Abstract
Stripe rust, caused by Puccinia striiformis f. sp. tritici (PST), has historically been the most frequently destructive disease of wheat (Triticum aestivum) in the western United States and has become a more frequent problem in the central and southeastern states since 2000. The race composition of PST has been determined every year from rust-infected leaf samples of wheat and grasses collected in the United States on a set of 20 differential wheat genotypes. In 2006, a total of 18 races were detected, of which five were detected for the first time. In 2007, a total of 30 races were detected, of which 11 were newly detected. Among the 16 new races detected in 2006 and 2007, PST-127 was the most important as it has the broadest virulence spectrum identified so far (virulent to all 20 differential genotypes except for ‘Moro’, AVS/6*Yr5 (Yr5), and ‘Tres’) and combined virulence factors to ‘Tyee’ (YrTye) and ‘Hyak’ (Yr17 and YrTye) and those common in the race group detected since 2000. The distribution, frequency changes, and evolutionary relationships for races detected from 2000 to 2007 were analyzed. Three major waves of race changes were identified during the eight-year period. From 2000 to 2002, the predominant races were PST-78 and PST-80, which were virulent on wheat genotypes ‘Lemhi’, ‘Heines VII’, ‘Lee’, ‘Fielder’, ‘Express’, AVS/6*Yr8, AVS/6*Yr9, ‘Clement’ and ‘Compair’. Race PST-80 is also virulent on ‘Produra’. From 2003 to 2006, the predominant race was PST-100, with the same virulence formula as PST-80 plus virulence on ‘Yamhill’ and ‘Stephens’. Starting in 2006, races with the same virulence formula of PST-100 plus virulence to Yr1 became predominant in California and races with the virulence of PST-100 plus virulence on Yr10 became predominant in the Pacific Northwest. During 2000 to 2007, races with more virulence factors became more predominant in the United States, indicating that races with increased virulence factors are at an advantage in the pathogen population over those with fewer virulence factors because they are able to infect more wheat cultivars.
Résumé:
La rouille jaune du blé, causée par Puccinia striiformis, f. sp. tritici (PST), a été, historiquement et le plus souvent, la maladie la plus destructrice du blé (Triticum aestivum) dans l'ouest des États-Unis, et est devenue de plus en plus problématique dans les États du centre et du sud-ouest depuis l'année 2000. La composition des races de PST a été établie chaque année à partir d'échantillons de feuilles de blé et de graminées infectées par la rouille, collectés aux États-Unis sur une série de 20 génotypes différentiels de blé. En 2006, 18 races ont été détectées en tout, dont cinq l'étaient pour la première fois. En 2007, 30 races ont été détectées en tout, dont 11 l'étaient pour la première fois. Parmi les 16 nouvelles races détectées en 2006 et en 2007, PST-127 était la plus importante, car elle affiche à ce jour le plus large spectre de virulence [virulent à l'égard des 20 génotypes différentiels, sauf ‘Moro’, AVS/6*Yr5 (Yr5) et ‘Tres’] et des facteurs combinés de virulence à l’égard de ‘Tyee’ (YrTye) et ‘Hyak’ (Yr17 et YrTye), en plus de ceux communs au groupe de race détecté depuis 2000. La distribution, la fréquence des changements et les relations évolutives relatives aux races détectées de 2000 à 2007 ont été analysées. Trois vagues de changements ont été décelées durant cette période de huit ans. De 2000 à 2002, les races prédominantes étaient PST-78 et PST-80 qui étaient virulentes à l’égard des génotypes de blé ‘Lemhi’, ‘Heines VII’, ‘Lee’, ‘Fielder’, ‘Express’, AVS/6*Yr8, AVS/6*Yr9, ‘Clement’ et ‘Compair’. La race PST-80 est également virulente à l’égard de ‘Produra’. De 2003 à 2006, la race prédominante était PST-100, qui affichait la même formule de virulence que PST-80, en plus d'être virulente à l’égard de ‘Yamhill’ et ‘Stephens’. À partir de 2006, les races affichant la même formule de virulence que PST-100, en plus de la virulence à l'égard de Yr1, ont prédominé en Californie et celles affichant la virulence de PST-100, en plus de la virulence à l'égard de Yr10, ont prédominé dans les États du nord-ouest du Pacifique. De 2000 à 2007, les races possédant davantage de facteurs de virulence ont de plus en plus prédominé aux États-Unis, ce qui démontre que les races qui possèdent plus de facteurs de virulence ont un avantage parmi les populations qui en possèdent moins parce qu'elles peuvent infecter un plus grand nombre de cultivars de blé.
Introduction
Stripe rust of wheat (Triticum aestivum L.), caused by Puccinia striiformis Westend. f. sp. tritici Eriks. (PST), was first recognized in the United States in 1915 (Carleton, Citation1915) and has caused frequent epidemics in the western United States (mainly Washington, Idaho, Oregon and California) since the late 1950s (Line & Qayoum, Citation1992). In the regions east of the Rocky Mountains, stripe rust occurred in the southern and central Great Plains in the late 1950s (Pady et al., Citation1957) and has increased in frequency since the early 1980s (Line & Qayoum, Citation1992), but it seldom caused major damage until 2000 (Chen et al., Citation2002). The disease is now important throughout most of the United States (Chen, Citation2005, Citation2007). As the disease also occurs in more than 60 countries and has caused devastating damage in Central, East and West Asia, Europe, East and South Africa, North America and South America, it is considered to be one of the most important diseases of wheat in the world (Stubbs, Citation1985; Chen, Citation2005).
Stripe rust can cause 100% yield loss, but damage most often ranges from 10–70% in a single field depending upon the duration and severity of infection during the growing season and susceptibility of the cultivar (Chen, Citation2005). In the United States, yield losses caused by stripe rust have been recorded since 1958 (http://www.cdl.umn.edu/loss.html). Before 2000, most of the major stripe rust damage occurred in the Pacific Northwest and California (Line, Citation2002; Chen, Citation2005). Since 2000, wheat stripe rust has become more prevalent throughout the United States (Chen et al., Citation2002; Chen Citation2005, Citation2007). The greatest distribution of stripe rust was in 2005 (Chen & Penman, Citation2006). From 2000 to 2007, stripe rust occurred in at least 15 states each year (Chen et al., Citation2003, Citation2004, Citation2007, Citation2008; Chen, Citation2005, Citation2007; Chen & Penman, Citation2005, Citation2006). Because of the wide distribution of the disease, yield losses and the cost of using fungicides to reduce stripe rust damage nationwide were substantial almost every year. The nationwide yield losses were estimated as 247, 026 t (9, 068, 500 bu) in 2000; 1, 080, 150 t, (39, 653,100 bu) in 2001; 217, 800 t (7, 995, 600 bu) in 2002; 2 ,421,121 t (88, 881,100 bu) in 2003; and 333,132 t (12 ,229, 500 bu) in 2004; 1, 996, 090 t (73, 277, 900 bu) in 2005; 119 ,396 t (4 ,383,100 bu) in 2006; and 297, 611 t (10 ,925 ,500 bu) in 2007 (Chen, Citation2005, Citation2007; http://www.cdl.umn.edu/loss/loss.html). Without the widespread use of fungicides, the yield losses would have been much higher than the above estimates.
Stripe rust epidemics are often caused by new races of the pathogen that are able to render previously resistant cultivars susceptible (Stubbs, Citation1985; Wellings & McIntosh, Citation1990; Line & Qayoum, Citation1992; Wan et al., Citation2004; Chen, Citation2005). Differences in virulence spectrum of PST was first recognized in the 1920s (Humphrey et al., Citation1924) and race determination using a set of wheat differential genotypes on a yearly basis were initiated in the late 1960s (Line et al., Citation1970; Line & Qayoum, Citation1992). Line & Qayoum (Citation1992) summarized races that were identified from 1967 to 1987. Line and his associates reported on the races that were identified from 1988 to 1999 (Line & Chen, Citation1996; Line, Citation2002). Chen et al. (Citation2002) described a group of races with virulences to Yr8 and Yr9, which were new to the United States and caused a widespread epidemic of stripe rust in 2000. Since then, races of the stripe rust pathogen in each year were reported to breeders and pathologists working on cereal crops, and summarized in abstracts presented at annual meetings of the American Phytopathological Society (Chen et al., Citation2003, Citation2004, Citation2007, Citation2008; Chen & Penman, Citation2005, Citation2006). The virulence formulae of PST races detected from 2000 to 2005 were summarized (Chen, Citation2005, Citation2007) and frequencies and distributions of races detected from 2000 to 2003 were previously reported (Chen, Citation2005). Here, we present information on the PST races and their distribution and frequencies detected in 2006 and 2007, compare distribution and frequencies of the races with those detected from 2000 to 2005, and discuss evolutionary changes and impacts of the pathogen population from 2000 to 2007 in the United States.
Materials and methods
Collection of stripe rust samples
Stripe rust monitoring was conducted by the authors and collaborators during the crop season every year throughout the wheat growing areas in the United States. Leaf samples of wheat, triticale (× Triticosecale Wittmack) and grasses (Aegilops spp., Hordeum spp. and Elymus spp.) infected by PST were collected from commercial fields, disease monitoring plots, and breeding nurseries throughout the United States. Effort was taken to collect samples from wheat cultivars or breeding lines that were previously resistant to stripe rust. In general, infected leaves for one sample were put into a glassine envelope or a regular paper envelope and sent to our lab for race identification. The location, growth stage, cultivar or genotype, severity and prevalence, and other related information were recorded whenever possible. Samples were kept at 4 °C for as short a period as possible before they were used to increase urediniospores.
The wheat cultivar ‘Nugaines’, which has been shown to be susceptible to all PST races in the seedling stage and has a seedling growth habit conducive for increasing rust spores, was used to increase urediniospores from each sample. Seedlings of ‘Nugaines’ at the two-leaf stage were inoculated with urediniospores from each sample using various techniques depending upon sample quality. Samples with a reasonably good number of sporulating uredia were directly inoculated on ‘Nugaines’ leaves. For samples with a limited number of uredia, the leaves were cut into pieces and placed on wet filter paper in a Petri dish. After treatment at 4 °C overnight or maximally 24 h, sample leaves with orange-coloured dews were used to inoculate seedlings of ‘Nugaines’. The inoculated plants were kept in a dew chamber in the dark at 10 °C for 16 to 24 h and then transferred to a growth chamber with a diurnal temperature cycle that gradually changed from 4 °C at 2:00 am to 20 °C at 2:00 pm with 16 h photoperiod as described by Chen et al. (Citation2002). Urediniospores were collected when sporulation was obvious on inoculated ‘Nugaines’ leaves, generally at 16 days after inoculation. The rust increase cycle was repeated until sufficient urediniospores (about 20 mg) were obtained for testing on differentials and for storage. Urediniospores contained in a glass vial without a cap were dried in a desiccator at 4 °C for 3 to 10 days, and then sealed in a small glass vial or foil bag and kept in liquid nitrogen for long-term storage. Fresh urediniospores or dried urediniospores kept at 4 °C for less than two months were used for virulence testing. If urediniospores stored in liquid nitrogen needed to be used, the glass vial or foil bag containing the spores was treated in water at 50–55 °C for 2 min to activate the spores before inoculation.
Differential testing
A set of 20 wheat genotypes () were used to differentiate PST races (Chen, Citation2005, Citation2007). Previously described experimental procedures and conditions (Line & Qayoum, Citation1992; Chen et al., Citation2002) were followed for identifying the races. To facilitate efficient testing, five differential genotypes were spot planted in a 7 × 7 cm plastic pot filled with a potting mixture of 24 L peat moss, 8 L perlite, 12 L sand, 12 L commercial potting soil, 16 L vermiculite and 250 g 14–14–14 Osmacote. About five to seven seeds of each differential genotype were planted. The seedlings were grown in a rust-free greenhouse and inoculated at the two-leaf stage with urediniospores mixed with talcum (Sigma, St. Louis, MO) at a ratio of about 1:20. The inoculated plants were incubated in a dew chamber at 10 °C in dark for 18 to 24 h and then grown in a growth chamber under the environmental conditions previously described (Chen & Line, 1992b). Infection types on each genotype were recorded based on a 0–9 scale (Line & Qayoum, Citation1992) at 18 to 22 days after inoculation. Infection types were converted to avirulence/virulence data. An isolate was considered avirulent on a specific differential genotype when there were no symptoms (IT 0) or there were necrotic or chlorotic flecks (IT 1), necrotic or chlorotic blotches without sporulation (IT 2), or necrotic or chlorotic blotches with only a trace of to slight sporulation (IT 3–4). An isolate was considered to be virulent if it caused moderate to abundant sporulation, with or without chlorosis or necrosis (IT 5, 6, 7, 8 or 9), as previously described (Line & Qayoum, Citation1992; Chen, Citation2005).
Table 1. Wheat genotypes used to differentiate races of Puccinia striiformis f. sp. tritici in the United States
Obtaining sub-isolates and single-pustule isolates
If an original isolate (urediniospores increased on ‘Nugaines’ from the field sample) showed a virulent/avirulent pattern different from previously identified races, key differential genotypes that displayed a unique combination of susceptible reaction were selected and inoculated to obtain sub-isolates. Sub-isolates were tested on the 20 differential cultivars to determine if the original isolate consisted of a mixture of two or more previously identified races or a new race. The isolation process was often repeated several times to obtain pure isolates for race designation. For isolates or sub-isolates that appeared to be new races, single-pustule isolates were also obtained and tested on the set of differential genotypes to confirm their new virulence patterns.
Data analyses
Frequencies were calculated for each race and each virulence factor (i.e. virulence on a single differential genotype rather than to a single Yr gene) on a yearly basis. Race dynamics were determined by comparing frequencies from 2000 to 2007 using the data previously published for 2000 to 2003 (Chen, Citation2005). The relationships based on virulence/avirulence formulae of the 20 wheat differential genotypes of all the races detected from 2000 to 2007 were determined using the NTSYSpc software (version 2.02a, Applied Biostatistics Inc., New York, NY). The virulence or avirulence on each of the 20 wheat differential genotypes was coded as 1 and 0, respectively. A similarity matrix based on simple matching was generated by the SIMQUAL (similarity for qualitative data) program, and cluster analysis was done using the unweighted pair group arithmetic mean (UPGMA) method in the SAHN (sequential, agglomerative, hierarchical, and nested) program of the software (Rohlf, Citation1992). The dendrogram with the best fit to a similarity matrix based on the cophenetic (COPH) values using a matrix comparison (MXCOP) program of NTSYS-pc was chosen. Groups and subgroups were determined using arbitrary points of similarity coefficients. Common virulence and avirulence factors were identified for each group and subgroup.
Results and discussion
Stripe rust distributions and severities
The distribution and relative severity of stripe rust in 2000 to 2007 are shown in . Compared with 2000 to 2005, stripe rust severities were in general relatively low throughout most of the United States wheat growing regions in both 2006 and 2007, although it was reported in more than 20 states in 2006 and in 17 states in 2007 (Chen et al., Citation2007, Citation2008).
Fig. 1 Distributions and its relative severity of wheat stripe rust, caused by Puccinia striiformis f. sp. tritici, in the United States from 2000 to 2007.
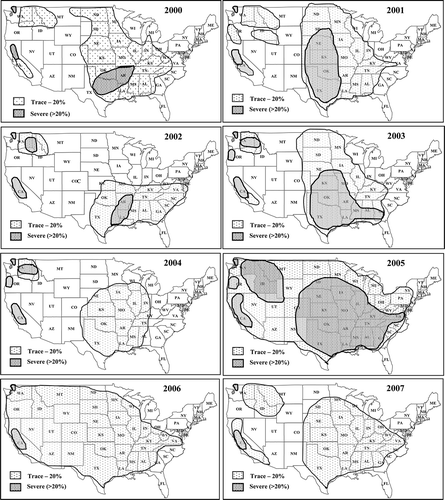
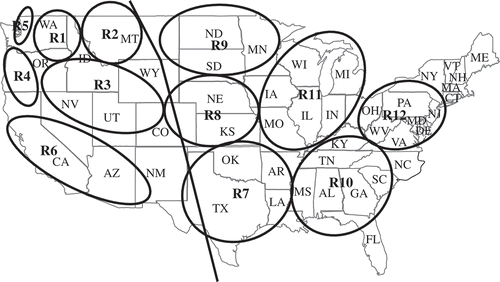
Because stripe rust was historically a major problem in the western United States and seldom caused major damage in the regions east of the Rocky Mountains, the western United States was separated into six epidemiological regions, while a huge area in the east was considered as a single region 7 (Line & Qayoum, Citation1992; Line, Citation2002). However, the distribution and severity of stripe rust epidemics from 2000 to 2007 revealed significant differences in disease patterns in areas east of the Rocky Mountains. Therefore, the area previously designated region 7 was separated into six regions (). The separation of these regions was also based on wheat crop systems, climatic conditions including wind directions, and source or reception of the pathogen inoculum. Region 7 covers Texas, Louisiana, Arkansas, Oklahoma, and eastern New Mexico. Region 8 includes Kansas, Nebraska and eastern Colorado. Region 9 covers South Dakota, North Dakota, Minnesota, eastern Montana, and southern Manitoba and southern Saskatchewan, Canada. Region 10 covers Mississippi, Alabama, Florida, Georgia, North Carolina, Tennessee and Kentucky. Region 11 includes Missouri, Illinois, Indiana, Iowa, Wisconsin, Michigan and southern Ontario, Canada. Region 12 covers Virginia, West Virginia, Ohio, Delaware, Maryland, Pennsylvania and New York. No stripe rust has been reported in the far northeastern states from Connecticut to Maine. If the disease is a concern there, these states could be included in region 12. In general, region 7 provides inoculum to regions 8–12. However, stripe rust epidemic patterns are often different as shown in because of different cropping systems and weather conditions.
Fig. 2 Epidemiological regions of stripe rust of wheat, caused by Puccinia striiformis f. sp. tritici, in the United States and Canada (see Line & Qayoum (Citation1992) for details). The previous region 7 (separated from other regions in the west by the line along the Rocky Mountains) is divided into regions 7 to 12. Region 1 (R1) = eastern Washington, northeastern Oregon, and northern Idaho; R2 = western Montana and southern Alberta, Canada; R3 = southern Idaho, southeastern Oregon, northern Nevada, northern Utah, western Wyoming, and western Colorado; R4 = western Oregon and northern California; R5 = northwestern Washington and southwestern British Columbia, Canada; R6 = central and southern California, Arizona, and western New Mexico; and R7 = Texas, Louisiana, Arkansas, Oklahoma, and eastern New Mexico; R8 = Kansas, Nebraska, and eastern Colorado; R9 = South Dakota, North Dakota, Minnesota, eastern Montana, and southern Manitoba and southern Saskatchewan, Canada; R10 = Mississippi, Alabama, Florida, Georgia, South Dakota, North Dakota, Tennessee, and Kentucky; R11 = Missouri, Illinois, Indiana, Iowa, Wisconsin, Michigan, and Ontario, Canada; and R12 = Virginia, West Virginia, Ohio, Maryland, Pennsylvania and New York.
Number of PST samples and races
summarizes the number of isolates of PST, total number of races and new races obtained from 2000 to 2007 in regions 1 to 12. A total of 2351 samples were collected, from which 1945 viable isolates were characterized over the eight years. The numbers of samples generally indicated how widespread stripe rust was in the United States in these years. For example, stripe rust was the most widely distributed in 2005, and we had the most samples in that year. There were a smaller number of viable isolates in 2006 and 2007 when the prevalence and severity of stripe rust was limited. In 2006, 18 races were detected and in 2007, 30 races were detected, of which 12 were detected in both years. A total of 115 PST races were detected from 2000 to 2007 (). The virulence formulae of the races detected from 2000 to 2005 were reported previously (Chen, Citation2005, Citation2007) and those in 2006 and 2007 are first reported in the present study. These races showed the great diversity and dynamics of virulence structures in the United States PST population.
Table 2. Numbers of viable isolates, races, and first detected races and the most predominant races of Puccinia striiformis f. sp. tritici in the United States from 2000 to 2007
Table 3. Virulences, the first year detected, and frequencies and distribution of races of Puccinia striiformis f. sp. tritici (PST) in the United States from 2000 to 2007
New PST races
New races were detected every year from 2000 to 2007. Five races were first detected in 2006 and 11 were detected in 2007 (). Most of the new races have similar, but different virulence spectra from the group of races that have been detected since 2000, indicating that the rust population has continued evolving. The most important group of new races detected in 2000 were those virulent to resistance genes Yr8 and Yr9 because those genes were effective against all the races identified before 2000 (Chen et al., Citation2002). Examples of these races include PST-77, PST-78, PST-79 and PST-80 (). In 2001, race PST-90 was first detected. This race had the same virulence formula as PST-78, plus virulence to ‘Tres’. In 2002, new races, such as PST-97, PST-98 and PST-99 that were similar to PST-77, PST-78 and PST-80 but with virulence to ‘Stephens’ or ‘Yamhill’, were detected. In 2003, PST-100 with the PST-80 virulence factors plus ‘Yamhill’ and ‘Stephens’ was detected. Since then, this race was the most predominant race. Races with the PST-100 virulence formula plus virulence to ‘Chinese 166’ (PST-101) or ‘Tres’ (PST-102) were also detected in 2003. These races later became predominant in most of the regions and evolved into other important races. In 2004, races with combinations of virulence to ‘Moro’ (PST-114) or ‘Paha’ (PST-115) and those of PST-100 were detected in region 1, but they have been generally restricted to that region. In 2005, PST-116 with virulence to both ‘Moro’ and ‘Paha’ and those of PST-100 was detected. This race has also been restricted to region 1. PST-117 had virulence to ‘Druchamp’ added to the virulence spectrum of PST-102. In 2006, PST-122, which had virulence combinations of PST-117 with virulence to ‘Chinese 166’, was detected in California (region 6). In 2007, five new races were detected having virulence to ‘Tyee’ (YrTye) and ‘Hyak’ (Yr17 and YrTye). Of these races, PST-127 was virulent on all 20 wheat differential genotypes except ‘Moro’, AVS/6*Yr5 and ‘Tres’, the broadest virulence spectrum identified so far. This race and PST-137 (similar to PST-127 but avirulent to ‘Express’ and moderately virulent to ‘Lee’) had the combinations of virulence to ‘Tyee’ and ‘Hyak’ and those to AVS/6*Yr8 and AVS/6*Yr9. Although these races were detected in the western United States and had not spread to the eastern United States in 2008 and 2009 (Wan and Chen, unpublished data), they pose a threat to wheat production because Yr17 has been widely used in breeding programmes.
Some of the other new races had narrower virulence spectra than in previously detected races and those discussed above. In general, these races have contributed less to the epidemics from 2000 to 2007. However, these races could have played important roles in the evolutionary process in the development of the predominant races discussed above.
Frequency changes of PST races and virulences
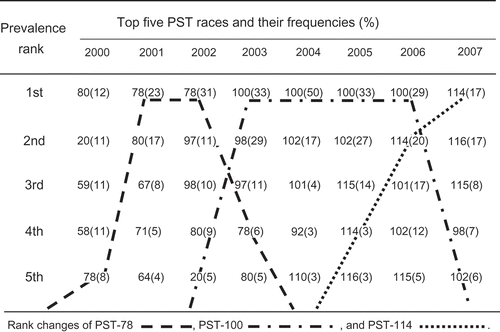
Frequencies of races detected from 2000 to 2007 are listed in . The most predominant race and its frequency for each region in each year are given in . The changes in the top five predominant races in the United States from 2000 to 2007 are shown in . In 2000, race PST-80 was the most predominant race with a frequency of 12% and PST-78 ranked No. 5 with 8%. PST-78 increased to the No. 1 frequent race in 2001 (23%) and 2002 (31%). This race decreased to No. 4 in 2003 (6%). PST-100 replaced PST-78 as No. 1 and was widely distributed in 2003 to 2006. It reached 50% in 2004 and was still common in some regions in 2007. PST-114 was first detected in 2004, increased to No. 4 in 2005 (3%), and No. 2 in 2006 (20%), and ranked No. 1 in frequency in 2007 (17%).
Fig. 3 Top five predominant races of Puccinia striiformis f. sp. tritici and their frequencies in the United States from 2000 to 2007.
As the majority of wheat genotypes that are used to differentiate PST races have two or more resistance genes (), a virulence factor is defined as virulence to a particular differential genotype. The frequencies of the 20 virulence factors corresponding to the 20 differential genotypes are listed in and their changes are shown in . Virulence factor to Yr5 (the number 7 differential) has not been detected, showing that resistance gene Yr5 is still effective against all races identified so far in the United States. The inclusion of the AVS/6*Yr5 line in the differential set was pro-active because the gene has been widely used in wheat breeding programmes in the United States (Chen, Citation2005).
Table 4. Frequencies of virulence factors in Puccinia striiformis f. sp. tritici from 2000 to 2007
Fig. 4 Frequency changes of virulence factors to the 20 wheat genotypes that were used to differentiate races of Puccinia striiformis f. sp. tritici in the United States from 2000 to 2007, showing a, virulence factors (1, 2, 6, and 12) with relatively stable frequencies; b, those (4, 5, 9, 13, 14, and 15) with frequencies changing from 0–10% to 4–80%; c, those (10, 17, 19 and 20) with frequencies changing from 10–30% to 80–100%; and d, those (3, 8, 11, 16, and 18) from 30–65% to 80–100%. The numbers in the figure represent the virulence factors corresponding to wheat differential number listed in ; 1 = ‘Lemhi’, 2 = ‘Chinese 166’, 3 = ‘Heines VII’, 4 = ‘Moro’, 5 = ‘Paha’, 6 = ‘Druchamp’, 7 = AVS/6*Yr5, 8 = ‘Produra’, 9 = ‘Yamhil'l, 10 = ‘Stephens’, 11 = ‘Lee’, 12 = ‘Fielder’, 13 = ‘Tyee’, 14 = ‘Tres’, 15 = ‘Hyak’, 16 = ‘Express’, 17 = AVS/6*Yr8, 18 = AVS/6*Yr9, 19 = ‘Clement’ and 20 = ‘Compair’.
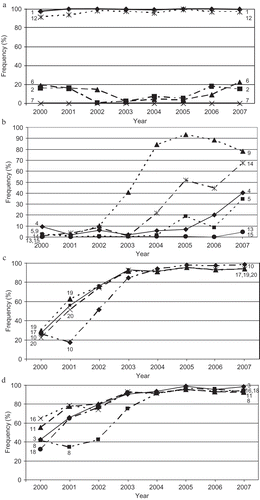
Virulence factors 1, 2, 6 and 12 ( a) remained at relatively stable frequencies over the eight years. Virulence factor 1 (to ‘Lemhi’) had the highest frequencies of 97.5–100% during the period of 2000 to 2007. With ineffective resistance gene Yr21 (Chen et al., Citation1995), ‘Lemhi’ is susceptible to all PST races except for PST-21 (Line & Qayoum, Citation1992; Chen, Citation2005, Citation2007). Similarly, virulence factor 12 (to ‘Fielder’) remained at high frequencies (91.2–99.5%) throughout the period of 2000 to 2007. Thus, virulence factors 1 and 12 commonly exist in past and current PST populations. The frequency of virulence factor 2 (to ‘Chinese 166’ with Yr1) decreased from 15.7% in 2000 to 0.3% in 2002 and then gradually increased to 17.6% in 2006 and 15.0% in 2007. The later increase of virulence factor 2 corresponded to the development of new races in the post-2000 group, such as PST-101, PST-104, PST-112, PST-113 and PST-127. The increase of virulence factor 2 was likely due to the increase of the hard white spring wheat cultivar ‘Blanca Grande’, which has Yr1 (Chen, unpublished data), in California. Similarly, the frequency of virulence factor 6 (to ‘Druchamp’) decreased from 18.9% in 2000 to 2.1% in 2003 and then increased gradually to 22% in 2007 as the virulence factor appeared in some recently identified races of the post-2000 group.
shows virulence factors 4, 5, 9, 13, 14 and 15, whose frequencies changed from 0–10% to 4–80%. Virulence factor 4 (to ‘Moro’ with Yr10 and YrMor) was first detected in 1972 (Line & Qayoum, Citation1992) and existed in predominant races such as PST-28 and PST-29 in the 1980s and PST-43 and PST-44 in the early 1990s in the Pacific Northwest (Line & Chen, Citation1996). This virulence factor decreased in frequency to almost undetectable until the appearance in 2004 of PST-114 that has virulence factor 4 combined with the common virulence factors of the post-2000 race group. The rapid increase of PST-114, together with PST-116, which was first detected in 2005, made virulence factor 4 the most frequent in the Pacific Northwest. A similar trend was observed for virulence factor 5 (to ‘Paha’). Virulence factor 9 (to ‘Yamhill’) increased in frequency from 2.5% in 2000 and 1.6% in 2001 to 78.0–93.5% in 2004 to 2007 as it appeared in many of the recently identified races. Similarly, the frequency of virulence factor 14 (to ‘Tres’) gradually increased from 1.3% to 67.6% in 2007. Virulence factors 13 (to ‘Tyee’ with YrTye) and 15 (to ‘Hyak’ with Yr17 and YrTye) were first detected in 1983 and 1990, respectively, and were in the predominant races in the Pacific Northwest in the 1980s and 1990s (Line & Qayoum, Citation1992; Line & Chen, Citation1996). These virulence factors decreased to undetectable levels until 2007 when races PST-127, PST-132, PST-135, PST-136 and PST-137 were found in the western United States at low frequencies. Virulence factors 13 and 15 occurred in combinations with the virulence factors of the post-2000 group races in PST-127 and PST-137, so these races had the broadest spectra of virulence. PST-127 increased in frequency in the western United States in 2008 and 2009 (Wan & Chen, unpublished data).
The frequencies of virulence factors 10 (to ‘Stephens’), 17 (to AVS/6*Yr8), 18 (AVS/6*Yr9), 19 (‘Clement’), and 20 (‘Compair’) increased from the 20–35% range in 2000 to over 90% from 2004 to 2007 ( c and ). The frequencies of virulence factors 3 (to ‘Heines VII’), 8 (to ‘Produra’), 11 (to ‘Lee’), and 16 (to ‘Express’) increased from the 40–65% range in 2000 to over 90% in 2003 to 2007. Such increases were due to the fact that virulence factors 3, 11, 16, 17, 18, 19 and 20 are commonly presented in races of the post-2000 group and virulence factor 10 is presented in the recent predominant races represented by PST-100. The increase in frequency of virulence factor 10 may be due to the fact that ‘Stephens’ has been widely grown in the Pacific Northwest and was used in developing ‘Jagger’, a major cultivar that has been widely grown in the Great Plains since the late 1990s.
Relationships of PST races
Cluster analysis classified the 115 races detected from 2000 to 2007 into various groups based on their virulence/avirulence patterns (). Using the 0.5 similarity coefficient as a separation point, the 115 races were classified into two groups, G1 and G2. The G1 group contained races that were avirulent to resistance genes Yr8 and Yr9 and first identified either before or after 2000, while the G2 group only contained races that had virulence to these genes and were first identified after 2000.
Fig. 5 Dendrogram of 115 races of Puccinia striiformis f. sp. tritici that were detected in the United States from 2000 to 2007 that have been arranged based on their virulence and avirulence to the set of 20 wheat differential genotypes, using the unweighted pair group arithmetic mean (UPGMA) program of NTSYS-pc (version 2.2.1; Rohlf, Citation1992).
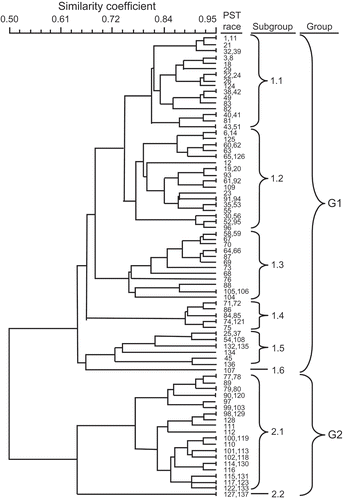
The G1 group was separated into subgroups 1.1 to 1.6 using a coefficient of about 0.74 as the separation point. Races in subgroup 1.1 have avirulence factors 7 (AVS/6*Yr5), 8 (‘Produra’), 10 (‘Stephens’), 13 (‘Tyee’), 15 (‘Hyak’), 16 (‘Express’), 18 (AVS/6*Yr9), 19 (‘Clement’) and 20 (‘Compair’) in common but do not have any common virulence factors. All races in this subgroup were first detected before 2000. Races in subgroup 1.2 have avirulence factors 7, 13, 15, 17 (AVS/6*Yr8), 18, 19 and 20 and virulence factor 1 in common. Races in subgroup 1.3 have avirulence factors 4 (‘Moro’), 5 (‘Paha’), 6 (‘Druchamp’), 7, 13, 14 (‘Tres’) and 15 and virulence factors 1 (‘Lemhi’), 12 (‘Fielder’) and 16 in common. Races in subgroup 1.4 have avirulence factors 2 (‘Chinese 166’), 3 (‘Heines VII’), 5, 7, 9 (‘Yamhill’), 11 (‘Lee’), 13, 14 and 15 and virulence factors 1, 8, 10, 12 and 18 in common. Races in subgroup 1.5 have avirulence factors 2, 7, 17, 18, 19 and 20 and virulence factors 1, 3 and 12 in common. Subgroup 1.6 had only race PST-107 that has virulence factors 1, 3, 4, 5, 9, 10 and 14 and avirulence factors 2, 6, 7, 8, 11, 12, 13, 15, 16, 17, 18, 19 and 20.
The G2 group was classified into two subgroups, 2.1 and 2.2, using a similarity coefficient of 0.74 as a cutting point. Races in subgroup 2.1 have avirulence factors 7 and 15 and virulence factors 1, 12, 16, 17, 18, 19 and 20 in common. Races PST-127 and PST-137 in subgroup 2.2 have avirulence factors 4, 7 and 14 and virulence factors 1, 2, 3, 5, 6, 8, 9, 10, 11, 12, 13, 15, 17, 18, 19 and 20 in common.
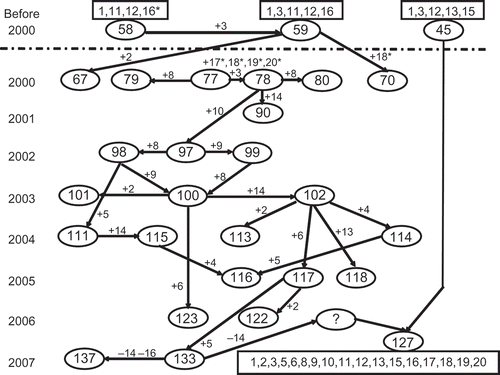
The results of cluster analysis indicate some evolutionary aspects of PST in the United States. Generally, relatively simple races with few virulence factors were displaced by complex races with more virulence factors among races in either G1 or G2. This trend can be illustrated with a simplified scheme of step-wise gains of virulence factors (). Evolution of any new races identified before 2000 can be postulated to arise from previously existing races by one-step mutation or somatic recombination (Chen et al., Citation2002; Chen, Citation2005, Citation2007). However, as shown in , the appearance of the post-2000 group of races, PST-77, 78, 79 and 80, could not be explained by single-step mutation or somatic recombination processes. PST-58 and PST-59, first identified in 1998 in California, are more closely related to the G2 races than other pre-2000 races, but they would have had to gain four virulence factors, 17–20, in a very short period to evolve into the new group of races. The possibility of such a rapid evolution is very low. The appearance of the post-2000 group of races in 2000 was more likely an introduction from outside of the United States as the Yr9 virulence had occurred in Africa, Asia, Europe and South America (Stubbs, Citation1985) long before its detection in the United States in 2000 (Chen et al., Citation2002; Chen, Citation2005, Citation2007). The clear separation of the races in G1 and G2 groups indicates that G2 did not likely evolve from races in G1 and supports the introduction hypothesis. Recently, Markell & Milus (Citation2008) showed that the post-2000 group of races in south-central states was different from the prior-2000 group using amplified fragment length polymorphism (AFLP) markers. We have studied a wide range of isolates using simple sequence repeat (SSR), gene-specific markers and sequences of selected genes in PST isolates. The preliminary results support the introduction of the post-2000 group of races (data not shown). Since this group of races was first detected in 2000, the population has continued evolving into more virulent races. As shown in , PST-97 likely evolved from PST-78 by gaining virulence factor 10 (to ‘Stephens’) and it likely evolved into PST-98 and PST-99 by adding virulence factors 8 (to ‘Produra’) and 9 (to ‘Yamhill’), respectively. From one of these two races, PST-100 was produced. PST-114 can be postulated to evolve from PST-102 by adding virulence factor 4 (to ‘Moro’) and PST-102 from PST-100 by adding virulence factor 14 (to ‘Tres’). It is not clear how PST-127 evolved. It might be through somatic recombination of races that have virulence factors 13 and 15, such as PST-45, with some races of the post-2000 group.
Fig. 6 Postulated evolutionary relationships of selected races of Puccinia striiformis f. sp. tritici to demonstrate the major virulence changes in the US population of the pathogen from 2000 to 2007. The numbers in oval cycles represent PST races (see ). The numbers in a rectangle represent the virulence formula for the race (see ). ‘+’ indicates addition of one or more virulence factors and ‘-‘ indicates loss of one or more virulence factors to the wheat differentials. Wheat differential numbers () are: 1 = ‘Lemhi’, 2 = ‘Chinese 166’, 3 = ‘Heines VII’, 4 = ‘Moro’, 5 = ‘Paha’, 6 = ‘Druchamp’, 7 = AVS/6*Yr5, 8 = ‘Produra’, 9 = ‘Yamhill’, 10 = ‘Stephens’, 11 = ‘Lee’, 12 = ‘Fielder’, 13 = ‘Tyee’, 14 = ‘Tres’, 15 = ‘Hyak’, 16 = ‘Express’, 17 = AVS/6*Yr8, 18 = AVS/6*Yr9, 19 = ‘Clement’ and 20 = ‘Compair’.
This study was focussed on the United States population of P. striiformis f. sp. tritici using the current set of 20 differential genotypes that has been evolving since the 1960s (Line et al., Citation1970; Line & Qayoum, Citation1992; Line, Citation2002; Chen, Citation2005). The use of this set of differentials has allowed us to compare the current populations with those from the past. Many of the genotypes were commercial cultivars and were added to the differential set when they became susceptible to new races. Races differentiated by such cultivars are directly relevant to breeding programmes and production because most new cultivars were developed using older adapted cultivars. However, the identification of new sources of resistance requires the continual addition of resistant genotypes to the differential series, which can make the set become too large to use efficiently in race identification. The second problem is that the use of commercial cultivars tends to result in the creation of different sets of differentials for different regions or countries (Wellings & McIntosh, Citation1990; Wellings et al., Citation2003; Wan et al., Citation2004; Chen, Citation2005), which makes the comparison of pathogen populations in different countries difficult. We have been conducting experiments to compare the United States isolates with a limited number of isolates from Canada, Chile, China, Mexico, Nepal, Pakistan, Russia, Spain and Turkey, and found that virulence to ‘Lemhi’, ‘Heines VII’, ‘Stephens’, ‘Lee’, ‘Fielder’, AVS/6*Yr8 and AVS/6*Yr9 are quite common, while virulence to ‘Moro’ was absent in other countries’ samples (data not shown). These results are generally in agreement with reports on virulence in Australia, China, Central and West Asia and Europe (Wellings et al., Citation2003; Wan et al., Citation2004; Hovmøller et al., Citation2008). The third problem is that many of the current differentials have more than one resistance gene. Sometimes, virulence to a specific resistance gene cannot be detected until the differential genotype becomes susceptible. We are currently testing PST isolates on the Yr near-isogenic lines in the ‘Avocet Susceptible’ background (Wellings et al., Citation2004) and genotypes each with a single gene to select genotypes as differentials, which may be suitable for keeping the continuation of the current system and also for providing more useful race information for breeding programmes.
Because of the limitations of different genotypes and sample collections, it is impossible to detect all the variation in virulence patterns in the rust population. On the other hand, not all races or mutants that arise in the pathogen population have an equal impact in subsequent epidemics. Some of the races may disappear or remain at a very low frequency while others become predominant and cause large-scale damage. The fate of a new race is determined by many factors, such as its virulence, fitness and aggressiveness of the race and host selection including the acreage of susceptible cultivars. Among these factors, the virulence of the pathogen and susceptibility of cultivars are the most important. Because PST is an obligate biotrophic pathogen, virulence on some currently grown cultivars is an essential requirement in order for a new race to become predominant. This is why the post-2000 group of races represented by PST-78 and 80 and their derivatives, such as PST-100, PST-101, PST-102, PST-114 and PST-116, have become predominant and caused large-scale, severe yield losses since 2000. Increased aggressiveness may also have been a factor, making this group of races more predominant and the disease more widespread (Milus et al., Citation2006).
This study and previous studies (Line & Qayoum, Citation1992; Line & Chen, Citation1996; Chen et al., Citation2002) demonstrate that PST is highly variable and new races appear frequently. This study also shows that races of the pathogen can spread over long distances between regions. Races of PST-78 and 80 in the post-2000 group were first detected in regions 6 and 7 in 2000 (Chen et al., Citation2002). In 2002, these races appeared in region 1 and caused severe damage on spring wheat cultivars that lacked high-temperature adult-plant (HTAP) resistance (Chen et al., Citation2003). PST-100 appeared in 2003 and quickly became widespread and caused the largest scale of damage throughout the country in 2003 to 2005. The recently detected races with virulence to resistance gene Yr1, such as PST-101, 112, 113 and 122, have still been restricted to California. PST-114 with virulence on Yr10 became the most predominant race in 2007, but its distribution has been limited to the Pacific Northwest thus far. Similarly, PST-127 and similar races with virulence to ‘Tyee’ (YrTye) and ‘Hyak’ (Yr17 and YrTye) have not been detected in regions east of the Rocky Mountains. However, caution should be raised on the use of these genes in breeding programmes and in their deployment as single genes because these races may spread to other regions. As the stripe rust pathogen changes its virulence patterns and new races spread very quickly, care should be taken on the development of race-specific resistance genes such as Yr5 and Yr15, which are still effective against all races identified so far in the United States. A more effective strategy to combat the changing populations of the stripe rust pathogen is to use non-race specific resistance, such as HTAP resistance that has been successfully used to reduce stripe rust damage in the Pacific Northwest and some other regions since the 1960s (Qayoum & Line, Citation1985; Chen & Line, 1995; Line & Chen, Citation1995; Line, Citation2002; Chen, Citation2005). The recently identified resistance genes or quantitative trait loci for HTAP resistance and their linked markers (Uauy et al., Citation2005; Chen & Zhao, 2007; Lin & Chen, Citation2007, Citation2009; Santra et al., Citation2008) should efficiently facilitate the incorporation of this durable type of stripe rust resistance into adapted cultivars in different wheat production regions.
Acknowledgements
This study was supported by the US Department of Agriculture, Agricultural Research Service (Projects No. 5348–22000–014–00D), Washington Wheat Commission, and Idaho Wheat Commission. PPNS No. 0515, Department of Plant Pathology, College of Agricultural, Human, and Natural Resource Sciences, Agricultural Research Center, Project No. WNP00823 (Projects 13C–3061–3923 and 13C–3061–5822), Washington State University, Pullman, WA 99164-6430, USA. The authors thank the following people for collecting and sending stripe rust samples: G. Aksland, R. Allan, D. Anderson, J. Avant, D. Bland, T. Blunt, R. Bowden, M. Bowman, B. Brown, C. Brown, K. Burch, J. Burns, O. Cantu, M. Carson, B. Carver, R. Cartwright, G. Cisar, A. Corey, J. Costa, A. Coulon, C. Cowger, B. Cunfer, E. DeWolf, B. Edge, J. Engle, D. Falk, S. Fichtner, A. Fritz, J. Gilbert, B. Grey, C. Griffey, J. Hancock, S. Haley, S. Harrison, R. Herrington, D. Herron, D. Hole, R. Hunger, L. Jackson, Y. Jin, J. Johnson, M. Johnston, R. Johnston, D. Jones, S. Jones, R. Karow, D. Kirby, A. Klatt, J. Kleinjan, T. Koehler, L. Kolb, M. Kolding, J. Kolmer, H. Lambert, M. Larson, M. Lazar, M. Lewis, R. Line, P. Lipps, Y. Liu, A. Loladze, D. Long, L. Lowe, S. Lyon, S. Markell, D. Marshall, R. Matchett, R. McIntosh, J. Miller, E. Milus, J. Moffat, S. Monfort, S. Mornell, C. Nelson, L. Osborne, B. Padgett, T. Paulitz, J. Peterson, S. Petrie, S. Pomoransky, L. Reddy, T. Reoper, T. Richard, J. Roberts, J. Rudd, D. Sanford, D. Santra, J. Schimelfenig, J. Schmierer, T. Schubert, R. Sears, G. Shaner, G. Shelton, R. Smiley, E. Souza, J. Stack, B. Steffenson, J. Stein, A. Steve, D. Tague, T. Tayson, A. Tenuta, V. Velasco, J. Vestal, M. Vuhoeven, M. Wang, J. Watkins, J. Windes, D. Wood, J. Youmans, R. Zemetra, X. Zhang and J. Zitzenitz. We also thank Drs Roland F. Line and Dennis A. Johnson for their critical review of the manuscript.
References
- Carleton , M.A. 1915 . A serious new wheat rust in this country . Science , 42 : 58 – 59 .
- Chen , X.M. 2005 . Epidemiology and control of stripe rust [Puccinia striiformis f. sp. tritici] on wheat . Can. J. Plant Pathol. , 27 : 314 – 337 .
- Chen , X.M. 2007 . Challenges and solutions for stripe rust control in the United States . Austral. J. of Agric. Res. , 58 : 648 – 655 .
- Chen , X.M. and Line , R.F. 1992a . Identification of stripe rust resistance genes in wheat cultivars used to differentiate North American races of Puccinia striiformis . Phytopathology , 82 : 1428 – 1434 .
- Chen , X.M. and Line , R.F. 1992b . Inheritance of stripe rust resistance in wheat cultivars used to differentiate races of Puccinia striiformis in North America . Phytopathology , 82 : 633 – 637 .
- Chen , X.M. and Line , R.F. 1992c . Genes for resistance to stripe rust in ‘Tres’ wheat . Crop Sci. , 32 : 692 – 696 .
- Chen , X.M. and Line , R.F. 1993 . Inheritance of stripe rust resistance in wheat cultivars postulated to have resistance genes at Yr3 and Yr4 loci . Phytopathology , 83 : 382 – 388 .
- Chen , X.M. and Line , R.F. 1995 . Gene action in wheat cultivars for durable high-temperature adult-plant resistance and interactions with race-specific, seedling resistance to stripe rust caused by Puccinia striiformis . Phytopathology , 85 : 567 – 572 .
- Chen , X.M. , Line , R.F. and Jones , S.S. 1995 . Chromosomal location of genes for stripe rust resistance in spring wheat cultivars Compair, Fielder, Lee, and Lemhi and interactions of aneuploid wheats with races of Puccinia striiformis . Phytopathology , 85 : 375 – 381 .
- Chen , X.M. , Line , R.F. , Shi , Z.X. and Leung , H. 1998 . “ Genetics of wheat resistance to stripe rust ” . In Proceedings of the 9th International Wheat Genetics Symposium , Edited by: Slinkard , A.E. Vol. 3 , 237 – 239 . Saskatchewan, , Canada : Saskatoon .
- Chen , X.M. , Moore , M. , Milus , E.A. , Long , D.L. , Line , R.F. , Marshall , D. and Jackson , L. 2002 . Wheat stripe rust epidemics and races of Puccinia striiformis f. sp. tritici in the United States in 2000 . Plant Dis. , 86 : 39 – 46 .
- Chen , X.M. , Moore , M.K. and Wood , D.A. 2003 . Epidemics and control of stripe rust on spring wheat in the Pacific Northwest in 2002 . Phytopathology , 93 : S16 (abstr.)
- Chen , X.M. , Moore , M.K. and Wood , D.A. 2004 . Stripe rust epidemics and races of Puccinia striiformis in the United States in 2003 . Phytopathology , 94 : S18
- Chen , X.M. and Penman , L. 2005 . Stripe rust epidemic and races of Puccinia striiformis in the United States in 2004 . Phytopathology , 95 : S19 (abstr.)
- Chen , X.M. and Penman , L. 2006 . Stripe rust epidemic and races of Puccinia striiformis in the United States in 2005 . Phytopathology , 96 : S23 (abstr.)
- Chen , X.M. , Penman , L.N. and Richardson , K.L. 2007 . Races of Puccinia striiformis, the stripe rust pathogen in the United States in 2006 . Phytopathology , 97 : S21 (abstr.)
- Chen , X.M. , Wan , A.M. and Richardson , K. 2008 . Virulent races of Puccinia striiformis identified in the United States in 2007 . Phytopathology , 98 : S36 (abstr.)
- Chen , X.M. and Zhao , J. 2007 . Identification of molecular markers for Yr8 and a gene for high-temperature, adult-plant resistance against stripe rust in the AVS/6*Yr8 wheat line . Phytopathology , 97 : S21 (abstr.)
- Hovmøller , M.S. , Yahyaoui , A.H. , Milus , E.A. and Justesen , A.F. 2008 . Rapid global spread of two aggressive strains of wheat rust fungus . Mol. Ecol. , 17 : 3818 – 3826 .
- Humphrey , H.B. , Hungerford , C.W. and Johnson , A.G. 1924 . Stripe rust (Puccinia glumarum) of cereals and grasses in the United States . J. Agric. Res. , 29 : 209 – 227 .
- Lin , F. and Chen , X.M. 2007 . Genetics and molecular mapping of genes for race-specific all-stage resistance and non-race specific high-temperature adult-plant resistance to stripe rust in spring wheat cultivar Alpowa . Theor. Appl. Genet. , 114 : 1277 – 1287 .
- Lin , F. and Chen , X.M. 2008 . Molecular mapping of genes for race-specific overall resistance to stripe rust in wheat cultivar Express . Theor. Appl. Genet. , 116 : 797 – 806 .
- Lin , F. and Chen , X.M. 2009 . Quantitative trait loci for non-race-specific, high-temperature adult-plant resistance to stripe rust in wheat cultivar Express . Theor. Appl. Genet. , 118 : 631 – 642 .
- Line , R.F. 2002 . Stripe rust of wheat and barley in North America: a retrospective historical review . Annu. Rev. Phytopathol. , 40 : 75 – 118 .
- Line , R.F. and Chen , X.M. 1995 . Successes in breeding for and managing durable resistance to wheat rusts . Plant Dis , 79 : 1254 – 1255 .
- Line , R.F. and Chen , X.M. 1996 . “ Wheat and barley stripe rust in North America ” . In Proceedings of the 9th European and Mediterranean Cereal Rusts & Powdery Mildews Conference Bulletin Bulletin, (Suppl.) , Edited by: Kema , G.H.J. , Nike , R.E. and Damen , R.A. Vol. 24 , 101 – 104 . Netherlands : Lunteren .
- Line , R.F. and Qayoum , A. 1992 . Virulence, aggressiveness, evolution, and distribution of races of Puccinia striiformis the cause of stripe rust of wheat in North America 1968–87 Technical Bulletin Number 1788 , Agricultural Research Service : United States Department of Agriculture .
- Line , R.F. , Sharp , E.L. and Powelson , R.L. 1970 . A system for differentiating races of Puccinia striiformis in the United States . Plant Dis. Rep , 54 : 992 – 994 .
- Markell , S.G. and Milus , E.A. 2008 . Emergence of a novel population of Puccinia striiformis f. sp. tritici in Eastern United States . Phytopathology , 98 : 632 – 639 .
- McIntosh , R.A. , Hart , G.E. , Devos , K.M. , Gale , M.D. and Rogers , W.J. 1998 . “ Catalogue of gene symbols for wheat ” . In Proceedings of the 9th International Wheat Genetics Symposium , Edited by: Slinkard , A.E. Vol. 5 , 1 – 235 . Saskatchewan, , Canada : Saskatoon .
- Milus , E.A. , Seyran , E. and McNew , R. 2006 . Aggressiveness of Puccinia striiformis f. sp. tritici isolates in the south-central United States . Plant Dis , 90 : 847 – 852 .
- Pady , S.M. , Johnson , C.O. and Rogerson , C.T. 1957 . Stripe rust of wheat in Kansas in 1957 . Plant Dis. Rep. , 41 : 959 – 961 .
- Qayoum , A. and Line , R.F. 1985 . High-temperature, adult-plant resistance to stripe rust of wheat . Phytopathology , 75 : 1121 – 1125 .
- Rohlf , F.J. 1992 . NTSYS-pc: numerical taxonomy and multivariate analysis system version 1.70 , New York : Exter Software .
- Santra , D.K. , Chen , X.M. , Santra , M. , Campbell , K.G. and Kidwell , K.K. 2008 . Identification and mapping QTL for high-temperature adult-plant resistance to stripe rust in winter wheat (Triticum aestivum L.) cultivar ‘Stephens’ . Theor. Appl. Genet. , 117 : 793 – 802 .
- Stubbs , R.W. 1985 . “ Stripe rust ” . In The cereal rusts , Edited by: Roelfs , A.P. and Bushnell , W.R. Vol. II , 61 – 101 . Orlando, FL : Academic Press .
- Uauy , C. , Brevis , J.C. , Chen , X.M. , Khan , I.A. , Jackson , L.F. , Chicaiza , O. , Distelfeld , A. , Fahima , T. and Dubcovsky , J. 2005 . “ High-temperature adult plant (HTAP) stripe rust resistance gene Yr36 from Triticum turgidum ssp. dicoccoides is closely linked to the grain protein content locus Gpc-B1 ” . In Theor. Appl. Genet Vol. 112 , 97 – 105 .
- Wan , A.M. , Zhao , Z.H. , Chen , X.M. , He , Z.H. , Jin , S.L. , Jia , Q.Z. , Yao , G. , Yang , J.X. , Wang , B.T. , Li , G.B. , Bi , Y.Q. and Yuan , Z.Y. 2004 . Wheat stripe rust epidemics and virulence of Puccinia striiformis f. sp. tritici in China in 2002 . Plant Dis. , 88 : 896 – 904 .
- Wellings , C.R. and McIntosh , R.A. 1990 . Puccinia striiformis f. sp. tritici in Australasia: pathogenic changes during the first 10 years . Plant Pathol. , 39 : 316 – 325 .
- Wellings, C.R., Singh, R.P., McIntosh, R.A., & Pretorius, Z.A. (2004). The development and application of near isogenic lines for the stripe (yellow) rust pathosystem. In Proceedings of the 11th International Cereal Rusts and Powdery Mildew Conference, Norwich, UK. Abstract A1.39, Cereal Rusts and Powdery Mildews Bulletin http://www.crpmb.org/icrpmc11/abstracts.htm (http://www.crpmb.org/icrpmc11/abstracts.htm)
- Wellings , C.R. , Wright , D.G. , Keiper , F. and Loughman , R. 2003 . First detection of wheat stripe rust in Western Australia, evidence for a foreign incursion . Aust. Plant Pathol. , 32 : 321 – 322 .