Abstract
The specificity of ascospore germination of Monosporascus cannonballus in the rhizosphere of 26 species/cultivars of plants belonging to eight families and 14 genera was examined. Results showed that ascospore germination occurred only in the rhizosphere of genera, species and cultivars belonging to the Cucurbitaceae family. Differences between the number of ascospore germlings attached to roots of the various cucurbit species/cultivars suggests qualitative and/or quantitative differences in a specific root exudate(s) which are presumably associated with the induction of ascospore germination.
Résumé
La spécificité de germination des ascospores de Monosporascus cannonballus dans la rhizosphère de 26 espèces ou cultivars de plantes appartenant à huit familles et 14 genres a été étudiée. Les résultats ont montré que la germination des ascospores s'est produite seulement dans la rhizosphère de genres, d'espèces et de cultivars de la famille des Cucurbitacées. Les différences quant au nombre de germes d'ascospores fixés aux différentes espèces ou cultivars de cucurbitacées suggèrent des différences qualitatives ou quantitatives chez des exsudats racinaires spécifiques qui sont apparemment associés au déclenchement de la germination des ascospores.
Introduction
Monosporascus cannonballus Pollack & Uecker, a root-infecting ascomycete, has been identified as the causal agent of a destructive disease of cucurbits (Martyn & Miller, Citation1996; Cohen et al., Citation2000). Ascospores, the only spore stage produced by this fungus, are considered to function as both the primary survival structure and inoculum for infection of roots in field soil (Merteley et al., Citation1993b ; Stanghellini et al., Citation1996, Citation2000; Beltrán et al., Citation2005, Citation2007). However, ascospores of M. cannonballus, unlike survival structures of most soil-borne fungal and fungal-like (i.e. oomycete) pathogens, do not germinate in axenic culture (Pollack & Uecker, Citation1974; Uecker & Pollack, Citation1975). However, they germinate readily in the rhizosphere of cantaloupe seedlings (Cucumis melo L.) growing in field soil (Stanghellini et al., Citation1996). Furthermore, our preliminary studies indicated that ascospore germination did not occur in the rhizosphere of several noncucurbit plant species. Germination of survival structures of most soil-borne fungal and fungal-like pathogens, with a single notable exception, i.e. the Sclerotium cepivorum–Allium interaction (Coley-Smith et al., Citation1968; King & Coley-Smith, Citation1968), is nonspecific and readily induced by one or more components of root exudates from host as well as nonhost plant species (Schroth & Hildebrand, Citation1964; Huisman, Citation1982).
The objective of this investigation was to document and provide quantitative data on the specificity of ascospore germination of M. cannonballus in the rhizosphere of cucurbit and noncucurbit plant species.
Materials and methods
Cucurbit and noncucurbit plant species
Twenty-six species or cultivars of plants belonging to eight families and 14 genera (many representing crops commonly grown in rotation with susceptible cucurbits) were evaluated for their ability to induce ascospore germination in field soil ().
Table 1. Germination and attachment of ascospores of Monosporascu cannonballu s to roots of diverse plant species
Ascospore germination assessment
A previously described method for assessing ascospore germination was used (Stanghellini et al., Citation1996). Two-day-old seedlings of the plants listed in were transplanted into a field soil (Meloland sandy loam) that had been infested artificially with culturally produced ascospores. Ascospore population density after soil infestation was estimated at c. 2400 ascospores g−1 soil. The infested soil (7 g) was placed in a polyethylene centrifuge tube (8 cm long and 1.7 cm diam.) with drainage holes drilled through the bottom of each tube, and one seedling added. Radical length of the seedlings ranged from 2 to 4 mm at transplant. The tubes were then placed in a growth chamber at 30 °C with a 12h photoperiod. The plants were irrigated every other day. After 10 days of incubation, seedlings were removed from the tubes and placed in Petri dishes containing sterile distilled water and observed with a dissecting microscope. The total length of each root system and the number of ascospore germlings attached to the root system of most plants were recorded ().
All treatments were replicated three times and repeated once. Data on the number of ascospore germlings attached to plant roots were log10 transformed and analyzed by the Kruskal–Wallis one way analysis of variance on rank, followed by the all-pairwise multiple comparison procedures (Student–Newman–Keuls method).
Results
Ascospore germination assessment
Ascospore germination, as assessed by observation of ascospore germlings attached to plant roots () occurred only in the rhizosphere of genera, species and cultivars belonging to the cucurbit family. Significant differences (P < 0.01) in the number of ascospore germlings attached to roots were found among the various cucurbits assayed (). Highest numbers of ascospore germlings were recorded on cantaloupe roots. However, not all members of the cucurbit family supported ascospore germination. No germination occurred in the rhizosphere of Cucurbita maxima ‘Brava’ or ‘Sakata #4’. In a separate experiment, a ‘Brava’ seedling (C. maxima) and a ‘Caravelle’ seedling (C. melo) were planted side-by-side in the same soil assay tube. After 10 days, the roots of both plants, which were intertwined, were removed and assessed for ascospore germination and attachment to roots. Ascospore germlings were attached only to roots of ‘Caravelle’.
Fig. 1. Ascospore germling(s) of Monosporascus cannonballus attached to a root of a susceptible melon cultivar (C. melo ‘Caravelle’). Ascospore diameters = 40–45 μm.
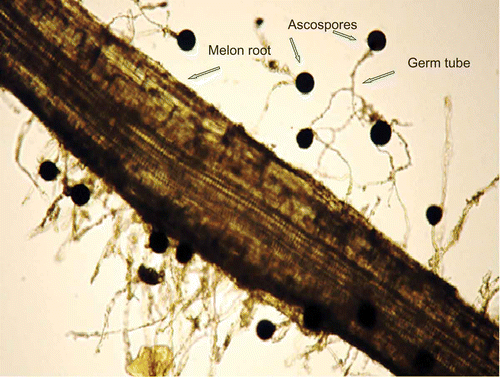
All plants had a radicle length of 2–4 mm at transplant and the root system of all plants increased at least 10-fold over the next 10 days ().
Discussion
Our study quantitatively documents that ascospore germination, and subsequent germling attachment to roots, occurs only in the rhizosphere of certain genera and species of plants belonging exclusively to the family Cucurbitaceae (). Specifically, ascospore germination occurred in all cucurbit genera and species assayed except for Cucurbita maxima. No ascospore germination occurred in the rhizosphere of any of the other diverse plant genera and species (including crops commonly used in rotation with melons) belonging to several other plant families. Additionally, it is apparent, based upon the number of ascospore germlings attached to melon roots, that differences in the quality and/or quantity of a specific exudate(s) exists among various cucurbit species. For example, the numbers of germlings attached to cantaloupe roots (C. melo) were minimally three-fold higher than on root systems of all other cucurbits. However, it would be presumptive to assume that low numbers of ascospore germlings attached to the roots of a specific cucurbit equate to some degree of field tolerance. For example, watermelons are very susceptible but their roots support only modest numbers of ascospore germlings.
The results of our investigation may have several practical applications. First, the specific but currently unidentified chemical(s) in cucurbit root exudate that induce ascospore germination could be used to stimulate inopportune ascospore germination during fallow periods. Germination in the absence of a host would result in death of the fungus and a concomitant decrease in the soil inoculum density and in subsequent disease severity. The latter strategy is currently employed for disease management of white rot of onion and garlic caused by Sclerotium cepivorum (Merriman et al., Citation1980; Hovius & McDonald, Citation2002). Second, the seedling assay could be employed as a rapid screening method to identify cucurbits that do not support ascospore germination. Lack of germination would preclude susceptibility. As previously mentioned, no ascospores germinated in the rhizosphere of C. maxima and cultivars of this cucurbit are currently employed as M. cannonballus-resistant rootstocks for grafting of susceptible melons scions (Edelstein et al., Citation1999; Cohen et al., Citation2000, Citation2005; Beltrán et al., Citation2008). Lack of ascospore germination, as documented in this study, provides direct evidence in support of the conclusion of Beltrán et al. (Citation2008) that resistance of squash rootstock resides in the ‘lack of recognition between the host and the pathogen’. Further, our experiments involving co-cultivation of ‘Brava’ and ‘Caravelle’ in the same soil assay tube demonstrated that exudates from ‘Brava’ did not inhibit ascospore germination and that ascospores stimulated to germinate by ‘Caravelle’ roots did not attach to ‘Brava’ roots. Third, the absence of ascospore germination in the rhizosphere of noncucurbit plants suggests that these plants would not be able to serve as hosts. However, several noncucurbit plants have been implicated as possible hosts (Merteley et al., Citation1993a ). Such data were derived from greenhouse pathogenicity studies involving ascospores and mycelial inoculum operating from a food base in steam-pasteurized potting medium. Since none of these noncucurbit plants have been documented to serve as susceptible hosts under field conditions over the past 16 years, it can be assumed that the latter method is not appropriate for assessment of the probable host range of M. cannonballus.
References
- Beltrán , R.A. , Vicent , A. , Sales , R. Jr. , García-Jiménez , J. and Armengol , J. 2005 . Population dynamics of Monosporascus cannonballus ascospores in marsh soils in eastern Spain . Eur. J. Plant Pathol. , 113 : 357 – 365 .
- Beltrán , R. , Vicent , A. , García-Jiménez , J. and Armengol , J. 2007 . Quantification of Monosporascus cannonballus ascospores in muskmelon fields in eastern Spain . J. Phytopathol. , 155 : 248 – 250 .
- Beltrán , R. , Vicent , A. , García-Jiménez , J. and Armengol , J. 2008 . Comparative epidemiology of Monosporascus root rot and vine decline in muskmelon, watermelon, and grafted watermelon crops . Plant Dis. , 92 : 158 – 163 .
- Cohen , R. , Burger , Y. , Horev , C. , Porat , A. and Edelstein , M. 2005 . Performance of Galia-type melons grafted on to Cucurbita rootstock in Monosporascus cannonballus-infested and non-infested soils . Ann. Appl. Biol. , 146 : 381 – 387 .
- Cohen , R. , Pivonia , S. , Burger , Y. , Edelstein , M. , Gamliel , A. and Katan , J. 2000 . Toward integrated management of Monosporascus wilt of melons in Israel . Plant Dis. , 84 : 496 – 505 .
- Coley-Smith , J.R. , Dickinson , D.J. , King , J.E. and Holt , R.W. 1968 . The effect of species of Allium on soil bacteria in relation to germination of sclerotia of Sclerotium cepivorum . Ann. Appl. Biol. , 62 : 103 – 111 .
- Edelstein , M. , Cohen , R. , Burger , Y. , Shriber , S. , Pivonia , S. and Shtienberg , D. 1999 . Integrated management of sudden wilt in melons, caused by Monosporascus cannonballus, using grafting and reduced rates of methyl bromide . Plant Dis. , 83 : 1142 – 1145 .
- Hovius , M.H.Y. and McDonald , M.R. 2002 . Management of Allium white rot (Sclerotium cepivorum) in onions on organic soil with soil-applied diallyl disulfide and di-N-propyl disulfide . Can. J. Plant Pathol. , 24 : 281 – 286 .
- Huisman , O.C. 1982 . Interrelations of root growth dynamics to epidemiology of root-infecting fungi . Annu. Rev. Phytopathol. , 20 : 303 – 327 .
- King , J.E. and Coley-Smith , J.R. 1968 . Effects of volatile products of Allium species and their extracts on germination of sclerotia of Sclerotium cepivorum Berk . Ann. Appl. Biol. , 61 : 407 – 414 .
- Martyn , R.D. and Miller , M.E. 1996 . Monosporascus root rot and vine decline, an emerging disease of melons worldwide . Plant Dis. , 80 : 716 – 725 .
- Merriman , P.R. , Isaacs , S. , MacGregor , R.R. and Towers , G.B. 1980 . Control of white rot in dry bulb onions with artificial onion oil . Ann. Appl. Biol. , 96 : 163 – 168 .
- Merteley , J.C. , Martyn , R.D. , Miller , M.E. and Bruton , B.D. 1993a . An expanded host range for the muskmelon pathogen Monosporascus cannonballus . Plant Dis. , 77 : 667 – 673 .
- Merteley , J.C. , Martyn , R.D. , Miller , M.E. and Bruton , B.D. 1993b . Quantification of Monosporascus cannonballus ascospores in three commercial muskmelon fields in South Texas . Plant Dis. , 77 : 766 – 771 .
- Pollack , F.G. and Uecker , F.A. 1974 . Monosporascus cannonballus, an unusual ascomycete in cantaloupe roots . Mycologia , 66 : 346 – 349 .
- Schroth , M.N. and Hildebrand , D.C. 1964 . Influence of plant exudates on root-infecting fungi . Annu. Rev. Phytopathol. , 2 : 101 – 132 .
- Stanghellini , M.E. , Kim , D.H. and Rasmussen , S.L. 1996 . Ascospores of Monosporascus cannonballus: germination and distribution in cultivated and desert soils in Arizona . Phytopathology , 86 : 509 – 514 .
- Stanghellini , M.E. , Kim , D.H. and Waugh , M. 2000 . Microbe-mediated germination of ascospores of Monosporascus cannonballus . Phytopathology , 90 : 243 – 247 .
- Uecker , F.A. and Pollack , F.G. 1975 . Development and cytology of . Monosporascus cannonballus. Bot. Gaz , 136 : 333 – 340 .