Abstract
Puccinia triticina, the causal agent of wheat leaf rust, is a damaging and rapidly changing pathogen in Canada and throughout the world. To determine the virulence present in this pathogen population, 381 single-pustule derived P. triticina isolates were made from infected leaf collections from across Canada in 2007. Five virulence phenotypes were identified from 11 isolates collected in British Columbia and Alberta, the most common of these was MBDS. There were 14 unique virulence phenotypes among 323 isolates from Manitoba and eastern Saskatchewan. The most common of these were TDBG (61.0%), TDBJ (15.2%), MDPS (5.3%), and MDLS (5.3%). Nine virulence phenotypes were identified among 33 isolates from Ontario, with MLDS (36.4%), TDBG (21.2%), and TDBJ (15.2%) being the most common. Five virulence phenotypes were identified among 10 isolates from Quebec. The most common of these were TDBJ and TDBG (three isolates each) and MFDS (two isolates). Each of the four isolates from Prince Edward Island represented a different virulence phenotype (MCNS, MFDS, MHNQ and MHNS). These were all unique to Prince Edward Island, except for MFDS. The frequencies of virulence to Lr2a, Lr2c and Lr24 have increased, whereas those to Lr14a, Lr16 and Lr17 have declined since 2000. The frequency of isolates virulent to Lr9 rose dramatically from 2.8% in 2006 to 8.7% in 2007. The frequencies of the two most predominant virulence phenotypes, TDBG (54.3%) and TDBJ (16.5%), remained unchanged from 2006, whereas the third most predominant phenotype MLDS increased from 1.4% in 2006 to 8.4% in 2007.
Résumé: Puccina recondita, agent causal de la rouille des feuilles du blé, est un agent pathogène nuisible, en rapide évolution, au Canada et partout dans le monde. Afin d'évaluer la virulence de la population de cet agent pathogène, en 2007, 381 isolats de P. triticina provenant d'une pustule unique ont été préparés à partir de collections de feuilles infectées de partout au Canada. À partir de 11 isolats collectés en Colombie-Britannique et en Alberta, cinq phénotypes de virulence ont été identifiés, dont le plus courant était MBDS. Il y avait 14 phénotypes de virulence uniques parmi les 323 isolats provenant du Manitoba et de l'est de la Saskatchewan, les plus courants étant TDBG (61,0 %), TDBJ (15,2 %), MDPS (5,3 %) et MDLS (5,3 %). Neuf phénotypes de virulence ont été identifiés chez les 33 isolats en provenance de l'Ontario : MLDS (36,4 %), TDBG (21,2 %) et TDBJ (15,2 %) étant les plus courants. Cinq phénotypes de virulence ont été identifiés parmi les 10 isolats du Québec. Les plus courants de ceux-ci étaient TDBJ et TDBG (trois isolats de chacun) et MFDS (2 isolats). Chacun des quatre isolats de l'Île-du-Prince-Édouard représentait un phénotype de virulence différent (MCNS, MFDS, MHNQ et MHNS). Ces derniers étaient tous spécifiques de l'Île-du-Prince-Édouard, sauf MFDS. Les fréquences de virulence pour les gènes Lr2a, Lr2c et Lr24 ont augmenté depuis 2000, tandis que celles pour Lr14a, Lr16 et Lr17 ont diminué. La fréquence des isolats virulents pour le gène Lr9 a augmenté de façon spectaculaire de 2,8 % en 2006 à 8,7 % en 2007. Les fréquences des deux phénotypes de virulence prédominants, TDBG (54,3 %) et TDBJ (16,5 %), n'ont pas changé depuis 2006, tandis que celles du troisième phénotype le plus courant, MLDS, sont passées de 1,4 % en 2006 à 8,4 % en 2007.
Introduction
Wheat leaf rust, caused by Puccinia triticina Eriks. (Anikster et al., Citation1997) (= P. recondita Rob. ex Desmaz. f. sp. tritici), occurs frequently on wheat in Canada and other parts of the world. It is a serious production problem in Canada, causing yield losses of 5–25%, depending on the growth stage of the crop at the time of infection and the susceptibility of the wheat crop (Samborski, Citation1985; Kolmer, Citation2001).
There were approximately 8.8 M ha seeded to wheat in Canada during 2007, including 6.2 M ha of hexaploid spring wheat (Triticum aestivum L.), 1.9 M ha of durum wheat (Triticum turgidum L. ssp. durum (Desf.) Husn.) and 0.7 M ha of winter wheat (T. aestivum) (Statistics Canada, Citation2007). Canada western red spring (CWRS) wheat class was grown on approximately 5.3 M ha of the 6.2 M ha of hexaploid spring wheat planted, with other wheat classes accounting for the remaining seeded area. The most commonly grown CWRS cultivars in 2007 were ‘Lillian’, ‘AC Barrie’ and ‘Superb’, grown on 14.8, 13.8 and 12.8%, respectively, of the total area seeded to CWRS cultivars (Anonymous, Citation2008). The genetics of leaf rust resistance in ‘Lillian’ is not known, but ‘AC Barrie’ is thought to have Lr13 and Lr16 (Kolmer, Citation2001), and ‘Superb’ to have Lr2a and Lr10 (B. McCallum, unpublished data). Resistance genes found commonly in western Canadian wheat cultivars include Lr1, Lr2a, Lr10, Lr13, Lr14a, Lr16, Lr21 and Lr34 (McCallum et al., Citation2007). There was also 0.4 M ha seeded to wheat in eastern Canada, with winter wheat in Ontario accounting for 0.3 M ha and the rest primarily spring wheat in the other eastern provinces (Statistics Canada, Citation2007). The genetic basis of leaf rust resistance in the eastern Canadian wheat cultivars has not been investigated.
Virulence surveys have been conducted annually since 1931 to monitor changes in the P. triticina population within Canada (Kolmer, Citation1991). These surveys can detect new virulence phenotypes, and provide a historic record of shifts in virulence to specific resistance genes. Knowledge on the virulence of the P. triticina population and the resistance genes in Canadian wheat cultivars (Kolmer, Citation1996) can be used to make informed decisions on breeding and developing new wheat cultivars with effective resistance. The main objective of this study was to determine the frequency and distribution of virulence phenotypes from P. triticina isolates collected across Canada in 2007.
Materials and methods
Infected wheat leaves were collected from individual fields and nurseries from June–September in 2007 in various locations throughout Prince Edward Island (PEI), Ontario, Quebec, Manitoba, Saskatchewan, Alberta and British Columbia. The leaves were air dried at 20–27 °C for approximately 12 to 24 h then stored separately for two to four months at 5 oC. Puccinia triticina urediniospores from individual collections were transferred with a metal spatula and a small amount of water to seven-day-old seedlings of the susceptible cultivar ‘Little Club’ as described previously (McCallum & Seto-Goh, Citation2005). Each pot of ‘Little Club’ seedlings was pretreated with 50 mL maleic hydrazide solution (0.36 g L−1) approximately five days after seeding to suppress the emergence of secondary leaves and enhance sporulation. A plastic cone, approximately 25 cm in height, with an open top was placed over each pot to minimize cross contamination. Inoculated seedlings were placed into a dew chamber (Model I-60D, Percival Scientific, Perry, Iowa) with nearly 100% relative humidity for approximately 17 h and then placed inside a greenhouse at 20 ± 4 °C with supplemental high-pressure sodium lighting. Approximately seven days after inoculation, the leaves were then trimmed so that single isolated uredinia remained on the upper edge of each trimmed leaf. Cross contamination was minimized by removing all extra leaves. At approximately 14 days after inoculation, urediniospores were collected from a single isolated uredinium into a 00 gelatin capsule, using a vacuum suction micro-collector.
Virulence phenotypes were determined according to the North American P. triticina nomenclature (Long & Kolmer, Citation1989). Urediniospores from single uredinia were mixed with approximately 350 μL of a light mineral oil (Bayol, Esso Canada, Toronto, ON) and sprayed onto a seven-day-old seedlings of ‘Thatcher’ and 16 standard ‘Thatcher’ near-isogenic differential lines with single resistance genes: [Set 1: Lr1 (RL6003a), Lr2a (RL6016), Lr2c (RL6047), Lr3 (RL6002); Set 2: Lr9 (RL6010), Lr16 (RL6005), Lr24 (RL6064), Lr26 (RL6078); Set 3: Lr3ka (RL6007), Lr11 (RL6053), Lr17 (RL6008), Lr30 (RL6049), Set 4: LrB (RL6051), Lr10 (RL6004), Lr14a (RL6013), Lr18 (RL6009)]. One to three (typically two) single uredinial isolates per collection were evaluated for virulence. All isolates were also inoculated onto ‘Thatcher’ and Lr1 +LrCen (RL6003b). The resistance gene temporarily named LrCen was previously identified in the Thatcher-Lr1 near-isogenic line RL6003 (McCallum et al., Citation2006; McCallum & Seto-Goh, Citation2006b). All plants of the line RL6003b have both Lr1 and LrCen. Approximately 12 seeds of each Thatcher' near-isogenic line were planted in a clump and the clumps were evenly spaced in a fibre flat (25 × 15 cm). After inoculation the plants were dried for at least 1 h to allow the oil to evaporate and then incubated and maintained as described above. Infection types produced on the 12 standards were added to provide additional virulence information about the isolates, resulting in a four letter code. Infection types to all the differential near-isogenic lines were rated 12 days after inoculation. Isolates that produced infection types ‘;’ (hypersensitive flecks), ‘1’ (small uredinia with necrosis) and ‘2’ (small- to medium-sized uredinia with chlorosis) were considered low or avirulent and those that produced infection types ‘3’ (medium-sized uredinia without chlorosis or necrosis) and ‘4’ (large uredinia without chlorosis or necrosis) were considered high (virulent) (Long & Kolmer, Citation1989). Isolates were retested if the infection type on any of the differential lines could not be determined.
At least one isolate from each of the unique virulence phenotypes identified was inoculated onto adult plants of ‘Thatcher’ and six ‘Thatcher’ near-isogenic lines (Lr12 [RL6011], Lr13 [RL4031], Lr22a [RL6044], Lr34 [RL6091], Lr35 [RL6082] or Lr37 [RL6081]). Single plants of four ‘Thatcher’ near-isogenic lines and ‘Thatcher’ were grown together in a 15 cm diameter pot and the other two near-isogenic lines and ‘Thatcher’ were grown in a second pot in the greenhouse at day/night temperatures of 25/18 °C with supplemental high-pressure sodium lighting. Plants were trimmed so that only two or three culms per plant remained. Flag leaves of two to three culms from each of the plants within a pot were inoculated with a single pustule isolate. The inoculated plants were incubated overnight in a dew chamber and grown in the greenhouse as described previously. Infection types were evaluated 14 days after inoculation. This subset of isolates was also tested on 12 additional ‘Thatcher’ near-isogenic lines at the seedling stage (Lr2b [RL6019], Lr3bg [RL6042], Lr14b [RL6006], Lr15 [RL6052], Lr19 [RL6040], Lr20 [RL6092], Lr21 [RL6043], Lr23 [RL6012], Lr25 [RL6084], Lr28 [RL6079], Lr29 [RL6080] and Lr32 [RL6086]). These isolates were retested on seedlings of the set of 16 ‘Thatcher’ near-isogenic lines mentioned above to confirm the reactions, since many of these resistance genes (particularly Lr18 and LrB) are sensitive to temperature and other conditions. Inoculation, incubation and rating were as described for seedling evaluation.
Results and discussion
Incidence and severity of wheat leaf rust in Canada
Wheat leaf rust was widespread and more severe than normal in Manitoba and eastern Saskatchewan (referred to as Manitoba and Saskatchewan hereafter) in 2007 (McCallum et al., Citation2008). In Manitoba, the level of severity was as high as 75% of the flag leaf area infected with an average of 15.7%. Foliar fungicide application was very common in Manitoba to protect the crop from leaf rust and fusarium head blight. Leaf rust was detected in 75% of the fields in a larger survey throughout Saskatchewan in 2007, which was higher than both 2005 and 2006 (Fernandez et al., Citation2008). Leaf rust was also common in Ontario, causing significant yield losses in unprotected fields (Xue et al., Citation2008).
Virulence to 16 standard differential lines at the seedling stage
In total, 381 single-pustule isolations were made from 252 wheat leaf collections from farm fields and nursery plots throughout Canada (). Most of these (323) were from Manitoba and Saskatchewan; there were also 11 from British Columbia and Alberta, 33 from Ontario, 10 from Quebec and four from PEI.
Table 1. Frequency and distribution of virulence phenotypes of Puccinia triticina identified by infection types to selected resistance genes in Canada in 2007
Five virulence phenotypes (MBDS, MBBJ, MBGJ, MLDS and TDBJ) were identified from 11 isolates collected in British Columbia and Alberta, with MBDS (four isolates) being the most common. All of these virulence phenotypes were previously identified in this region from 1998 to 2006 (McCallum & Seto-Goh, Citation2002, 2003, Citation2004, 2005, Citation2006a, 2006b, Citation2008, Citation2009) except for MLDS, which is virulent to Lr9. Virulence to Lr9 was not detected in British Columbia and Alberta from 1998 to 2006 (McCallum & Seto-Goh, Citation2002, 2003, Citation2004, 2005, Citation2006a, 2006b, Citation2008, Citation2009), but was relatively common in the USA during 2007 and previous years (Kolmer et al., Citation2009). The appearance of virulence to Lr9 in this region may be due to selection of this phenotype on cultivars in the USA with Lr9 and subsequent movement into Canada, since inoculum typically travels from the northern USA into Canada during the growing season on prevailing winds (Samborski, Citation1985).
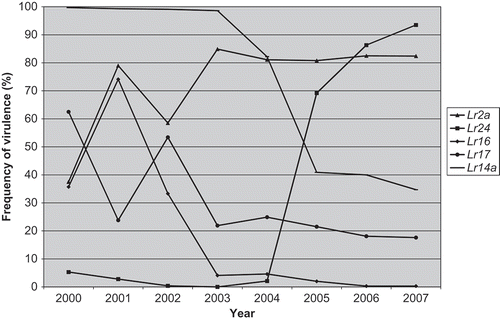
There were 14 different virulence phenotypes identified among 323 isolates from Manitoba and Saskatchewan. The most common of these were TDBG (61.0%), TDBJ (15.2%), MDPS (5.3%) and MLDS (5.3%) (). In 2006, TDBG (56.2%) and TDBJ (18.7%) were also very common in this region, whereas MDPS (3.2%) was at relatively lower levels. All the virulence phenotypes identified in 2007 were also found in this region from 1995 to 2006 except for MDPN and TDBQ. Phenotypes TDGB, TDBJ and MLDS were among the most commonly isolated virulence phenotypes from a similar study done in a north central area of the USA, directly south of Manitoba and Saskatchewan (Kolmer et al., Citation2009). The frequencies of virulence to Lr1, Lr2a, Lr2c, Lr3, Lr24 and Lr10 were over 80% in this region (). Frequencies of virulence to Lr2a, Lr2c and Lr24 rose substantially from 2000 when 37.5% of the isolates were virulent to Lr2a and Lr2c and only 5.3% of the isolates were virulent to Lr24. This increase in the frequency of virulence started in 2001 for Lr2a, but not until 2004 for Lr24 (). Selection for virulence on wheat cultivars with Lr24 in the USA could be a contributing factor driving this change (Kolmer et al., Citation2008). Resistance gene Lr24 is not thought to be used in western Canada (McCallum et al., Citation2007), but could be in some winter wheat cultivars in Ontario, including ‘Vienna’ (B. McCallum, unpublished data). Alternatively, the frequencies of virulence to Lr14a, Lr16 and Lr17 have declined from 2000 when they were 99.7%, 35.7% and 62.5%, respectively, to the 2007 levels of 34.7%, 0.3% and 17.6% (). Phenotype TDBG was the most common and TDBJ the second or third most common virulence phenotype found in Manitoba and Saskatchewan during 2005–2007 (). Both of these virulence phenotypes have some unique virulence characteristics. All isolates of TDBG and some isolates of TDBJ are avirulent to LrCen. Phenotype TDBG isolates were also avirulent to Lr14a, whereas nearly all other isolates are virulent.
Table 2. Frequencies of virulence of Puccinia triticin a in Canada in 2007 to lines of wheat with single Lr genes for leaf rust resistance
Table 3. Frequency (%) of predominant P. triticina virulence phenotypes in Canada from 2000 to 2007
Fig. 1. Frequency of virulence (%) from 2000–2007 in the Manitoba and Saskatchewan population of P. triticina to near-isogenic lines containing Lr2a, Lr14a, Lr16, Lr17 or Lr24. Data from McCallum & Seto-Goh (Citation2003, Citation2004, 2005, Citation2006a, 2006b, Citation2008, Citation2009).
Nine virulence phenotypes were identified among 33 isolates collected in Ontario. The most common were MLDS (36.4%), TDBG (21.2%) and TDBJ (15.2%). Only MFPS and TDBJ were found in this region previously during the period 1998 to 2006 (McCallum & Seto-Goh, Citation2002, 2003, Citation2004, 2005, Citation2006a, 2006b, Citation2008, 2009). Although MLDS, TDBG and TDBJ were not found recently in Ontario, they were common phenotypes in Manitoba and Saskatchewan in 2006 and 2007. The frequency of virulence to Lr24 (60.6%) has dramatically increased in Ontario since 2004, when no Lr24 virulent isolates were detected (McCallum & Seto-Goh, Citation2006b). Virulence to Lr9 was either not detected or present at low levels in Ontario, from 1998 to 2006 (McCallum & Seto-Goh, Citation2002, 2003, 2004, 2005, Citation2006a, 2006b, 2008, 2009) but was at a relatively high frequency (36.4%) in 2007. This increase may be due to selection on wheat cultivars with Lr9 in the USA, as outlined above, and subsequent movement of this inoculum into Ontario.
Five virulence phenotypes were identified among 10 isolates from Quebec, with TDBJ and TDBG (three isolates each) being relatively common (). The other three phenotypes, MFDS (two isolates), MFGJ and MLDS were not found in Quebec from 1999 to 2006 (McCallum & Seto-Goh, Citation2002, 2003, Citation2004, 2005, Citation2006a, 2006b, 2008, 2009). All virulence phenotypes identified from Quebec were virulent to Lr24, except for MLDS. The frequency of virulence to Lr24 in this region was higher than during 1999 to 2005 (McCallum & Seto-Goh, Citation2002, 2003, Citation2004, 2005, 2006a, 2006b, 2008, 2009). However, in 2006, all isolates collected from Quebec were virulent to Lr24. This increase in virulence to Lr24 may be due to selection on cultivars with Lr24 either in the USA or in Canada. Selection in the USA is likely a factor since a similar rise in the frequency of virulence to Lr24 has been seen in the USA survey of virulence (Kolmer et al., Citation2009).
Each of the four isolates from PEI represented a different virulence phenotype – MCNS, MHNQ and MHNS, which were unique to PEI, and MFDS, which was also found in Quebec. Collections from PEI were analyzed in 2006 and only three of nine virulence phenotypes found in PEI, were found in other regions of Canada. Phenotype MFDS was found in both years. These results indicate that the PEI population of P. triticina is distinct from the populations in Quebec and Ontario, with only a few virulence pathotypes in common. Two of the four isolates collected in 2007 from PEI were virulent to Lr16, which was rare in the rest of Canada, although most isolates collected in Canada have an intermediate level of virulence for Lr16. Three of the four PEI isolates were also virulent to Lr3ka which had a relatively low frequency of virulence in the other regions.
Virulence to six adult and 12 additional seedling differential lines
There were 69 isolates, representative of all the virulence phenotypes found in 2007, tested on the six ‘Thatcher’ near-isogenic lines with single adult plant resistance genes and 12 additional differential lines with single seedling resistance genes. The results for 39 of these isolates are presented in . If more than one isolate from the same virulence phenotype had the same reactions, results of only one isolate are shown. At the adult plant stage, all isolates tested were avirulent to Lr22a, Lr34 and Lr35, except 243-2 MLDS which was virulent to Lr35. The isolates varied in their reaction to Lr12, Lr13 and Lr37, but most isolates were virulent on all three of the lines with these genes. Resistance genes Lr13, Lr22a and Lr34 are used in Canadian wheat cultivars (McCallum & DePauw, Citation2008). Since 2002, a representative selection of isolates have been tested for virulence to Lr35 and Lr37 (McCallum & Seto-Goh, Citation2005, Citation2006a, Citation2006b, Citation2008, Citation2009). Throughout these years, Lr35 has provided effective resistance to nearly all isolates while there was an increasing frequency of virulence to Lr37. All 2007 isolates were also avirulent to Lr19, Lr21, Lr25, Lr29 and Lr32, virulent to Lr15 and Lr14b except 221-2 MDPS which was avirulent to Lr14b, and varied in reaction to Lr3bg, Lr20, Lr23 and Lr28 (). Isolates had similar reactions to the lines with Lr2b or Lr2a. The resistance gene Lr21, discovered in Aegilops squarrosa L. (Triticum tauschii (Coss.) Schmal.,) and transferred into Triticum aestivum L. by Canadian researchers in 1974 (Rowland & Kerber, Citation1974), is currently deployed in a number of Canadian cultivars including ‘AC Cora’, ‘McKenzie’ and ‘CDC Alsask’ (McCallum & DePauw, Citation2008), and has provided effective resistance since the registration of ‘AC Cora’ in 1994.
Table 4. Infection types of 39 representative isolates from Canada in 2007 tested on near isogenic lines of ‘Thatcher’ with adult plant resistance genes and additional seedling resistance genes
Overall, the most predominant virulence phenotypes found in Canada during 2007, TDBG (54.3%) and TDBJ (16.5%), were found at very similar frequencies in 2006. Prior to 2005, however, they were rarely detected (). The third most predominant virulence phenotype found in 2007, MLDS (8.4%) was also rarely found from 2000 to 2006 (). Phenotype MLDS is virulent to Lr9, whereas the majority of isolates are avirulent. Gene Lr9 is not common in current Canadian wheat cultivars (McCallum et al., Citation2007), but is found in wheat cultivars throughout the US (Kolmer et al., Citation2009) which could account for the increase in virulence to this gene. All other virulence phenotypes identified in 2007 have been found in Canada in recent years (1995 to 2006), except for MFRJ (from Ontario), MDPN (from Manitoba and Saskatchewan), and MHNQ and MHNS (both from PEI). Puccinia triticina has only been collected in PEI for two years and therefore it is not surprising that novel virulence phenotypes were detected. The P. triticina populations in Ontario, Manitoba and Saskatchewan have been sampled more extensively therefore MFRJ and MDPN may represent new virulence combinations in these regions.
While some leaf rust resistance genes have become ineffective due to increased frequencies of virulence in the pathogen population over time, other genes such as Lr18, Lr19, Lr21, Lr22a, Lr25, Lr29, Lr32, Lr34 and Lr35 are still very effective in controlling wheat leaf rust. Canadian cultivars with the most durable leaf rust resistance have typically involved combinations, or pyramids, of effective leaf rust resistance genes (McCallum et al., Citation2007). Future wheat cultivars should have good protection from leaf rust with combinations of effective genes, particularly those involving the durable race non-specific gene Lr34. It has provided effective leaf rust resistance in Canadian wheat cultivars since its deployment in the cultivar ‘Glenlea’ in 1972 (McCallum & DePauw, Citation2008) and enhances the expression of other resistance genes (German & Kolmer, Citation1992).
Acknowledgements
Thanks to André Comeau and Richard Martin who sent collections for analysis, and other cooperators who grew trap rust nurseries.
References
- Anikster , Y. , Bushnell , W.R. , Eilam , T. , Manisterski , J. and Roelfs , A.P. 1997 . Puccinia recondita causing leaf rust on cultivated wheats, wild wheats, and rye . Can. J. Bot. , 75 : 2082 – 2096 .
- Anonymous . 2008 . “ Canadian Wheat Board, wheat variety survey 2007–2008 ” . In Available from the Canadian Wheat Board, 423 Main St., P.O. Box 816 Station Main, Winnipeg, MB, R3C 2P5, Canada
- Fernandez , M.R. , Dusabenyagasani , M. , Pearse , P.G. and Holzgang , G. 2008 . Leaf and stripe rust diseases of common wheat in Saskatchewan in 2007 . Can. Plant Dis. Surv. , 88 : 83
- German , S.E. and Kolmer , J.A. 1992 . Effect of gene Lr34 in the enhancement of resistance to leaf rust of wheat . Theor. Appl. Genet. , 84 : 97 – 105 .
- Kolmer , J.A. 1991 . Phenotypic diversity in two populations of Puccinia recondita f. sp. tritici in Canada during 1931–1987 . Phytopathology , 81 : 311 – 315 .
- Kolmer , J.A. 1996 . Genetics of resistance to wheat leaf rust . Annu. Rev. Phytopathol. , 34 : 435 – 455 .
- Kolmer , J.A. 2001 . Physiologic specialization of Puccinia triticina in Canada in 1998 . Plant Dis. , 85 : 155 – 158 .
- Kolmer , J.A. , Long , D.L. and Hughes , M.E. 2008 . Physiologic specialization of Puccinia triticina on wheat in the United States in 2006 . Plant Dis. , 92 : 1241 – 1246 .
- Kolmer , J.A. , Long , D.L. and Hughes , M.E. 2009 . Physiologic specialization of Puccinia triticina on wheat in the United States in 2007 . Plant Dis. , 93 : 538 – 544 .
- Long , D.L. and Kolmer , J.A. 1989 . A North American system of nomenclature for Puccinia recondita f. sp. tritici . Phytopathology , 79 : 525 – 529 .
- McCallum , B.D. , Cloutier , S. , Rampitsch , C. and Bykova , N. 2006 . Evidence for a second seedling leaf rust resistance gene in the Thatcher-Lr1 near-isogenic wheat line RL6003 . Can. J. Plant Pathol. , 27 : 472 – 473 .
- McCallum , B.D. and DePauw , R.M. 2008 . A review of wheat cultivars grown in the Canadian prairies . Can. J. Plant Sci. , 88 : 649 – 677 .
- McCallum , B.D. , Fetch , T. and Chong , J. 2007 . Cereal rust control in Canada . Austr. J. Agric. Res. , 58 : 639 – 647 .
- McCallum , B.D. , Pearse , P.G. and Seto-Goh , P. 2008 . Leaf rust and stripe rust of wheat in Manitoba and eastern Saskatchewan in 2007 . Can. Plant Dis. Surv. , 88 : 88
- McCallum , B.D. and Seto-Goh , P. 2002 . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 1999 . Can. J. Plant Pathol. , 24 : 205 – 210 .
- McCallum , B.D. and Seto-Goh , P. 2003 . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2000 . Can. J. Plant Pathol. , 25 : 91 – 97 .
- McCallum , B.D. and Seto-Goh , P. 2004 . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2001 . Can. J. Plant Pathol. , 26 : 109 – 120 .
- McCallum , B.D. and Seto-Goh , P. 2005 . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2002 . Can. J. Plant Pathol. , 27 : 90 – 99 .
- McCallum , B.D. and Seto-Goh , P. 2006a . Physiologic specialization of wheat leaf rust (Puccinia triticina) in Canada in 2003 . Can. J. Plant Pathol. , 28 : 208 – 213 .
- McCallum , B.D. and Seto-Goh , P. 2006b . Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2004 . Can. J. Plant Pathol. , 28 : 566 – 576 .
- McCallum , B.D. and Seto-Goh , P. 2008 . Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2005 . Can. J. Plant Pathol. , 30 : 124 – 132 .
- McCallum , B.D. and Seto-Goh , P. 2009 . Physiologic specialization of Puccinia triticina, the causal agent of wheat leaf rust, in Canada in 2006 . Can. J. Plant Pathol. , 31 : 80 – 87 .
- Rowland , G.G. and Kerber , E.R. 1974 . Telocentric mapping hexaploid wheat of genes for leaf rust resistance and other characters derived from Aegilops squarrosa . Can. J. Genet. Cytol. , 16 : 137 – 144 .
- Samborski , D.J. 1985 . “ Wheat leaf rust ” . In The cereal rusts, Vol. 2. Diseases, distribution, epidemiology, and control , Edited by: Roelfs , A.P. and Bushnell , W.R. 39 – 59 . Orlando, FL : Academic Press .
- Canada , Statistics . 2007 . “ Field crop reporting series – 3401 ” . In Table 001–0010
- Xue , A.G. , Chen , Y. and Voldeng , H.D. 2008 . Diseases of spring wheat in eastern Ontario in 2007 . Can. Plant Dis. Surv. , 88 : 95 – 96 .