Citation2013), several questions remained about the regional flow system in order to define a more representative conceptual model. The geochemical and isotopic groundwater data were thus expected to provide information on the following remaining issues related to the flow system:
| • | Even though recharge was estimated using the infiltration model HELP (Schroeder et al. Citation1994; Croteau et al. Citation2010; Carrier et al. Citation2013), the location of groundwater recharge and discharge zones needed to be confirmed through the geochemical and isotopic signatures respectively associated with young and evolved groundwaters (Tóth Citation1999); | ||||
| • | Although numerical simulations of the flow system have predicted the presence of nested ‘Tothian’ (Tóth Citation1963) local, intermediate and regional flow subsystems (Laurencelle et al. Citation2013), the hydraulic conductivity of the regional aquifer appeared to exponentially decrease with depth due to fewer open fractures as depth increases (Laurencelle Citation2018). There was thus a need for field evidence that could determine whether groundwater flow is restricted to the shallow, more permeable part of the rock aquifer or rather has a relatively deep regional component. The geochemical signature of groundwater and especially its radiocarbon content were intended to provide information on groundwater residence time, according to Plummer and Glynn (Citation2013), Clark (Citation2015) and Han and Plummer (Citation2016); | ||||
| • | Complementary to the determination of recharge and discharge zones, as well as the occurrence of regional groundwater flow, there was also a question remaining about the potential communication between the geological contexts present in the study area. In particular, it was not known whether the presence of the Monteregian granitoids and their associated dyke network has caused a regionally more fractured zone that would have favored long and deep flow paths between the Appalachian Uplands and the adjacent Lowlands; | ||||
| • | Finally, the impact of the marine invasion on groundwater outside of the 2200 km2 area where brackish groundwater is found in the regional rock aquifer was unknown. The geochemical characterization was thus also meant to help in better understanding the physical processes involved in the penetration of marine water in the regional rock aquifer and its following long-term leaching by fresh groundwater (Laurencelle Citation2018), as in other areas of the St. Lawrence Lowlands (Cloutier et al. Citation2010). | ||||
Materials and methods
Sampled boreholes
The characterization of groundwater geochemistry and isotopes was carried out through the sampling of groundwater from boreholes (see next subsection), mostly installed in the regional fractured rock aquifer that was the focus of the study. Some of the sampled boreholes were drilled within the framework of the project (and are now integrated into the observation well network of the Quebec Environment Ministry), but most of the boreholes were private wells supplying households. All boreholes had a steel casing across surficial sediments, which was anchored with a casing shoe at the top of the rock aquifer, and the portion of boreholes through the rock aquifer was open and without screens. Although the casing of observation wells was sealed through surficial sediments, this is not generally the case for private wells as the sealing of casings has only become mandatory in recent years.
Carrier et al. (Citation2013) and Laurencelle (Citation2018) provide information about the general conditions of wells in the study area. Surficial sediment thickness is more important in the Lowlands (5 to 20 m) compared to the Appalachians (2 to 10 m); it controls the length of steel casing above the open-hole section in the rock aquifer. Static water level is generally around 5 m below ground and rarely exceeds 10 m. Boreholes are relatively shallow, being generally less than 50 m deep in the rock aquifer, although boreholes can be deeper in the Appalachians. Hydraulic conductivity ranges from about 10−6 m/s in the shallow part of the rock aquifer to about 10−8 m/s at a depth of about 50 m in the rock aquifer, due to the decrease in the occurrence of open fractures with depth. However, there is a lot of variability in fracturing and thus in hydraulic properties.
Jackson and Heagle (Citation2016) warned about the limitations of using private wells for the purpose of assessing baseline groundwater quality in relation with shale gas exploitation. However, at the regional scale it would be prohibitive to install boreholes dedicated to the establishment of groundwater geochemistry to obtain adequate spatial coverage. McIntosh et al. (Citation2014) showed that the sampling of private wells could actually provide very important data about regional groundwater geochemistry. Still, the implications of using open boreholes to sample groundwater have to be recognized. An important implication is that groundwater samples represent aggregates of groundwater coming from different depths in the rock aquifer. As the shallow part of the rock aquifer is more fractured and permeable, it is likely that more active groundwater flow could take place in that zone, where more recently recharged groundwater could be present. Another implication is that preferential infiltration could take place along the steel casing and affect the groundwater geochemistry. However, very few wells actually have a geochemical signature that would indicate anthropic contamination, such as the presence of nitrate (Carrier et al. Citation2013; Beaudry Citation2013). Furthermore, results presented in this paper show groundwater geochemistry that is coherent with the hydrogeological context, such as the presence of reducing groundwater under confined conditions and radiocarbon proportions indicative of the dominance of evolved groundwater with a long residence time. Thus, the nature of sampled wells found in the study area did not preclude the definition of representative groundwater geochemical conditions.
Data collection
Groundwater from 206 wells drilled into the regional fractured rock aquifer was sampled in 2010 and 2011, including 178 private wells and 28 observation wells. Each sample is representative of the complete water column of the well and was sampled according to a standard sampling procedure (Beaudry Citation2013; Carrier et al. Citation2013). The analytical program for each sample included physicochemical parameters such as alkalinity; major, minor and trace inorganic elements; 30 metals; nutrients; and sulfur. Stable isotopes of water (δ2H and δ18O) were analyzed for a subset of 90 samples. Tritium (3H) and radiocarbon (14C-DIC) were analyzed as indicators of groundwater residence time in 44 and 43 samples, respectively, but five of the 14C analyses were performed on samples with poor ionic balance that were not included in the geochemical interpretation (there are three more 3H and four more 14C analyses than reported by Beaudry Citation2013). The selection of samples for isotopic analyses was based on the aim to cover the different hydrogeological contexts, as well as on knowledge of the area. After verification of the availability of isotopic analyses for the water types that are defined later in this paper, the addition of three 3H and four 14C analyses was done to better characterize all water types.
Geochemical results were compiled into a database, including the site geographic location, well ID (that refers to the full well description in the hydrogeological database), and the analytical method used. Concentration of carbonate and bicarbonate ions, total dissolved solids (TDS), hardness, and ionic balance (in meq/L) were determined using simple equations in a spreadsheet (Beaudry Citation2013). The ionic balance acceptance limit was fixed at ± 15% for further use of geochemical results. A total of 19 samples exceeded that limit; they were therefore rejected at first, then treated separately and considered for the interpretation. All results reported as ‘undetected’ (below the detection limit imposed by the analytical method) were replaced by a value corresponding to 50% of the detection limit (Sanford et al. Citation1993).
Data interpretation techniques
A statistical method based on Cloutier et al. (Citation2008) was first used to sort all results due to the large amount of geochemical data. As suggested by Güler et al. (Citation2002), the multivariate statistical techniques were combined with graphical hydrogeochemical interpretation to meet the objectives of the study.
Multivariate statistical analysis (MSA) can be done with a combination of physical and chemical parameters. Parameters considered in the present study were selected based on three criteria:
| • | Parameters available for most of the samples; | ||||
| • | A maximum of 10% of a parameter with undetected concentrations; | ||||
| • | Independence of parameters (for example, Eh and pe are dependent). | ||||
| • | Availability of values for all 16 selected parameters; | ||||
| • | Acceptability of the ionic balance (within ± 15%). | ||||
The hierarchical cluster analysis (HCA) MSA method was initially carried out with Statistica 6.1® (StatSoft Inc. Citation2004) to group data into families having common characteristics (Davis Citation1986). To do so, Ward’s method was selected as a linkage rule, with Euclidian distances used as a similarity measurement as done by Cloutier et al. (Citation2008). This analysis provides a tree diagram representing the linkage distance between each sample. This diagram starts with a single cluster including all samples with zero loss of information. When two clusters are joined, information is lost since differences within a given cluster are disregarded. A phenon line, determined by visual inspection, is used to find the right compromise between loss of information and a manageable number of groups.
Then, the principal components analysis (PCA) MSA method was carried out with the same data matrix. PCA is a statistical method used to observe trends in a multivariate data set. It simplifies the understanding of the significance of the 16 parameters by creating new components (called principal components) that better explain the variability of the chemical composition between samples (Davis Citation1986). PCA also helps identify relationships between water groups. Results of the PCA are presented in terms of loading values. Only the most significant components were kept for the interpretation.
Results
Multivariate statistical analysis
At a linkage distance (phenon line) of 18, the cluster analysis defined eight water groups (Figure A). Figure B shows that the spatial distribution of these water groups is generally coherent with the hydrogeological contexts of the region (Figures and ) and corresponds well to the area where brackish groundwater is found (Figure ). The names assigned to these water groups are related to their geochemistry (Figure ) and their spatial distribution, which will be further discussed later (Figure B): A1 (light blue), A2 (blue) and A3 (purple) are water groups associated with the Appalachians (Uplands and Piedmont); M1 (red), M2 (orange) and M3 (yellow) are Monteregian and ‘Mixed’ types of water groups; the CS (light green) water group is close to the original Champlain Sea water geochemistry; and the LL (green) water group mostly occupies the Southern part of the Lowlands. Colors assigned to water groups are the same for all graphs presented in the paper.
Figure 4. Results of multivariate statistical analysis of geochemical parameters. A, Cluster analysis tree diagram defining the eight water groups below a phenon line of 18. B, Spatial distribution of the 190 samples with colored areas belonging to a water group. C, Values of the first and second components of the principal component analysis for the 190 samples identified with their water group. The names of water groups were assigned on the basis of their spatial distribution (Figure B) and their geochemical characteristics (Figure ): three ‘Appalachian’ groups, A1, A2 and A3; a ‘Lowland’ group, LL; a ‘Champlain Sea’ group, CS; and three ‘Mixed’ or ‘Monteregian’ groups, M1, M2 and M3.
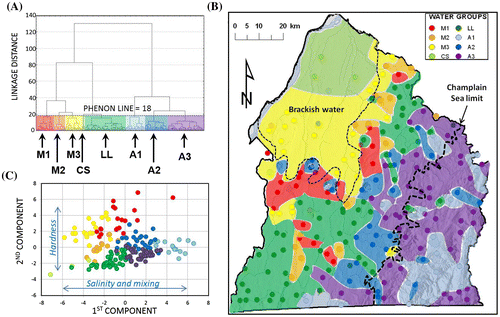
Figure 5. Major ions in groundwater. A, Proportions of major ions for each sample, associated with its water group (color), represented on a Piper diagram. B, Average ionic composition for each water group represented by Stiff diagrams (ions represented and concentration scale shown to the left of diagrams). The order of Stiff diagrams is based on relations between water groups and geochemical evolution paths that will be discussed later.
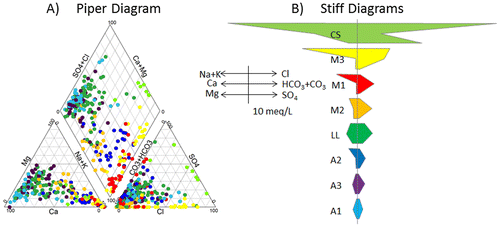
Table presents loading values of each parameter for the first five components of the PCA. Components 1 and 2 together explain more than 61% of the variance. The third component only adds 8% and was therefore neglected, as were subsequent components. The principal component scores for these first two components from each sample (associated to one of the eight water groups by a color) are illustrated in Figure C. This graph shows that water groups defined by clustering have distinct and coherent global geochemical characteristics according to the first two principal components obtained from PCA.
Table 1. Loadings of the 16 geochemical parameters for the first five components (C1 through C5) of the principal component analysis (bold values indicate dominant parameters).
As illustrated in Figure C, cluster analysis and PCA together provide a first basis for data interpretation. By considering the important loading values of TDS and other salinity parameters (Table ), the first component can be interpreted as a combination of both fresh and saline water. Saline waters have negative first component values, whereas low-salinity water has positive values. The second component seems to be associated with hardness (Table ). Negative values of the second component correspond to high calcium (Ca) and magnesium (Mg) concentrations, and thus high hardness, whereas high positive values correspond to sodium (Na) concentrations larger than those of Ca and Mg. This figure will also be discussed later to support the interpretation of groundwater origins and geochemical evolutions, after the geochemical characteristics of water groups are further described.
Geochemical characteristics and spatial distribution of water groups
Descriptive statistics were calculated for each of the eight water groups (Beaudry Citation2013). The complete geochemical data set can be found in electronic format in Beaudry (Citation2013) and can be accessed online. Several hydrogeochemical graphs distinguishing water groups were used to illustrate the distinct geochemical natures of water groups and to help in understanding the mechanisms responsible for the geochemical evolution of groundwater in the fractured rock aquifer system.
Based on the water groups defined by HCA, median geochemical characteristics of water groups were defined. Table summarizes the median values of physico-chemical properties and component concentrations for the water groups. The median concentrations, calculated for each major ion, provide an indication of the geochemical profile of each water group. To help visualize differences between water groups, Figure shows the proportions of major ion concentrations on a Piper diagram for each sample (Figure A), and the average concentrations of major ions on Stiff diagrams for each group (Figure B). The implications of the general geochemical composition of water groups illustrated by Figure will also be discussed in this section in relation with the spatial distribution of each water group represented on Figure B. The pH and pe will also be mentioned to better describe the geochemical conditions characterizing water groups (Figure A). The extent of water group polygons were defined on the basis of each of the 190 rock aquifer groundwater samples (location and water group), but also by considering groundwater flow directions indicated by the potentiometric map (Carrier et al. Citation2013).
Table 2. Median values of physico-chemical parameters and component concentrations (mg/L) for the water groups defined in Figure 4 (geochemical data and water group statistics are available in Beaudry Citation2013).
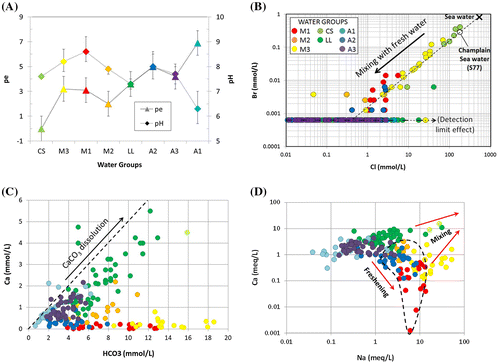
Figure 6. Geochemical conditions for the water groups found in Montérégie Est. A, Average pe and pH values for the eight water groups. B, Champlain Sea water mixing. C, Carbonate dissolution mechanism indicated by a Ca/HCO3 ratio (in mmol/L) of 1:2 (dashed line). D, Evidence of groundwater freshening due to Na–Ca ion exchange.
Brackish water groups CS and M3
The brackish groundwater area, located in the Northern Lowlands, is associated with water groups CS (Champlain Sea) and M3, which are characterized by significant concentrations of Na+ and Cl− in areas under confined conditions (Figure ). CS waters are found at the northern extremity of the study area, whereas M3 waters are found in the southern part and eastern fringe of the brackish groundwater area. The CS group is dominated by the Na+, Cl−, and SO42− ions (Figure B; Table ), and has the highest concentrations of TDS as well as reducing conditions (Figure A). The M3 group is dominated by Na+, HCO3− and Cl− ions (Figure B; Table ) and has a lower concentration of TDS than the CS group, but it is quite alkaline (Figure A). M3 has the highest HCO3− concentrations of all water types. CS waters show significant concentrations of Sr, Fe, As, S, B and Mn, in decreasing order of importance relative to concentrations found in the study area (Table ). M3 waters have relatively high concentrations of F, Ba, B and As (Table ). CS and M3 waters are non-potable.
Monteregian and Lowland water groups M1, M2 and LL
The area containing brackish groundwater is surrounded by water groups M1 and M2 and, farther south and east, by the LL water group in the Southern Lowlands and northwestern part of the Piedmont. M1 and M2 waters are found in areas that are confined or semi-confined, whereas LL waters are found under semi-confined and unconfined conditions. The M1 group has the most alkaline waters of the study area (Figure A) and is dominated by Na+ and HCO3− ions (Figure B; Table ). M1 waters have extremely low concentrations of Ca+, Mg2+ and Mn2+, but have relatively high concentrations of F and S (Table ). The M2 group is of Na-HCO3− water type, with very low sulfate concentrations, but relatively high concentrations of Ba, F, S and Sr (Table ). The LL water group has a Ca2+-HCO3− water type, which is common for recently recharged groundwater, as indicated by the relatively low residence time of these waters (discussed later). LL waters have a relatively high TDS average concentration that is indicative of a remnant of the Champlain Sea water signature. LL waters have relatively high concentrations of SO4, Fe, Mn, Si and Ba and variable concentrations of Cl (Table ). The Si concentration of LL waters is actually the highest of the water groups found in the study area, which is inferred to be related to a slow water infiltration rate through thick till deposits.
Appalachian water groups A1, A3 and A2
The Appalachian groups (A) have quite distinctive geochemical conditions relative to other water groups. Group A1 waters are of Ca-HCO3 water type, which is consistent with recent recharge and as shown by tritium and 14C data (discussed later). A1 waters are found mostly in the southeast of the Appalachian Uplands and in the Monteregian Hills that are mostly under unconfined to semi-confined conditions. The low pH and high pe of A1 indicate oxidizing and acidic conditions (Figure A). Water groups A3 and A2 are relatively more evolved waters, compared to A1, although A3 is still a Ca-HCO3 water type, whereas A2 is an Na-HCO3 water type. A3 waters are dominant in the Appalachian Uplands and in the northeast part of the Piedmont, which are under semi-confined or unconfined conditions. A2 waters have relatively high concentrations of Mn, Fe, S and As (Table ). Water group A2 is found mostly at the western fringe of the southern half of the Piedmont, as well as on the Monteregian Hills. A2 waters are mostly found within the limit of the Champlain Sea maximum transgression (Figure B). A2 waters have relatively high concentrations of F, S, Mn and U (Table ).
Major geochemical processes
Geochemical conditions
Figure presents four geochemical graphs further illustrating the geochemical nature of water groups and allowing an interpretation of important geochemical processes occurring in this study area. Figure A shows variations of pH (log of hydrogen activity) and pe (log of electron activity) for all water groups (see also Table ), which are good indicators of groundwater conditions and evolution. Figure A shows the median values of pe and pH for each water group, as well as the main range of values (‘error bars’ indicate the values of the 25th and 75th percentiles). Each water group identified in Montérégie Est shows pe values from mildly to strongly reducing waters (Hounslow Citation1995). Water groups are positioned in Figure A according to the spatial associations of water groups and their inferred geochemical evolution path based on residence time indicators (discussed in the section on ‘Water origins and ages’) and other elements discussed in the present section or the next. The A1 water group shows the most oxidizing conditions with the highest dissolved oxygen concentration (Table ), which is consistent with a typical Ca-HCO3 recharge water type. On the other hand, the CS water group has strongly reducing conditions, with the lowest dissolved oxygen concentration (Table ).
The geochemical processes further discussed here are the mixing of brackish water with fresher waters, carbonate dissolution and ionic exchanges between Na+ and Ca2+ ions. Since the former presence of the Champlain Sea over the study area exerts an important control over groundwater geochemistry in Montérégie Est, the origin of groundwater salinity will first be discussed.
Origin of groundwater salinity
The study area was in large part covered by the Champlain Sea approximately 13,000 to 11,000 years ago (Occhietti and Richard Citation2003; see Figure for marine limit). As demonstrated by Cloutier et al. (Citation2010) for an area located 80 km west of Montérégie Est, the Champlain Sea water could have invaded the fractured rock aquifer and imparted an important geochemical signature to the groundwater. Cloutier et al. (Citation2010) show that the Champlain Sea water was 34% sea water mixed with 66% fresh water from melting glaciers and precipitation. Their calculation was based on the Br/Cl ratio that remained constant in the sea water, with original concentrations of 0.8385 mmol/L of Br for 535.92 mmol/L of Cl. Based on the numerical modelling of marine water invasion of the rock aquifer, Laurencelle (Citation2018) suggests that the Champlain Sea may have had a normal marine water concentration, with dilution occurring in the aquifer rather than the sea. Figure B shows the Br versus Cl concentrations (in mmol/L) for groundwater sampled in the study area. The black cross represents the global sea water composition (Hem Citation1985); the white circle represents sample S77 found in the Cloutier et al. (Citation2010) study area that is considered representative of the Champlain Sea water composition after mixing with fresh water. Samples from water groups CS and M3 plot close to the dashed dilution line – between Champlain Sea water and fresh water – which means that the brackish area in the north of Montérégie Est originated from the Champlain Sea invasion. The composition of the CS group is similar to that of the diluted Champlain Sea, and the M3 group is further diluted sea water with fresh water. So, the CS water group is considered an end member representing the original composition of brackish Champlain Sea water in the regional rock aquifer in the areas formerly covered by the sea (Figure ).
Carbonate dissolution
Limestones and dolomites contain Ca and Mg minerals (responsible for the water hardness) that dissolve relatively easily in groundwater, especially in recharge areas, where low-pH rainwater infiltrates in the aquifer (Appelo and Postma Citation2005; Clark Citation2015). Beaudry (Citation2013) found that saturation indexes of dolomite and calcite (both carbonates) were especially undersaturated with respect to carbonates for group A1, which means that if carbonates are present in the aquifer, they will potentially dissolve. Group LL seems to be more in equilibrium with dolomite and somewhat undersaturated with respect to calcite. Calcite (CaCO3) dissolution occurs according to the following reaction:
(1)
This reaction explains why the type of water associated with recharge is generally Ca2+-HCO3−. Water groups LL and A1 are good examples of the carbonate dissolution mechanism, as shown by the Ca versus HCO3 graph of Figure C. Water groups A3 and M2 also seem to be affected by calcite dissolution, but with less intensity.
The mechanism of carbonate dissolution controls Ca and HCO3 concentrations for water group A1, while other processes may influence these concentrations for water group LL. Both groups are located in unconfined to semi-confined conditions in areas contributing to the regional aquifer recharge (Carrier et al. Citation2013). Water groups A1 and LL are thus both considered end members representing fresh recharge water input into the regional rock aquifer system, even though LL is also subject to mixing with sea water.
Freshening/ion exchange
Calcium concentration, relative to sodium, increases due to carbonate dissolution, as discussed previously. On the other hand, sources of sodium are limited to sea water (characterized by halite dissolution, NaCl), some silicates and a few rarer minerals (Hounslow Citation1995). Water group CS represents sea water with significant TDS and sodium (Na) concentrations (Figure ; Table ). Water group LL is partly the result of mixing between fresh water with geologic influence and, to a lesser extent, sea water. However, freshening due to Na–Ca ion exchange is responsible for the radical increase of sodium relative to calcium (Figure D), which occurs with a limited increase in TDS concentration (not shown), especially for water groups M1 and A2.
Some of the aquifer materials such as clay minerals (abundant in shales of the Lowlands; Globensky Citation1985), organic matter, and metal oxy-hydroxides have the property to sorb ions (Appelo and Postma Citation2005; Clark Citation2015). An adherence is created between cations and the solid surface of the material. This is called the adsorption phenomenon. Under steady-state chemical conditions, the exchanger (site of adsorption) is occupied by the dominant cations. In fresh aquifers, Ca2+ is often dominant. When sea water infiltrates the aquifer, Na+ becomes a dominant cation and gets adsorbed. When freshening occurred, after the Champlain Sea had receded, Ca2+ moved Na+ out of the exchanger to get re-adsorbed. The consequence of this ionic exchange, called freshening, is a rise in the amount of sodium found in solution, accompanied by a drop in calcium concentration as shown by Figure D. Those Na-rich water groups (M1, M2, M3 and A2) are generally associated with confined to semi-confined conditions, in bedrock containing clay minerals, inside the Champlain Sea transgression limit (Figure B). The M1 group has the highest freshening level and, although it is not an end member representing a provenance of groundwater, it represents the ultimate state of groundwater evolution due to Na–Ca ion exchange.
Na–Ca ion exchange occurs during the flushing by newly recharged fresh water into parts of the aquifer system that were formerly invaded by high-salinity marine water (Na–Cl water type). Thus, the freshening process at the origin of Na–Ca ion exchange also involves the mixing of fresh Ca-HCO3 recharge water with Na–Cl water having high salinity. It follows that the sum of cations in groundwater that has undergone these combined processes has a higher sum of cations (ultimately only Na) than the sum of cations that were present dominantly as Ca in the fresh recharge water, even though that water has an increased concentration of Ca as it evolves and further dissolves calcite along its flow path. This is seen by the larger concentration of Na relative to Ca in Figure D. When comparing water groups A3 and A2, where the former evolves into the latter which is subjected to Na–Ca ion exchange, the sum of Ca and Na ions stays relatively constant between 2 and 5 meq/L. However, when Na becomes the dominant cation and shows sign of mixing with marine water (higher Cl), then the sum of cations increases.
Mixing and relations between water groups
Figure A shows again samples from water groups relative to the first and second components of the PCA. This graph is used here to illustrate mixing and geochemical evolution between water groups. This graph also identifies the inferred groundwater end members recognized in the study area, as suggested by Valder et al. (Citation2012).
Figure 7. Mixing and relations between water groups and groundwater geochemical end members. A, Samples of water groups defined in Figures and according to the first and second components of the principal component analysis (PCA). B, Relative concentrations of major cations (x-axis) and major anions (y-axis), similar to a Piper plot, supporting relations shown on the PCA graph. Inferred geochemical end members are superposed on both graphs: sea water, lowland recharge, Monteregian water and Appalachian recharge.
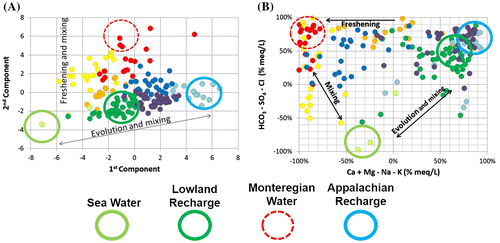
By associating the first component of Figure A with salinity and TDS, the mixing process can be described. TDS, or salinity, decreases from the left side of the graph to the right: between brackish compositions of the CS end member (Champlain Sea water), to the fresh water of the A1 end member (recharge in the Appalachians). The A1 group is characterized by the lowest TDS concentrations among the water groups. The LL end member is found between the A1 and the CS groups. The LL water group features similar characteristics to A1 (recharge water with low pH and calcite dissolution mechanism), but with a much higher TDS, representative of diluted sea water. The mixing with residual Champlain Sea water (which is still potentially trapped in the rock aquifer and overlying till), and the presumed slow flow where topography is nearly flat, could explain the high TDS concentrations of the LL group that still bears the geochemical signature of the Champlain Sea water.
The same reasoning can be applied to major ions shown in Figure B. Similar to the traditional Piper diagram, the x-axis shows the relative concentrations of major cations and the y-axis shows the relative concentrations of major anions (in percentages and calculated in meq/L). Three circles (with a continuous line) identify the three end members, A1, LL and CS. Based on the ionic composition of these groups, the LL water group plots between the two others, supporting the mixing hypothesis introduced earlier, although the fact that LL represents recently recharged water makes it also an end member.
The second component of PCA of Figure A has been associated with hardness. The figure shows how M1 (red), M2 (orange), M3 (yellow) and A2 (dark blue) groups have important differences in their hardness, which have been related to a freshening mechanism compared to other groups. The M1 group is associated with the highest level of Ca–Na ion exchange; it is identified as a water evolution end member, with a dashed-line circle. The freshening is also clearly identified in Figure B where sodium-rich waters are found in the top left corner of the graph. Based on the fact that the highest concentrations of sulfates and chlorides are associated with the CS water type, it can be presumed that vertical variations on that graph are mainly explained by its mixture with sea water (brackish or residual).
Therefore, water group M3 is probably a mixture, or an overlap of groups M1 and CS. For the same reasons, M2 is probably a mixture of groups M1 and LL. Geographically, such mixing makes sense. Due to the geographic distance between A2 and M1, A2 is not a mixture between A1 and M1. These relations will be reviewed later, in the conceptual model section.
Water origins and age
Water origins
As a part of the water cycle, groundwater can be characterized according to its isotopic composition (δ2H and δ18O; Clark and Fritz Citation1997). A comparison with local meteoric water lines (LMWL) can help in understanding water origin in terms of climate or altitude. The most representative LMWL found for Montérégie Est is the one defined by Cloutier et al. (Citation2006) for the Basses-Laurentides region, located some 80 km to the west and with similar elevations. The Basses-Laurentides meteoric water line (BLMWL) and isotopic composition of samples from the eight water groups of Montérégie Est are displayed in Figure A.
Figure 8. Isotopic composition of groundwater. A, Stable isotopes of groundwater by groups compared to the Basses-Laurentides meteoric water line (BLMWL; Cloutier et al. Citation2006). B, Residence time of water groups indicated by tritium (3H is in tritium units, TU) and radiocarbon (non-corrected 14C ages in years before present, BP). Samples analyzed for only one of the two parameters are still represented in the left and bottom margins of the graph. Ellipses indicate the distribution of samples for water groups.
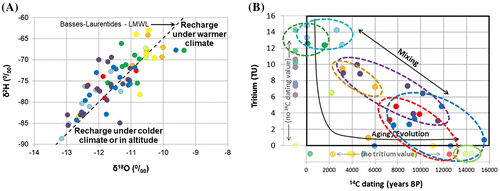
Based on the previously mentioned two thirds dilution hypothesis of the Champlain Sea water (Cloutier et al. Citation2010), it is expected that the CS water group will be a mixture of low isotope fractionation water (sea water; Vienna Standard Mean Ocean Water of δ2H = 0%0 and δ18O = 0%0) and high isotope fractionation water (melting glaciers and precipitation at high latitude). According to Figure A, the CS water group, as well as the Lowlands-related water groups M2, M3 and LL, plots near the top of the graph, with the lowest isotopic δ2H and δ18O fractionation. On the other hand, water groups associated with topographic highs (Monteregian Hills, Piedmont and Uplands) plot on the lower part of the graph, which is representative of a recharge under colder conditions or at high altitude. The M1 water group plots together with A1 to A3 water groups, which would support the hypothesis that the M1 water group originates from mixing between fresh water recharged in the Uplands and saline water from the Lowlands.
Groundwater age
Isotopic indicators of residence time can also help in inferring groundwater origin and evolution of the water groups found in Montérégie Est and their interrelations. Tritium and radiocarbon can provide such indications of groundwater residence time, for young water (less than about 50 years for tritium) and older water (up to 50,000 years for 14C), respectively (Clark and Fritz Citation1997). Overall, 42 samples were analyzed for tritium and 43 for radiocarbon, with 28 samples subjected to both analyses. Figure B relates tritium and uncorrected radiocarbon ages. Samples analyzed for only one of those two parameters are also represented, in the left and bottom margins of the graph. Ranges of values obtained for tritium and radiocarbon data for each water group are provided in Table .
Table 3. Ranges of tritium (3H), uncorrected (lab.) and corrected 14C ages for the water groups.
In order to have a better indication of the residence time that is representative of the different water groups, radiocarbon data were interpreted according to the guidelines provided by Han and Plummer (Citation2016) and with the use of the graphs proposed by Han et al. (Citation2012). Calculations of corrected radiocarbon ages were made using the interpretation spreadsheet developed by Janos (Citation2017). Basically, this approach identifies the processes affecting radiocarbon and uses the correction method suitable for these processes. The interpretation graphs used for the correction of radiocarbon ages and results are documented in a new appendix added to the thesis of Beaudry (Citation2013), which is accessible online. Table summarizes the range of corrected 14C residence times that were obtained for all water groups, which are also illustrated in Figure B. Vautour et al. (Citation2015) found similar ranges of groundwater residence times in another area of the St. Lawrence Lowlands, although the maximum ages were not as high in their study area, which did not include remnants of Champlain Sea water.
Figure 9. Geochemical evolution paths and residence time. A, Na/Ca ratio versus uncorrected 14C ages. B, Corrected 14C age range of water groups (Table ). Sample colors are related to water groups as defined in Figures A and .
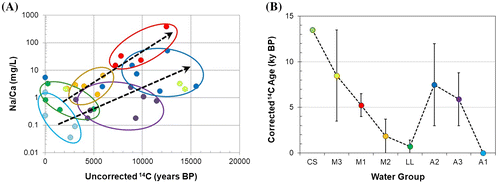
Water groups LL and A1 have a Ca-HCO3 water type that can be indicative of non-evolved recent-recharge groundwater (Clark and Fritz Citation1997; Cloutier et al. Citation2010). Data from these water types plot in the top left corner of the graph in Figure B, with over 10 tritium units (TU), uncorrected 14C ages of less than 4000 years before present, and corrected 14C ages corresponding mostly to modern water (Table ), which confirm they are young groundwaters in recharge areas or that they have had relatively short-duration flow paths from recharge areas. Both water groups show evidence of carbonate dissolution in an open CO2 system (appendix of Beaudry Citation2013), which is compatible with indications provided by Figure C and the undersaturated conditions relative to calcite and dolomite (appendix of Beaudry Citation2013), especially for water group A1.
At the other end of the age spectrum, Champlain Sea water group CS indicates two results (radiocarbon only) of either uncorrected or corrected 14C ages around 14,000 years, which are quite consistent with the Champlain Sea period (13 to 11 ky BP; Occhietti and Richard Citation2003; Laurencelle Citation2018). Intermediate tritium and 14C ages for water groups M2, A3, M1 and A2 indicate the importance of young and old water mixing affecting the residence time of these water groups, which allowed them to evolve geochemically. Overall, Table shows that the residence time increases from A1 to A3 and A2, which would lead to more evolved waters. The increase in residence time from LL to M2, to M3 and to CS (Table ) is consistent with groundwater evolution and mixing with old Champlain Sea water. Corrected 14C ages for water group M1 have the largest change compared to uncorrected ages, due to carbonate dissolution associated with Na–Ca ion exchange (Clark Citation2015). This process also affected the 14C ages of water group A3 to some extent. The 14C ages of water groups A2 and A3 were affected by calcite precipitation, which is consistent with their pCO2-pH relationship and their saturation indexes relative to calcite (appendix of Beaudry Citation2013).
The M1 water group is somewhat puzzling since it has relatively long residence times, despite the presence of tritium showing some mixing with young groundwater. The M1 water group is mostly located near the Monteregian Hills, which are presumed to represent preferential recharge areas. A source of old groundwater that can only be from great depth or long travel paths is thus needed to lead to such evolved groundwater among a preferential recharge zone. Pinti et al. (Citation2013) found more helium in groundwater in the Montérégie Est region compared to other parts of the St. Lawrence Lowlands between Quebec City and Montreal. Such helium concentrations could represent a preferential migration path from the Precambrian bedrock, which could perhaps result from the effect of a large mafic dyke network associated with the Monteregian Hills that are found over a large part of Montérégie Est (Séjourné et al. Citation2013).
Geochemical evolution and residence time
Even though the geochemical evolution (Figures and ) and residence time (Figure B) of water groups have been discussed successively, the intent here is to briefly wrap up the description of water groups by combining these two concepts. For that purpose, Figure shows one indicator of geochemical evolution (Na/Ca in Figure A) versus the 14C uncorrected age (in order to show data from water groups LL and A1 that have modern waters), which provides an indication of groundwater residence time. Figure B graphically shows the corrected 14C ages for each water group to demonstrate that the geochemical evolution illustrated in Figure A is quite coherent with the range of residence times of the water groups. Figure A clearly shows that geochemical evolution paths are distinct in the Lowlands and Appalachians, as the geochemical indicators are quite different for the two domains. Also, Figure A and B show together that the level of geochemical evolution stems directly from the groundwater residence time. Although water groups are statistically different, there is a continuous evolution of groundwater through time (as well as through space, as shown by the distribution of water types in the study area in Figure B). For instance, water group A1, characteristic of recharge zones, leads to older waters of group A3 found within the Appalachians, and then the older and still more evolved group A2 water, which is found in the Piedmont at the western edge of the Appalachians. A similar, but not as clear, groundwater evolution path may relate recharge water represented by the Lowlands LL water group with the M2 and M1 water groups. Actually, the evolution path related to these water types is clearer in Figure , which indicates that water group M1 could be related to the Appalachian groundwater evolution path as well as to the Lowlands evolution path. The relationships between water groups and the implications of groundwater evolution paths are integrated in a conceptual model of groundwater geochemical evolution in the next section.
Conceptual model and discussion
The integration of all available geochemical data and their interpretation provided the basis for the development of a conceptual model of regional groundwater geochemical evolution in the study area. Figure summarizes the relations among all water groups and represents the main mechanisms responsible for groundwater evolution.
Figure 10. Conceptual model of groundwater geochemical evolution in Montérégie Est. Each of the eight water groups is represented with the direction of evolution (arrows) and the main mechanisms involved (mixing, geological influence, ion exchange) and the corrected radiocarbon age range (see Table ).
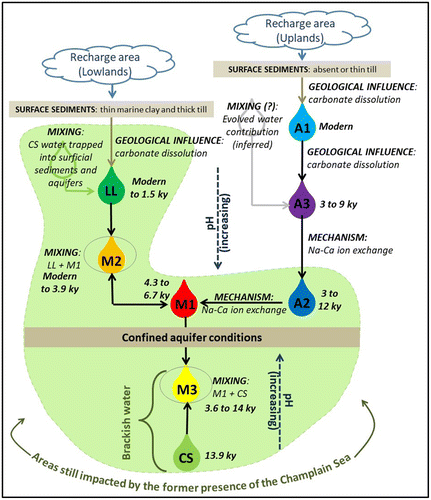
Figure is a schematic representation of the Montérégie Est regional flow system based on the hydrogeochemical interpretation made in the present study. The cross section starts from the Appalachian Uplands, to the east, goes through the Rougemont Monteregian Hill, and then branches northward through the brackish groundwater zone, toward the St. Lawrence River. Black arrows show groundwater flow directions. The remainder of this section further describes the geochemical conceptual model and its implications for the functioning of the regional aquifer system.
Figure 11. Geochemical conceptual model represented by a schematic cross section from the Appalachian Uplands to the St. Lawrence River. Water group locations are illustrated with associated colors, and flow directions are represented by arrows. The limit of the former Champlain Sea is indicated by dashed lines on the map and section.

Champlain Sea invasion area (water groups CS, M1, M2, M3, LL and A2)
The area of the aquifer system at lower altitude is still affected by the Champlain Sea invasion (Northern and Southern Lowlands, Piedmont; Figures and ) and by slower groundwater flow due to the generally flat topography. This area also has low aquifer recharge (Carrier et al. Citation2013), especially in the northern part of the Lowlands under confined conditions due to the relatively thick marine clay and silt cover (Figure ). Furthermore, important groundwater discharge seems to occur at the western edge of the Piedmont, due to either a topographic effect or the presence of the thrust fault zone, so the low-lying area farther west or northwest does not receive a major contribution of groundwater originating from the Uplands. The rock aquifer in the area within the limit of the former Champlain Sea contains either brackish groundwater, which is well preserved under confined conditions (CS water group), or water with traces of marine influence under semi-confined to unconfined conditions (LL water group).
Groundwater within that area is also characterized by important concentrations of sodium (and other residual marine components) in solution or adsorbed by the clay minerals of the rock aquifer made up in large part of shales and mudstones. Groundwater freshening occurs when fresh water mixes with brackish water in aquifers containing clay minerals (water groups M1 and A2; see section ‘Geochemical conditions’). When bedrock does not contain clay minerals, or when freshening has already been completed, the mixing of trapped or residual brackish water with fresh water occurs more as a simple dilution (LL water group). Water groups M2 and M3 represent transition (or mixing) between those three contexts, as also supported by 14C ages (Table ): well-preserved brackish water (CS with original Champlain Sea age; Table ), freshening contexts (M1 and A2) and brackish water simply diluted by the local recharge of fresh water with geologic influence (LL). The local recharge is easily identified by young (modern 14C; Table ) and fresh Ca-HCO3 water, with relatively low pH and with a composition controlled by carbonate dissolution (LL). However, according to the geochemical characteristics, the LL water group does not seem to be the fresh water source responsible for the water-freshening mechanism. The origin of the fresh water will be discussed next.
High-altitude areas (water groups A1 and A3)
High-altitude recharge is affected by geologic influence, which is not overshadowed by the high TDS concentrations of sea water as in the low-altitude recharge areas. The physiographic contexts associated with high-altitude recharge are the Appalachian Uplands (mainly southeast), the eastern and southern parts of the Piedmont and some of the Monteregian Hills. This recharge water is associated with the presence of tritium (typical of young water; Table ), a Ca-HCO3 water type with relatively low pH and a composition controlled by carbonate dissolution (A1). For water group A1, it is presumed that TDS increases slightly due to the relatively fast flow under unconfined to semi-confined conditions and the high topographic relief. On the other hand, the A3 water group, which is more evolved and corresponds to water of longer residence time (Table ), with higher TDS, is dominant in the Uplands and the northeast of the Piedmont, under semi-confined conditions as well as under unconfined conditions. As expected, the TDS increase is caused by the dissolution of common minerals, generating a moderate increase in the concentrations of major ions (Gibbs Citation1970). pH also increases along flow paths, accompanied by a decrease in dissolved oxygen and pe (Figure A). Two assumptions remain to be verified relative to the origin of the A3 water group, considering the relatively long residence time of that group as indicated by 14C data (Table ):
| 1. | Either the presence of a long and relatively deep flow path in the Appalachians, originating in part from the A1 water group, would lead to the evolution of the A3 water group having a long residence time; or | ||||
| 2. | The A3 water group represents the mixing of modern water with evolved groundwater from another source (e.g. continuous upward release of groundwater related to recharge at large depth of ice melting water from the last glaciation). | ||||
Hydraulic link between high altitude and Champlain Sea areas
The link between the high- and low-altitude systems, or the freshwater contribution, is presumed to be made by the A2 and the M1 water groups, which, as discussed previously, probably originate from high-altitude zones. The A2 water group would originate from the A3 water group, which discharges in the Appalachian Piedmont into the former Champlain Sea environment. The M1 water group would originate in part from high-altitude recharge in the adjacent Monteregian Hills. The A2 water group is observed in the Piedmont, whereas the M1 water group is locally present in the Southern Lowlands and especially around some of the Monteregian Hills. The confluence of fresh water (A3) with the former Champlain Sea environment involves ionic exchange mechanisms. The resulting water is characterized by an alkaline Na-HCO3 water type, with high sodium concentrations and low calcium concentrations. The A2 water type is associated with a lower level of ionic exchange and the M1 water type represents the highest level of effects from ionic exchange, which makes the M1 water type appear to be an end member among the water groups (Figure A and B).
Overall, the groundwater composition in Montérégie Est is largely related to the hydrogeologic conditions (confined, semi-confined or unconfined) and to the Champlain Sea influence (associated with topographic lows). This conceptual model of the groundwater flow system (Figure ) led to a revision of the initial conceptual model based on hydrogeological data alone. For instance, the Piedmont is not a preferential recharge area, as presumed initially at the onset of the project, but rather a discharge area for deep Appalachian groundwater. Also, in the Appalachian Uplands, due to relatively low hydraulic conductivity and the limited depth of natural fracturing, it was initially presumed that groundwater flow would be of a limited vertical extent and therefore strictly associated with young recharge groundwater, but geochemical data showed that, on the contrary, relatively evolved groundwater with long residence times is found in the uplands (A3), even though the origin of this water is not yet fully understood.
The residence time (evaluated with tritium and 14C ages) increases from A1 to A3 and A2 (Figures B and B; Table ), which would lead to more evolved waters. The increase in residence time from LL to M2, to M3 and to CS (Figures B and B; Table ) is consistent with groundwater evolution and mixing with old Champlain Sea water. The M1 water group has relatively long residence times, despite the presence of tritium showing some mixing with young groundwater. An uncertainty still remains about the actual origin of the M1 water group and the reason for its long residence time despite its location near some preferential recharge areas of the Monteregian Hills. One potential hypothesis is that deep waters upflowing along fractured Monteregian dykes could impart the long 14C residence times to this water type, even though the presence of tritium also shows the contribution of recent recharge to this water type. As mentioned, high helium concentrations (Pinti et al. Citation2013) in this area may provide indications that a preferential migration pathway is present in the region, which would allow deep flow originating from the Appalachians to reach not only the Piedmont (water group A2) but also the center of the study area (water group M1) along the Richelieu River and around the Monteregian Hills (Figure B).
Conclusions
The multivariate statistical analysis of geochemical parameters from groundwater samples in the Montérégie Est study area helped in defining water groups and understanding mechanisms controlling groundwater geochemistry. The identification of four end members (two recharge waters, A1 and LL; a brackish Champlain Sea water, CS; and a geochemical evolution end member, M1), the aquifer confinement levels and the limit of the Champlain Sea paleoenvironment appear to be the main elements controlling groundwater geochemical evolution. Even if only a few geochemical mechanisms were identified and discussed, it was demonstrated that ionic exchange and mixing have a dominant influence on the overall groundwater composition. Isotopic data were useful to support hydrogeochemical hypotheses. In particular, indications of residence times from radiocarbon and tritium confirmed the long residence time of water groups having indications of geochemical evolution and further supported their relationships.
This hydrogeochemical investigation significantly contributed to the understanding of the regional flow system. The most important contributions that can be attributed to the interpretation of the geochemical results and that would likely have been missed solely based on physical hydrogeological data, are the following:
| • | Identification of the areas of aquifer recharge, which are characterized by young (high tritium and modern 14C) water with relatively low pH and with a composition controlled by carbonate dissolution (Ca-HCO3 water); | ||||
| • | Delineation of a brackish (non-potable) water zone corresponding to a remnant of Champlain Sea water that had invaded the rock aquifer. This zone is now under fully confined conditions and without significant groundwater flow. The 14C age of this water corresponds to the Champlain Sea period; | ||||
| • | Identification of a potentially regional freshwater flow path, originating from the Appalachian Uplands and emerging into the Piedmont and locally in the Lowlands (around the Monteregian Hills), which would be responsible for the freshening mechanism. The geochemical data thus support the occurrence of Tothian nested flow systems of local, intermediate and regional scales; | ||||
| • | Evidence that the Piedmont is not a preferential recharge area as presumed initially at the onset of the project, but rather a regional discharge area for relatively deep Appalachian groundwater flow with long residence time, which emerges at the Appalachians’ western edge, in the vicinity of the thrust fault zone; | ||||
| • | Development of a conceptual model of the groundwater flow system integrating the geochemical interpretation, which gave rise to a major revision of the original model, and significantly improved the understanding of the regional aquifer dynamics. | ||||
Supplemental data
The underlying research materials for this article can be accessed at http://espace.inrs.ca/1363/ where the M.Sc. thesis of Beaudry (2013), the full geochemical dataset and the radiocarbon age interpretation can be obtained.
Acknowledgements
This study was supported by the regional groundwater resources assessment program (Programme d’acquisition de connaissances sur les eaux souterraines, PACES) of the Quebec Environment Ministry (Ministère du Développement durable, de l’Environnement et de la Lutte contre les Changements climatiques, MDDELCC) as well as the Groundwater Mapping Program of the Geological Survey of Canada. Numerous regional partners also supported the study through in-kind as well as monetary contributions, notably the Conférence régionale des élus (CRÉ) de la Montérégie Est. The authors would also like to acknowledge the well owners who allowed the sampling of groundwater. Finally, we want to thank the two anonymous reviewers who made constructive comments that improved the paper significantly.
References
- Appelo, C. A. J., and D. Postma. 2005. Geochemistry, groundwater and pollution, 649. Boca Raton (FL), USA: CRC Press.
- Beaudry, C. 2013. Hydrogéochimie de l’aquifère rocheux régional en Montérégie est, Québec [In French and English]. M.Sc. thesis, INRS, Centre Eau Terre Environnement, Québec, Canada, 196 pp. http://espace.inrs.ca/1363/ (Accessed March, 2018)
- Beaudry, C., X. Malet, R. Lefebvre, C. Rivard. 2011. Délimitation des eaux souterraines saumâtres en Montérégie Est, Québec, Canada [In French]. Geological Survey of Canada, Open File Report 6970, August 2011, 26 pp., doi: 10.4095/289123.
- Blanchette, D., R. Lefebvre, M. Nastev, and V. Cloutier. 2010. Groundwater quality, geochemical processes and groundwater evolution in the Chateauguay River watershed, Quebec, Canada. Canadian Water Resources Journal 35 (4): 503–526. doi:10.4296/cwrj3504503.
- Brisebois, D., Nadeau, J. 2003. Géologie de la Gaspésie et du Bas-Saint-Laurent [In French]. Ministère des Ressources Naturelles, de la Faune et des Parcs, Québec, Report DV 2003-08, Map scale 1/250 000.
- Carrier, M.-A., R. Lefebvre, C. Rivard, M. Parent, J.-M. Ballard, N. Benoit, H. Vigneault, C. Beaudry, X. Malet, M. Laurencelle, J.-S. Gosselin, P. Ladevèze, R. Thériault, I. Beaudin, A. Michaud, A. Pugin, R. Morin, H. Crow, E. Gloaguen, J. Bleser, A. Martin, D. Lavoie. 2013. Portrait des ressources en eau souterraine en Montérégie Est, Québec, Canada [In French]. INRS Report INRS R-1433, June 2013, 318 pp., ISBN: 978-2-89146-764-3. http://espace.inrs.ca/1639/ (Accessed March, 2018)
- Clark, I. 2015. Groundwater Geochemistry and Isotopes, 438. USA: CRC Press, Taylor & Francis Group, Boca Raton, FL.
- Clark, I. D., and P. Fritz. 1997. Environmental Isotopes in Hydrogeology, 328. Boca Raton, FL, USA: CRC Press.
- Clark, T. H., Y. Globensky, H. Hofmann. 1979. Stratigraphie Paléozoïque des Basses-terres du Saint-Laurent du Québec [In French], ed. Université Laval, Ste-Foy. Québec: Geological Association of Canada, Field Trip Guidebook.
- Cloutier, V., R. Lefebvre, M. Savard, É. Bourque, and R. Therrien. 2006. Hydrogeochemistry and groundwater origin of the Basses-Laurentides sedimentary rock aquifer system, St. Lawrence Lowlands, Québec, Canada. Hydrogeology Journal 14 (4): 573–590.
- Cloutier, V., R. Lefebvre, R. Therrien, and M. Savard. 2008. Multivariate statistical analysis of geochemical data as indicative of the hydrogeochemical evolution of groundwater in a sedimentary rock aquifer system. Journal of Hydrology 353 (3-4): 294–313.
- Cloutier, V., R. Lefebvre, M. M. Savard, and R. Therrien. 2010. Desalination of a sedimentary rock aquifer system invaded by Pleistocene Champlain Sea water and processes controlling groundwater geochemistry. Environmental Earth Sciences 59 (5): 977–994.
- Croteau, A., M. Nastev, and R. Lefebvre. 2010. Groundwater recharge assessment in the Chateauguay river watershed. Canadian Water Resources Journal 35 (4): 451–468. doi:10.4296/cwrj3504451.
- Davis, J. C. 1986. Statistics and Data Analysis in Geology. New York, NY: John Wiley & Sons Inc..
- Feininger, T., and A. K. Goodacre. 1995. The eight classical Monteregian hills at depth and the mechanism of their intrusion. Canadian Journal of Earth Sciences 32 (9): 1350–1364.
- Gaucher, E. 1984. Compilation de la géologie du Quaternaire - Région des Appalaches. Ministère de l’Énergie et des Ressources du Québec, Report DV 84-10, 89 maps (scale 1/50 000).
- Gibbs, R. J. 1970. Mechanisms controlling world water chemistry. Science 170 (3962): 1088–1090.
- Globensky, Y. 1985. Géologie des Basses-terres du Saint-Laurent [In French]. Gouvernement du Québec, Ministère de l’Énergie et des Ressources, Direction générale de l’Exploration géologique et minérale (Ed.), Map no. 1999 of Report MM 85-02.
- Güler, C., G. Thyne, J. McCray, and K. Turner. 2002. Evaluation of graphical and multivariate statistical methods for classification of water chemistry data. Hydrogeology Journal 10 (4): 455–474.
- Han, L. F., and L. N. Plummer. 2016. A review of single-sample-based models and other approaches for radiocarbon dating of dissolved inorganic carbon in groundwater. Earth-Science Reviews 152 (2016): 119–142. doi:10.1016/j.earscirev.2015.11.004.
- Han, L. F., L. N. Plummer, and P. Aggarwal. 2012. A graphical method to evaluate predominant geochemical processes occurring in groundwater systems for radiocarbon dating. Chemical Geology 318-319 (2012): 88–112. doi:10.1016/j.chemgeo.2012.05.004.
- Hem, J. D. 1985. Study and interpretation of the chemical characteristics of natural water. U.S. Geological Survey, Water Supply Paper 2254, Alexandria, VA, 263 pp.
- Hounslow, A. W. 1995. Water Quality Data: Analysis and Interpretation, 397. Boca Raton, Florida, USA: Lewis Publisher.
- Jackson, R. E., and D. J. Heagle. 2016. Sampling domestic/farm wells for baseline groundwater quality and fugitive gas. Hydrogeology Journal 24 : 269–272.
- Janos, D. 2017. Regional groundwater flow dynamics and residence times in Chaudière-Appalaches, Québec, Canada: insights from numerical simulations. M.Sc. thesis, Laval University, Quebec City, Canada, 103 pp.
- Laurencelle, 2018. Propriétés hydrauliques et processus d’invasion par la Mer de Champlain du système aquifère rocheux fracturé régional de la Montérégie Est, Québec, Canada. Ph.D. thesis, INRS, Centre Eau Terre Environnement, http://ete.inrs.ca/ete/publications/theses-memoires#L
- Laurencelle, M., R. Lefebvre, C. Rivard, M. Parent, P. Ladevèze, N. Benoit, M.-A. Carrier. 2013. Modeling the evolution of the regional fractured-rock aquifer system in the Northern Lake Champlain watershed following last deglaciation. GéoMontréal2013, 66th Canadian Geotechnical Conference and the 11th Joint CGS/IAH-CNC Groundwater Conference, Montreal, Quebec, Canada, Sept. 29 to Oct. 3, 2013.
- Lefebvre, R., Rivard, C., Carrier, M. A., Gloaguen, E., Parent, M., Pugin, A., Pullan, S., Benoit, N., Beaudry, C., Ballard, J. M., Chasseriau, P., Morin, R. H. 2011. Integrated regional characterization of the Montérégie Est aquifer system, Quebec, Canada. Geohydro2011, Joint IAH-CNC, CANQUA and AHQ conference, Quebec City, Canada, August 28-31, 2011, 8 p.
- McCormack, R. 1980. Étude hydrogéologique du bassin versant de la Richelieu [In French]. Service des eaux souterraines, Direction générale des inventaires et de la recherche, Ministère de l’Environement, Gouvernement du Québec, Québec, 47 pp.
- McIntosh, J. C., S. E. Grasby, S. M. Hamilton, and S. G. Osborn. 2014. Origin, distribution and hydrogeochemical controls on methane occurrences in shallow aquifers, southwestern Ontario, Canada. Applied Geochemistry 50 : 37–52.
- Ministère du Développement durable, de l’Environnement et de la Lutte contre les changements climatiques (MDDELCC). 2017. Programme de connaissances sur les eaux souterraines du Québec [In French]. Web site, http://www.mddelcc.gouv.qc.ca/eau/souterraines/programmes/acquisition-connaissance.htm (Accessed March, 2018).
- Montcoudiol, N., J. Molson, J. M. Lemieux, and V. Cloutier. 2015. A conceptual model for groundwater flow and geochemical evolution in the southern Outaouais Region, Québec, Canada. Applied Geochemistry 58 : 62–77. doi:10.1016/j.apgeochem.2015.03.007.
- Occhietti, S., and P. J. Richard. 2003. Effet réservoir sur les âges 14C de la Mer de Champlain à la transition Pléistocène-Holocène : révision de la chronologie de la déglaciation au Québec méridional. Géographie physique et Quaternaire 57 (2-3): 115–138.
- Palmer, S., S. Campeau, V. Cloutier, R. Daigneault, Larocque, M., R. Lefebvre, J. M. Lemieux, J. Molson, C. Rivard, Rouleau, A., R. Therrien. 2011. Collaborative approaches to groundwater knowledge acquisition in Quebec: Inter-regional characterization. Geohydro2011, Joint IAH-CNC, CANQUA and AHQ conference, Quebec City, Canada, August 28-31, 2011, 6 p.
- Paradis, D., L. Tremblay, R. Lefebvre, E. Gloaguen, A. Rivera, M. Parent, J. M. Ballard, Y. Michaud, and P. Brunet. 2014. Field characterization and data integration to define the hydraulic heterogeneity of a shallow granular aquifer at a sub-watershed scale. Environmental Earth Sciences 72 (5): 1325–1348.
- Parent, M., Occhietti, S., 1988. Late wisconsinan deglaciation and champlain sea invasion in the St. Lawrence Valley, Québec. Géographie physique et Quaternaire, 42(3): 215–246.
- Pétré, M. A., A. Rivera, R. Lefebvre, M. J. Hendry, A. J. B. Folnagy. 2016. A unified hydrogeological conceptual model of the Milk River transboundary aquifer, traversing Alberta (Canada) and Montana (USA). Online 29 June 2016, Hydrogeology J., 24(7), 1847–1871, doi: 10.1007/s10040-016-1433-8.
- Pinti, D. L., Y. Gélinas, M. Larocque, D. Barnetche, S. Retailleau, A. Moritz, J. F. Helie, R. Lefebvre. 2013. Concentrations, sources et mécanismes de migration préférentielle des gaz d’origine naturelle (méthane, hélium, radon) dans les eaux souterraines des Basses-Terres du Saint-Laurent [In French]. Volet géochimie, Étude E3-9, FQRNT ISI n° 171083, UQAM, U. Concordia, INRS, Août 2013, 94 p. http://www.bape.gouv.qc.ca/sections/mandats/gaz_de_schiste-enjeux/documents/PR3.6.9.pdf (Accessed in March, 2018)
- Plummer, L. N., P. D. Glynn, 2013. Radiocarbon dating in groundwater systems. Chapter 4, 33-89, In Dating Old Groundwater: A Guidebook, IAEA, Vienna, ISBN 978–92–0–137210–9.
- Prichonnet, G. 1984. Dépôts quaternaires de la région de Granby, Québec [In French]. Commission géologique du Canada, ed. Étude 83–30, Ottawa, Canada, 8 pp.
- Rey, N., E. Rosa, V. Cloutier, R. Lefebvre. 2017. Using water stable isotopes for tracing surface and groundwater flow systems in the Barlow-Ojibway Clay Belt, Quebec, Canada. Online 11 December 2017, Canadian Water Resources J., 22 pp., doi: 10.1080/07011784.2017.1403960.
- Sanford, R. F., C. T. Pierson, and R. A. Crovelli. 1993. An objective replacement method for censored geochemical data. Mathematical Geology 25 (1): 59–80.
- Schroeder, P. R., N. M. Aziz, C. M. Lloyd, and P. A. Zappi. 1994. The hydrologic evaluation of landfill performance (HELP) model: Engineering documentation for version 3. EPA/600/R-94/168b, 126. Washington, D.C.: U.S. Environmental Protection Agency, Office of Research and Development.
- Séjourné, S., Lefebvre, R., Malet, X., Lavoie, D., 2013. Synthèse géologique et hydrogéologique du Shale d’Utica et des unités sus-jacentes (Lorraine, Queenston et dépôts meubles), Basses-Terres du Saint-Laurent, Province de Québec. Geological Survey of Canada, Open File 7338, 156 p.
- Slivitzky, A., P. Saint-Julien. 1987. Compilation géologique de la région de l’Estrie-Beauce [In French]. Ministère de l’Énergie et des Ressources, Report MM 85-04, Québec, Canada, 48 pp., 1 map (1/ 250 000).
- StatSoft Inc. 2004. STATISTICA (Data Analysis Software System), Version 6.
- Tóth, J. 1963. A theoretical analysis of groundwater flow in small drainage basins. Journal of Geophysical Research 68 (16): 4795–4812. doi:10.1029/JZ068i016p04795.
- Tóth, J. 1999. Groundwater as a geologic agent: An overview of the causes, processes, and manifestations. Hydrogeology Journal 7 (1): 1–14. doi:10.1007/s100400050176.
- Tremblay, L., R. Lefebvre, D. Paradis, and E. Gloaguen. 2014. Conceptual model of leachate migration in a granular aquifer derived from the integration of multi-source characterization data (St-Lambert, Canada). Hydrogeology J. 22 (3): 587–608.
- Valder, J. F., A. J. Long, A. D. Davis, and S. J. Kenner. 2012. Multivariate statistical approach to estimate mixing proportions for unknown end members. Journal of Hydrology 460-461 : 65–76.
- Vautour, G., D. L. Pinti, P. Méjean, M. Saby, G. Meyzonnat, M. Larocque, M. C. Castro, C. M. Hall, C. Boucher, E. Roulleau, F. Barbecot, N. Takahata, and Y. Sano. 2015. 3H/3He, 14C and (U–Th)/He groundwater ages in the St. Lawrence Lowlands, Quebec, Eastern Canada. Chemical Geology 413 (2015): 94–106.
