Abstract
Garden pea leaf blight, caused by Mycosphaerella pinodes, is a severe disease of peas worldwide. Detection of this pathogen from seed, plant debris and soil is difficult using non-selective agar media. This study was conducted to develop a semiselective medium for the isolation of this pathogen. Of the 14 carbon sources and 18 nitrogen sources tested, maltose and soluble starch were the most suitable carbon sources and casein was the most important nitrogen source for mycelial growth of three isolates of M. pinodes. Among 13 pesticides and two antibiotics tested, atrazine, mepronil, paraquat, propamocarb and TMTD showed no adverse effect on growth of M. pinodes. Based on these results, a starch–casein semiselective medium (designated as the SC-semiselective medium) was developed by amendment of 1 L Czapek–Dox agar with 30 g soluble starch, 2 g casein, 25 μg mL-1 propamocarb, 10 μg mL-1 benomyl, 50 μg mL-1 mepronil, 300 μg mL-1 atrazine, 100 μg mL-1 streptomycin sulfate and 200 μg mL-1 neomycin sulfate. This SC-semiselective medium was effective for detection of M. pinodes in artificially and naturally infested field soils, diseased seeds, leaves, and stems of peas. It should have potential for applications in research on biology, ecology and control of M. pinodes in peas.
Résumé
L'anthracnose du pois de jardin, causée par Mycosphaerella pinodes, est une grave maladie des pois qui cause des ravages dans tous les pays. Il est difficile de détecter cet agent pathogène à partir de semences, de débris de plants et de sol lorsqu'on utilise un milieu de culture non sélectif. Cette étude a été menée afin de développer un milieu de culture semi-sélectif pour isoler l'agent pathogène. Des 14 sources de carbone et des 18 d'azote testées, le maltose et l'amidon soluble ont été les sources de carbone les plus appropriées et la caséine a été la source d'azote la plus propice à la croissance mycélienne de trois isolats de M. pinodes. Parmi les 13 pesticides et les 2 antibiotiques testés, l'atrazine, le mépronil, le paraquat, le propamocarbe et le TMTD n'ont eu aucun effet négatif sur la croissance de M. pinodes. À partir de ces résultats, un milieu semi-sélectif à base d'amidon et de caséine (désigné « milieu semi-sélectif AC ») a été développé en modifiant 1 L de gélose Czapek–Dox avec 30 g d'amidon soluble, 2 g de caséine, 25 μg mL–1 de propamocarbe, 10 μg mL–1 de bénomyl, 50 μg mL–1 de mépronil, 300 μg mL–1 d'atrazine, 100 μg mL–1 de sulfate de streptomycine et 200 μg mL–1 de sulfate de néomycine. Ce milieu semi-sélectif AC a permis de détecter M. pinodes dans le sol de champs infectés artificiellement et naturellement ainsi que dans des semences, des feuilles et des tiges de pois contaminées. Ce milieu devrait offrir des possibilités quant aux applications utilisées en recherches sur la biologie et l’écologie de M. pinodes chez le pois ainsi que dans la lutte contre cet agent pathogène.
Introduction
Leaf blight of garden pea (Pisum sativum L.), caused by Mycosphaerella pinodes (Berk. & Blox) Vester., is widespread worldwide (Jones, Citation1927; Dickinson & Sheridan, Citation1986; Hagedorn, Citation1989). It often causes severe losses in seed yield and seed quality (Tivoli et al., Citation1996; Beasse et al., Citation1999; Bretag et al., Citation2006). Infected pea plants showed symptoms of foot rot and necrotic spots on leaves, stems and pods (Bretag & Ramsey, Citation2001). The pathogen survives on infected pea seeds (Ali et al., Citation1982; Agarwal & Sinclair, Citation1987; Chen & Huang, Citation1994; Chen et al., Citation1994), dead pea haulm (Punithalingam & Hallidey, Citation1972; Sheridan, Citation1973) or other organic matter (Wallen, Citation1974; Dickinson & Sheridan, Citation1986), or in the soil (Wallen et al., Citation1967; Wallen, Citation1974; Dickinson & Sheridan, Citation1986).
Isolation of M. pinodes from seed, plant debris and soil is difficult due to the interference of saprophytic fungi and bacteria. Sensitive and specific techniques such as the enzyme-linked immunosorbent assay (ELISA) (Faris-Mokaiesh et al., Citation1995) and polymerase chain reaction (PCR) (Onfroy et al., Citation1999) have been developed for rapid detection of seed-borne M. pinodes, but these techniques are very expensive. Furthermore, the techniques neither provide information about viability nor allow the isolation of M. pinodes. Potato dextrose agar (PDA) and PDA amended with 0.5 g L-1 oxgall (Tinivella et al., Citation2009) are the common growth media used to isolate M. pinodes from seeds and other plant tissues. These media, however, are non-selective and they are difficult to use to detect M. pinodes from field specimens or soils because of frequent contamination of samples by other microorganisms. To our knowledge, no information is available on formulation of selective media for the detection of this pathogen. The objective of this study was to develop a semiselective medium for the isolation of M. pinodes from seed, plant debris and soil.
Materials and methods
Microorganisms and pesticides
Mycosphaerella pinodes isolates MP-12, MP-61 and MP-72 were obtained from diseased leaves of peas collected in Changhwa county, in central Taiwan and used in this study. Stock cultures were maintained on potato dextrose agar (PDA) and stored at −20 °C. Thirteen pesticides () and two antibiotics (neomycin sulfate and streptomycin sulfate) were evaluated for their effects on mycelial growth of M. pinodes.
Table 1. List of common names, chemical names and manufacturers of pesticides used in this study
Effect of carbon source on mycelial growth of M. pinodes
A modified Czapek–Dox medium (2 g NaNO3, 1 g K2HPO4, 0.5 g KCl, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 15 g agar (Difco, France) and 1 litre distilled water) without sucrose was used as a base for supplementing different saccharides (30 g L-1). The saccharides tested were: (i) the monosaccharides fructose, galactose, glucose, mannose, mannitol, galacturonic acid and xylose; (ii) the disaccharides cellobiose, lactose, maltose, sucrose and trehalose; (iii) polysaccharide soluble starch and raffinose. To determine the effect of various saccharides on the growth of M. pinodes, the sucrose in Czapek–Dox agar was substituted by each saccharide at a concentration of 3% (w/v). Agar discs (5 mm diam.) containing mycelial mats of each of the three isolates of M. pinodes were removed from seven-day-old PDA cultures of each isolate and inoculated on each saccharide-amended Czapek–Dox medium in Petri dishes, 1 disc/dish. After incubation for six days at 24 °C, mycelial growth was determined by measuring colony diameter. Mycelial growth of M. pinodes on Czapek–Dox medium without sucrose was used as controls. The experiment was repeated twice with four replicates for each treatment.
Effect of nitrogen source on mycelial growth of M. pinodes
The nitrogen sources tested at 0.2% (2 g L-1, w/v) were: (i) amino acids including arginine, asparagine, aspartic acid, methionine, glycine, phenylalanine, tyrose and tryptophan; (ii) inorganic nitrogen sources including calcium nitrate, potassium nitrate, sodium nitrate, sodium nitrite, ammonium chloride, ammonium phosphate, ammonium nitrate, ammonium sulfate and urea; and (iii) organic nitrogen including casein. Each nitrogen source was added to the base Czapek–Dox medium without sodium nitrate containing 3% (w/v) soluble starch. Three isolates of M. pinodes described previously were tested for mycelial growth on each nitrogen-amended Czapek–Dox medium using the same procedure described in the carbon source experiment. The growth of M. pinodes on Czapek–Dox medium containing soluble starch was used as controls. The experiment was repeated twice with four replicates for each treatment.
Susceptibility of M. pinodes to pesticides
Mycosphaerella pinodes isolate MP-61 was tested for sensitivity to 13 commercial pesticides, which did not cause reduction in mycelial growth of the pathogen but inhibited growth of several fungi associated with seeds or soils. The pesticides tested were: (i) herbicides including atrazine and paraquat; and (ii) fungicides including benomyl, bitertanil, iprodione, mancozed-metalaxyl, mepronil, PCNB, procymidone, propamocarb, thylate (TMTD), triforine and vinclozolin. Each pesticide was added to the base Czapek–Dox medium containing 3% (w/v) soluble starch and 0.2% (w/v) casein at rates of 50 and 100 μg mL-1. Mycosphaerella pinodes was tested for mycelial growth on each pesticide-amended Czapek–Dox medium using the same procedure described in the carbon source experiment. The growth of M. pinodes on Czapek–Dox medium containing soluble starch and casein was used as a control. The experiment was repeated twice with four replicates for each treatment.
Effect of pesticides and antibiotics on germ tube elongation and mycelial growth of M. pinodes
Seven pesticides (benomyl, mepronil, propamocarb, TMTD, triforine, paraquat and atrazine) and two antibiotics (neomycin sulfate and streptomycin sulfate) were tested for their effects on mycelial growth of M. pinodes, isolate MP-61. Antibiotics were dissolved in water and filter-sterilized using 0.22 μm filters (Millex, Milipore, USA) to prepare stock solutions. The Czapek–Dox medium containing 3% (w/v) soluble starch and 0.2% (w/v) casein supplemented with various pesticides and antibiotics at different concentrations were poured into Petri dishes (90 mm diam.). Concentrations of pesticides and antibiotics were 0, 25, 50 and 100 μg mL-1 for meprohil, propamocarb, TMTD and triforine; 0, 50, 100 and 200 μg mL-1 for neomycin sulfate and paraquat; 0, 10, 25 and 50 μg mL-1 for benomyl; 0, 50, 100, 250 and 500 μg mL-1 for atrazine; and 0, 100, 200 and 300 μg mL-1 for streptomycin sulfate. Mycosphaerella pinodes was tested for mycelial growth on each pesticide- or antibiotic-amended Czapek–Dox medium following the same procedure described in the carbon source experiment. The experiment was repeated twice with four replicates for each treatment.
To test conidial germination, M. pinodes isolate PM-61 was grown on PDA in Petri dishes and incubated at 24 °C under a 12h photoperiod for six days. Conidial suspensions were prepared by flooding each dish with 20 mL sterile distilled water and filtering through a double-layer cheesecloth. The suspensions were adjusted to 5 × 104 spores mL-1 using sterile distilled water. Aliquots (100 μL) of conidial suspensions were spread over the surface of each pesticide- or antibiotic-amended Czapek–Dox medium. After incubation at 24 °C for 12 h, each pesticide- or antibiotic-amended Czapek–Dox medium was stained using 0.1% (w/v) cotton blue. Germinated conidia were examined and measured for length of germ tubes using a compound microscope. The experiment was repeated twice with four replicates for each treatment.
Formulation and evaluation of a SC-semiselective medium for detection of M. pinodes in seed, plant debris and soil
The medium that satisfied the criteria specified above was named the SC-semiselective medium and had the following composition per litre: 2 g NaNO3, 1 g K2HPO4, 0.5 g KCl, 0.5 g MgSO4.7H2O, 0.01 g FeSO4.7H2O, 15 g agar, 30 g soluble starch, 3 g casein, 25 μg mL-1 propamocarb, 10 μg mL-1 benomyl, 50 μg mL-1 mepronil, 300 μg mL-1 atrazine, 100 μg mL-1 streptomycin sulfate and 200 μg mL-1 neomycin sulfate. The SC-semiselective medium was used for the following experiments.
To test recovery of the pathogen in soil, a spore suspension (2.35–2.48 × 104 spores mL-1) of M. pinodes isolate 61 was added to either Tali (sandy loam) or Wufeng soil (sandy loam), 20 mL per 100 g soil. Ten grams of artificially infested soil were suspended in 90 mL of sterile distilled water in each 250 mL flask and mixed thoroughly on a rotary shaker (250 rpm) at room temperature for 1 h. The suspension was serially diluted, spread on SC-semiselective medium and incubated at 24 °C for five days. The recovery of M. pinodes on SC-semiselective medium was recorded. The experiment was repeated twice with four replicates for each treatment.
To test recovery of the pathogen in diseased tissues, stems (two-months-old) and leaves (two-months-old) of garden pea showing symptoms of leaf blight were collected from fields naturally infested with M. pinodes in Hsinyi, Hsihu and Hsinshe counties, central Taiwan. Plant samples were surface sterilized in 1% (v/v) sodium hypochlorite for 2 min, rinsed with sterile distilled water, air-dried, cut into pieces measuring 0.5 cm long for stems and 0.5 × 0.5 cm for leaves, and placed on the SC-semiselective medium in Petri dishes, 10 pieces/dish. After incubation at 24 °C for five days, the number of tissues with growth of M. pinodes was recorded. There were 10 replicates for each treatment. The experiment was repeated twice.
To test recovery of the pathogen from infected seeds, samples of garden pea (‘Known-You Taichung No. 13', ‘Known-You Taichung No. 11’, ‘Hao-Nong-Gu No. 1’ and ‘Taichung No. 11’) were collected from commercial fields in Hsihu, Hsinyi and Hsinshe counties, central Taiwan. These seed samples were collected from fields with known or suspected infection of pea plants by M. pinodes. Fifty seeds per seedlot were tested on SC-semiselective medium, five seeds per dish. After incubation at 24 °C for five days, the number of seeds infected by M. pinodes in each seedlot was recorded. The experiment was repeated twice with 10 replicates for each treatment.
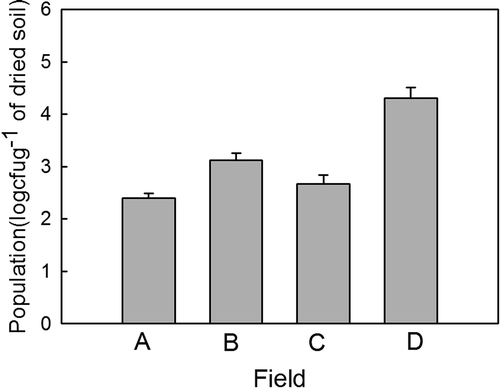
To test recovery of the pathogen from infested soil, samples of surface soil (top 10–15 cm) were collected from four pea fields (designated as A, B, C and D) in Hsinyi county, central Taiwan. Garden pea ‘Taichung No. 13’ (Known-You Seed Co., Taiwan) was grown in these fields and incidence of plants showing symptoms of leaf blight was about 30–50% when surveyed at harvest. Soil samples were processed and tested for infestation of M. pinodes by the method described above. The experiment was repeated twice with four replicates for each treatment.
Statistical analysis
Data from experiments on colony size and germ tube elongation were analyzed using analysis of variance (ANOVA) for a completely randomized design. Data from repeats of each experiment were pooled when the variances were homogeneous. Statistical significance was tested with untransformed data (population). Tukey's multiple range test (TMRT) or regression analysis (SAS Institute Inc., Cary, NC, 1997) was used to compare means of the treatments in each experiment.
Results
Effect of carbon source on mycelial growth of M. pinodes
Mycelial growth of M. pinodes varied with carbon sources in Czapek–Dox medium without sucrose (). Among the 14 carbon sources tested, maltose and soluble starch were the most effective for enhancing mycelial growth of M. pinodes. For example, colony diameter of M. pinodes isolates MP-12, MP-61 and MP-72 was 4.2, 3.8 and 4.3 cm, respectively, on the medium containing maltose and 4.0, 4.3 and 4.2 cm, respectively, on the medium containing soluble starch, compared with 1.7, 2.3 and 2.3 cm for the control (). Galacturonic acid was the least effective carbon source with colony diameter of 1.1 cm for all three isolates of M. pinodes (). The 11 other saccharides promoted mycelial growth, but they were not as effective as maltose and soluble starch.
Table 2. Effect of carbon source on mycelial growth of Mycosphaerella pinodes (isolates MP-12, MP-61 and MP-72)
Effect of nitrogen source on mycelial growth of M. pinodes
Mycelial growth of M. pinodes varied with nitrogen sources in Czapek–Dox medium containing soluble starch as carbon source (). Among the 18 nitrogen sources tested, all nine inorganic nitrogen compounds and eight amino acids caused a significant (P < 0.05) reduction in mycelial growth of M. pinodes, whereas the organic nitrogen compound, casein, was the only chemical with no inhibitory effect on the growth of this pathogen. The colony diameter of M. pinodes isolates MP-12, MP-61 and MP-72 was 5.2, 5.3 and 5.6 cm, respectively, for the treatment of casein, compared with 5.2, 5.6 and 5.6 cm, respectively, for the untreated control (). Urea and aspartic acid showed the highest suppressive effects on the growth of M. pinodes among all nitrogen chemicals tested.
Table 3. Effect of nitrogen source on mycelial growth of Mycosphaerella pinodes (isolates MP-12, MP-61 and MP-72)
Susceptibility of M. pinodes to pesticides
Among the 13 pesticides tested at the rate of 50 and 100 μg mL-1, the fungicides with highest toxicity to the growth of M. pinodes were iprodione, mancozeb-metalaxyl and procymiode, followed by Bitertanil, PCNB and Vinclozolin (). For example, the colony diameter of M. pinodes on Czapek–Dox medium amended with 50 μg mL-1 of pesticide was 0.83, 0 and 0.95 cm for iprodione, mancozeb-metalaxyl and procymiode, respectively, compared with 4.45 cm for the untreated control. The herbicides atrazine and paraquat and the fungicides mepronil, propamocarb and TMTD showed no suppressive effects on the growth of M. pinodes at both rates of 50 and 100 μg mL-1.
Table 4. Sensitivity of Mycosphaerella pinodes (isolate MP-61) to pesticides
Effect of pesticides and antibiotics on growth of germ tubes and mycelia of M. pinodes
The relationships between concentrations of pesticides and antibiotics against the germ tube elongation and mycelial growth of M. pinodes are shown in . Procampcarb and mepronil at 25–100 μg mL-1, streptomycin sulfate at 100–300 μg mL-1 and neomycin sulfate at 50–200 μg mL-1 did not inhibit the growth of germ tubes of M. pinodes isolate MP-61. A slight inhibition of germ tube elongation was observed with benomyl at 25–50 μg mL-1 (R 2 = 0.62, P < 0.0019), TMTD at 50–100 μg mL-1 (R 2 = 0.66, P < 0.0074) and triforine at 50–100 μg mL-1 (R2 = 0.79, P < 0.0001); whereas atrazine at 300 μg mL-1 enhanced the growth of germ tube of M. pinodes ().
Table 5. Effectiveness of pesticides and antibiotics on germ tube elongation and mycelial growth of Mycosphaerella pinodes isolate MP-61
No inhibition of mycelial growth of M. pinodes isolate MP-61 was observed in the treatments of procampcarb at 25–100 μg mL-1, triforine at 25–100 μg mL-1, TMTD at 50–100 μg mL-1, mepronil at 25–100 μg mL-1, atrazine at 50–500 μg mL-1, streptomycin sulfate at 100–300 μg mL-1 and neomycin sulfate at 50–200 μg mL-1 (). Compared to the controls, a slight reduction of mycelial growth of M. pinodes isolate MP-61 occurred in the treatments of benomyl at 25–50 μg mL-1 (R 2 = 0.94, P < 0.0001) and paraquat at 150–200 μg mL-1 (R 2 = 0.94, P < 0.0001).
Formulation of SC-semiselective medium for M. pinodes
Propamocarb at 25 μg mL-1 and mepronil at 50 μg mL-1 did not affect mycelial growth of M. pinodes and pycnidial formation on the SC-semiselective medium. Benomyl at 25 μg mL-1 or higher slightly reduced mycelial growth of M. pinodes and therefore, a concentration of 10 μg mL-1 was used in further experiments. The broad-spectrum antibiotics used in this medium were streptomycin sulfate at 100 μg mL-1 and neomycin sulfate at 200 μg mL-1. Atrazine was tested at 300 μg mL-1 to delay the germination of pea seeds. Thus, the SC-semiselective medium was composed of Czapek–Dox agar plus other ingredients, including 30 g soluble starch, 2 g casein, 25 μg mL-1 propamocarb, 10 μg mL-1 benomyl, 50 μg mL-1 mepronil, 300 μg mL-1 atrazine, 100 μg mL-1 streptomycin sulfate and 200 μg mL-1 neomycin sulfate per litre of the medium. Streptomycin sulfate and neomycin sulfate were added after the medium was autoclaved and cooled to 50 °C. This semiselective medium enhanced the sporulation of M. pinodes (data not shown).
Evaluation of SC-semiselective medium for isolation of M. pinodes
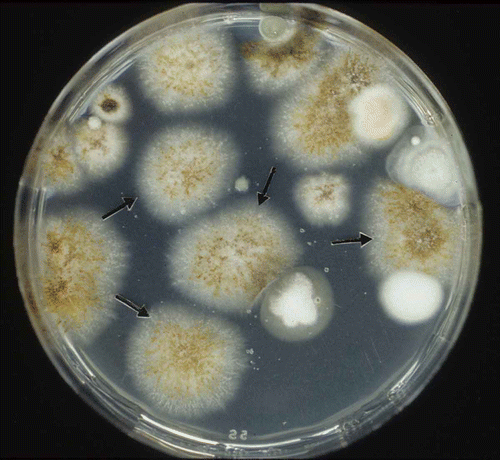
The SC-semiselective medium was used to detect M. pinodes in soil artificially infested with spores of this pathogen. Based on colony forming units that developed on the SC-semiselective medium, the recovery rate of M. pinodes was 83.3 and 93.6% for soil samples of Wufeng and Tali, respectively (). Mycosphaerella pinodes formed orange-pink or reddish pycnidia that were arranged in threads on this medium (). Several saprophytic soil fungi, including Cladosporium spp., Penicillium spp., and Alternaria spp. were detected in these soil samples ().
Table 6. Recovery of Mycosphaerella pinodes from two artificially infested soils on starch–casein (SC)-semiselective medium
Fig. 1. Isolation of Mycosphaerella pinodes from artificially infested soil on the starch–casein (SC)-semiselective medium. Note sporulation on colonies of M. pinodes (arrows) after incubation for five days at 24 °C.
Six seed samples from pea crops grown in the counties of Hsinyi, Hsinshe and Hsihu, Taiwan, were tested on the SC-semiselective medium and results showed that M. pinodes was detected in 0 to 2% of the seeds tested (). Colonies of M. pinodes were readily identified by their morphological characteristics. Other microorganisms on pea seeds either failed to grow on the medium or had distinctly different colony morphology (). Using SC-semiselective medium for isolation of pea plants grown in commercial fields, M. pinodes was found in 70–98% of the leaf-debris and 10–50% of stem-debris (). The samples of stem-debris were contaminated by Colletotrichum pisi Pat., ranging from 50 to 90%.
Table 7. Isolation of Mycosphaerella pinodes and Colletotrichum pisi from naturally infected pea tissues (seeds, leaves and stems) on SC-semiselective medium
Isolation of M. pinodes from naturally infested soils collected from pea-growing fields was successful. Population level was estimated from 2.5 × 102 to 2 × 104 cfu g-1 of dried soil in the four fields of peas in Hsinyi county, Taiwan ().
Discussion
Development of a medium for selective isolation of specific fungi is achieved by using nutrients and antimicrobial chemicals that are capable of enhancing the growth of desirable target microorganisms and suppressing the growth of undesirable microorganisms (Tsao, Citation1970). The SC-semiselective medium developed in this study is useful because it effectively inhibited the growth of most saprophytes, while allowing the growth and development of colonies of M. pinodes with a distinct morphology. This SC-semiselective medium has potential for applications in detecting M. pinodes in soil, commercial pea seed lots and other parts of pea tissues such as diseased leaves and stems. A few other fungal species in soil samples were detected, but they could be readily distinguished from M. pinodes because of their differences in colony size and colour.
This study reveals that the most important source of carbon and nitrogen for growth of M. pinodes is soluble starch and casein, respectively. Consequently, they were used as the primary source of carbon and nitrogen in the SC-semiselective medium. Sporulation of M. pinodes was considered a desirable attribute because fruiting bodies are the most important diagnostic feature for M. pinodes in the presence of other microbial contaminants. The SC-semiselective medium meets this requirement as the pesticides and antibiotics used in this medium have no adverse effects on colony development and sporulation of M. pinodes. Although benomyl had been used for treatment of pea seeds to control seed-borne M. pinodes (Gorfu & Sangchote, Citation2003), this fungicide at 10 μg mL-1 did not have an adverse effect on the colony appearance and pycnidial formation of this pathogen. The herbicides (paraquat and atrazine) did not inhibit the growth and sporulation of M. pinodes, but paraquat could not prevent the growth of Phomopsis spp., Cercospora spp. and Colletotrichum spp. (Cerkauskas & Sinclair, Citation1980; Cowling et al., Citation1984; Bigga, Citation1995). Paraquat was therefore not included in the development of the SC-semiselective medium.
Roger & Tivoli (Citation1996) demonstrated that M. pinodes can develop on all parts of the pea plant and is spread by wind-borne ascospores produced on senescent pea leaves. The levels of seed infestation by M. pinodes varied year to year and between seedlots, depending on the amount of rainfall between flowering and maturity (Bretag et al., Citation1995). Our results with naturally infected seeds and plant debris of pea plants showed that typical M. pinodes colonies could be easily detected by plating seeds directly on SC-semiselective medium (). Meanwhile, the SC-semiselective medium facilitated the differentiation of M. pinodes from Colletotrichum pisi, another important seed-borne pathogen of pea, as the latter had a slow growth rate on this medium and produced a black-brown colony (data not shown). The antibiotics, streptomycin sulfate and neomycin sulfate, in the SC-semiselective medium effectively inhibited the growth of most bacteria. Since bacteria in the soil or on plant tissues interfere with isolation of M. pinodes, addition of these two antibiotics in the SC-semiselective medium would alleviate the problem of bacterial contamination and thereby improve efficiency of isolation of M. pinodes from soil or pea tissues. Therefore, the SC-semiselective medium should have potential for application in research on the biology, ecology and control of M. pinodes in peas.
Acknowledgements
This research was funded by Grant No. NSC95-2313-B-005-015 from the National Science and Technology Program for Agricultural Biotechnology, National Science Council, Taiwan. Dr H. C. Huang is Chair Professor of the Department of Plant Pathology, National Chung-Hsing University, Taichung, Taiwan.
References
- Agarwal , V.K. and Sinclair , J.B. 1987 . Principle of seed pathology , Boca Raton, FL : CRC Press .
- Ali , S.M. , Paterson , J. and Crosby , J. 1982 . A standard technique for detecting seed-borne pathogens in peas, chemical control, and testing commercial seed in South Australia . Aust. J. Exp. Agri. Anim. Husb. , 22 : 348 – 352 .
- Beasse , C. , Ney , B. and Tivoli , B. 1999 . Effects of pod infection by Mycosphaerella pinodes on yield components of pea (Pisum sativum) . Ann. Appl. Biol. , 135 : 359 – 367 .
- Bigga , A.R. 1995 . Detection of latent infection in apple fruit with paraquat . Plant Dis. , 79 : 1062 – 1067 .
- Bretag , T.W. , Keane , P.J. and Price , T.V. 2006 . The epidemiology and control of ascochyta blight in field peas: a review . Aust. J. Agri. Res. , 57 : 883 – 902 .
- Bretag , T.W. , Price , T.V. and Keane , P.J. 1995 . Importance of seed-borne inoculum in the etiology of the Ascochyta blight complex of field peas (Pisum sativum L.) grown in Victoria . Aust. J. Exp. Agri. , 35 : 525 – 530 .
- Bretag , T.W. and Ramsey , M. 2001 . “ Foliar diseases caused by fungi: Ascochyta spp ” . In Compendium of pea diseases , Edited by: Kraft , J.M. and Pfleger , P.M. 24 – 28 . St. Paul, MN : American Phytopathological Society .
- Cerkauskas , R.F. and Sinclair , J.B. 1980 . Use of paraquat to aid detection of fungi in soybean tissues . Phytopathology , 70 : 1036 – 1038 .
- Chen , M.H. and Huang , J.W. 1994 . Factors affecting seed transmission of leaf blight pathogen of garden peas, Mycosphaerella pinodes . Plant Prot. Bull. , 36 : 189 – 200 .
- Chen , M.H. , Huang , J.W. and Yein , C.F. 1994 . The pathway and influence factors in pod and seed infections of garden peas by Mycosphaerella pinodes . Plant Pathol. Bull. , 3 : 133 – 139 .
- Cowling , W.A. , Wood , P.M. and Brown , A.G.P. 1984 . Use of a paraquat-diquat herbicide for the detection of Phomopsis leptostromiformis infection in lupins . Aust. Plant Pathol. , 13 : 45 – 46 .
- Dickinson , C.H. and Sheridan , J.J. 1986 . Studies on the survival of Mycosphaerella pinodes and Ascochyta pisi . Ann. Appl. Biol. , 62 : 473 – 483 .
- Faris-Mokaiesh , S. , Corbiere , R. , Lyons , N.F. and Spire , D. 1995 . Evaluation of an enzyme-linked immunosorbent assay for detection of Mycosphaerella pinodes in pea seeds . Ann. Appl. Biol. , 127 : 441 – 455 .
- Gorfu , D. and Sangchote , S. 2003 . Effects of seed treatment fungicides on Ascochyta pinodes of field pea under controlled and field conditions . Kasetsart J. Nat. Sci. , 37 : 429 – 444 .
- Hagedorn , D.J. 1989 . Compendium of pea diseases , St. Paul, MN : American Phytopathological Society Press .
- Jones , L.K. 1927 . Studies on the nature and control of blight, leaf and foot rot of peas caused by species of Ascochyta . New York Agr. Exp. Bull. , 574 : 1 – 46 . (Rev. Appl. Mycol., 7, 611–612, 1928)
- Onfroy , C. , Tivoli , B. , Corbiére , R. and Bouznad , Z. 1999 . Cultural, molecular and pathogenic variability of Mycosphaerella pinodes and Phoma medicaginis var. pinodella isolates from dried pea (Pisum sativum) in France . Plant Pathol. , 48 : 218 – 229 .
- Punithalingam , E. and Hallidey , P. 1972 . Mycosphaerella pinodes . CMI Descriptions of Pathogenic Fungi and Bacteria No. , : 340
- Roger , C. and Tivoli , B. 1996 . Spatio-temporal development of pycnidia and perithecia and dissemination of spores of M. pinodes on pea . Plant Pathol. , 45 : 518 – 528 .
- Sheridan , J.J. 1973 . The survival of Mycosphaerella pinodes on pea haulm buried in soil . Ann. Appl. Biol. , 75 : 195 – 203 .
- Tinivella , F. , Hirata , L.M. , Celan , M.A. , Wright , S.A.I. , Amein , T. , Schmitt , A. , Koch , E. , van der Wolf , J.M. , Groot , S.C. , Stephan , D. , Garibald , A. and Gullino , M.L. 2009 . Control of seed-borne pathogens on legumes by microbial and other alternative seed treatments . Eur. J. Plant Pathol. , 123 : 139 – 151 .
- Tivoli , B. , Beasse , C. , Lemarchand , E. and Masson , E. 1996 . Effect of ascochyta blight (Mycosphaerella pinodes) on yield components of single pea (Pisum sativum) plants under field conditions . Ann. Appl. Biol. , 129 : 207 – 216 .
- Tsao , P.H. 1970 . Selective media for isolation of pathogenic fungi . Annu. Rev. Phytopathol. , 8 : 157 – 186 .
- Wallen , V.R. 1974 . Influence of three Ascochyta diseases of peas on plant development and yield . Can. Plant Dis. Surv. , 54 : 86 – 90 .
- Wallen , V.R. , Wong , S.I. and Jeun , J. 1967 . Isolation, incidence and virulence of Ascochyta spp. of peas from the soil . Can. J. Bot. , 45 : 2243 – 2247 .