Abstract
Blackleg and blackspot are economically important diseases of canola caused by Leptosphaeria maculans and Alternaria brassicae, respectively, and can lead to significant crop losses. In this study, we investigated the effects of cytokinins on the symptoms caused by these two pathogens. We observed that cytokinin, especially 6-benzyl amino purine, was able to significantly reduce disease symptoms and mycelial growth within plant tissues. This cytokinin was also able to inhibit the in vitro growth of both fungi. Other cytokinins such as kinetin or adenine hemisulfate were unable to inhibit fungal growth in vitro, suggesting that the presence of a benzene ring structure is required for the inhibitory effects observed. Our findings are discussed within the context of plant–pathogen interactions.
Résumé
La jambe noire et l'alternariose sont des maladies du canola causées par Leptosphaeria maculans et Alternaria brassicae , respectivement qui peuvent engendrer de lourdes pertes de rendement, d'où leur grande importance économique. Dans cette étude, nous avons examiné les effets des cytokinines sur les symptômes causés par ces deux agents pathogènes. Nous avons observé que la cytokinine, particulièrement la 6-benzylaminopurine, pouvait atténuer notablement les symptômes de la maladie et la croissance mycélienne dans les tissus de la plante. Cette cytokinine pouvait également inhiber la croissance in vitro des deux champignons. D'autres cytokinines, comme la kinétine ou l'hémisulfate d'adénine, ont pu inhiber la croissance fongique in vitro , ce qui suggère que, pour induire les effets inhibiteurs, un noyau benzénique est requis. Nos résultats sont examinés en fonction des interactions plante-agent pathogène.
Introduction
Senescence is a process which involves a progressive change in a plant's physiological status and can affect sensitivity of plants to pathogens. For example, several necrotrophic fungi such as Alternaria alternata (Fr.) Keissl. (Stavely & Slana, Citation1971), A. brassicae (Berk.) Sacc. (Conn et al., Citation1990) and Botrytis cinerea (De Bary) Whetzel (Barna & Gyorgyi, Citation1992) are known to infect senescing plants due to increased susceptibility of the senescing tissue. Phytohormones such as cytokinins (CKs) play an essential role in sustaining juvenility of plant tissues and have been investigated to understand the relationship between senescence and susceptibility towards several plant pathogens (Pogany et al., Citation2004). For example, the formation of green islands, which is a characteristic feature of interactions between plants and biotrophic fungi such as rusts and mildews, is characterized by high levels of CKs, presumably aiding pathogen establishment and growth (Dekhuijzen, Citation1976; Greene, Citation1980). Moreover, the exogenous application of CKs has been reported to enhance plant resistance during some viral and fungal infections (Dekker, Citation1963; Clarke et al., Citation1998). However, the effects of CKs on the tolerance of plants to diseases caused by the hemibiotrophic fungus Leptosphaeria maculans (Desm.) Ces. & De Not. and the necrotrophic fungus Alternaria brassicae (Berk.) Sacc. have not been previously investigated.
Leptosphaeria maculans and A. brassicae cause blackleg and Alternaria blackspot diseases, respectively, in the economically important Brassica species, B. napus. Blackleg disease has been observed to occur on both winter and spring cultivars of oilseed rape, which are grown under a broad range of climates and using varying agricultural practices (West et al., Citation2001). Leptosphaeria maculans colonizes the tissue initially as a biotroph but acts as a necrotroph by producing pycnidia (asexual fruiting bodies) in the dead tissue at a later stage (Hammond et al., Citation1985; Hammond & Lewis, Citation1987). Alternaria blackspot disease causes chlorotic and necrotic lesions mostly on aerial parts of the plant (Verma & Saharan, Citation1994) and is responsible for substantial reduction in seed yield and oil quality. Up to 30% yield losses in canola have been reported in western Canada (Tewari & Conn, Citation1988). Strategies such as application of fungicides and developing resistant cultivars have been employed to control these diseases; however, the former is not environmentally sustainable and is expensive whereas the latter has been overcome by the evolution of several new races of the pathogen (Sprague et al., Citation2006). The development of durable resistance therefore has become important in combating these diseases of canola.
Studies have suggested that approaches aimed at reducing senescence could be advantageous for reducing the damage caused by A. brassicae. For example, A. brassicae is known to produce a complex mixture of phytotoxins including destruxin B (Bains & Tewari, Citation1987), which cause chlorosis in infected plants (Ayer & Rodriguez, Citation1987). In addition, Dahiya et al. (Citation1988) reported that A. brassicae synthesizes abscisic acid (ABA) which helps to accelerate senescence (El-Antalby et al., Citation1967; Rudnicki & Pieniazek, Citation1968; Arditti et al., Citation1971; Smart, Citation1994). Smigocki et al. (Citation1993) reported CK-mediated resistance to Manduca sexta (Linnaeus) and Myzus persicae (Sulzer) in transgenic Nicotiana plumbaginifolia (Viv.) plants expressing an isopentenyl transferase gene (ipt; involved in CK biosynthesis). In addition, higher levels of mRNA transcript of chitinase (known to hydrolyse chitin, a major component of the fungal cell wall and considered as a plant defence-related gene) and PR-1 (pathogenesis-related protein) in tobacco shoots have been reported as a result of exogenous application of CKs (Memelink et al., Citation1987).
Since it is well known that CKs antagonize the effects of ABA and delay senescence and induce the expression of defence-related genes, we investigated whether CKs can be used to delay the development of symptoms caused by A. brassicae in B. napus and B. carinata. We also investigated whether CKs could be used to delay symptoms caused by L. maculans since there are no reports in the literature investigating the effects of CKs on the L. maculans–B. napus pathosystem. We also investigated the effect of exogenous CK on growth of the two fungi.
Materials and methods
Plant material and pathogens
Brassica napus ‘Westar’ and B. carinata plants were used as susceptible genotypes for A. brassicae experimentation, whereas only B. napus plants were used for L. maculans experiments because of the resistance of B. carinata to this pathogen (Sacristan & Gerdemann, Citation1986; Rimmer & van den Berg, Citation1992). Seedlings of both species were grown in plastic inserts (7.5 cm × 5 cm; one seed per insert) filled with Metro Mix® 290 (Grace Horticultural Products, Ajax, Ontario) in the greenhouse (22 °C day/18 °C night; 16 h photoperiod) for three weeks. Plants were fertilized after two weeks with 200 ppm Peters® 20-20-20. Two different isolates of A. brassicae (UAMH 7476 and Ontario) and a virulent isolate of L. maculans (PG2; 77-33) were generously provided by Dr J.P. Tewari, Department of Agricultural, Food and Nutritional Science, University of Alberta and were used in all experiments. All experiments were repeated in three independent biological replicates.
Preparation of fungal inoculum
All fungal strains were cultured at room temperature (21 ± 2 °C) on V8 juice–rose bengal agar medium (Degenhardt et al., Citation1974). Leptosphaeria maculans was cultured under light supplied by cool white-fluorescent tubes, with a 12 h photoperiod, for 10 days whereas A. brassicae isolates were cultured in the dark for 12 days. Fungal spore suspensions were prepared in water by scraping spores and mycelia from the plates with the help of a glass-rod. This suspension was filtered through four layers of cheesecloth in order to remove mycelia and agar pieces. The suspension was centrifuged twice at 2000 g for 5 min in a Sorvall GLC-2 centrifuge (Sorvall; Allied Scientific, Canada) and in order to remove media and metabolites from the preparation. The washed, pelleted spores were resuspended in water containing 0.05% Tween-20 and counted using a haemocytometer.
Detached leaf and whole plant experiments
Detached leaves were obtained from three-week-old B. napus and B. carinata plants which were grown in the greenhouse as described previously. The petiole of the excised leaf was covered with an absorbent cotton ball and placed in Petri dishes (100 mm; two leaves/dish). 6-benzyl amino purine (BAP, Sigma; 3 mL of 0, 0.2 mM, 0.5 mM or 1.0 mM) was applied to the cotton balls. Four hours later, each leaf was wounded at two places on either side of the midrib using a sterile pipette tip and was inoculated with fungal spore suspension. A final spore concentration of 107 spores mL-1 was used in the case of L. maculans and 4 × 105 spores mL-1 for both A. brassicae isolates. We used 15 μL of L. maculans and 25 μL of A. brassicae spore-suspension in all these experiments. Mock-infected controls (un-inoculated) were treated with sterile distilled water containing 0.05% Tween-20 at the wounding site. Photographs were taken on the fifth day post-inoculation for leaves treated with A. brassicae and 11th day post-inoculation for leaves treated with L. maculans.
For whole-plant experiments, three-week-old plants (which were in the humidity chamber for at least 24 h prior to the experiment) were placed in the greenhouse, allowed to dry for 1 h, and sprayed to drenching with 25 mL of BAP (0, 0.2 mM, 0.5 mM and 1.0 mM). Plants were then allowed to remain in the greenhouse for an additional 4 h. First or second true leaves were wounded as described above, the fungal suspension applied and the plants were returned to the humidity chamber for 24 h. Plants were then moved back to the greenhouse and monitored for symptoms. Photographs were taken after 10 days for A. brassicae and after 11 days post-inoculation for L. maculans. Disease scoring was conducted using APS Assess software (Image Analysis Software for Plant Disease Quantification; The American Phytopathological Society).
Fungal growth on cytokinin-supplemented media
The V8 juice–rose bengal medium was used in these experiments. Three concentrations (0.2, 0.5 and 1.0 mM) of the cytokinins (CKs) 6-benzyl amino purine (BAP) and kinetin (Sigma-Aldrich Co. Ltd, St. Louis, MO) were prepared by dissolving in a small amount of 1N NaOH after which the final volume was adjusted with distilled water to achieve the appropriate concentration of CKs and were filter-sterilized using small filter units (25 mm, 0.22 μm pore size; Fisher Scientific, Ireland). Control plates with no CK supplementation contained distilled water with similar amounts of 1N NaOH as in the CK solutions. The pH of the CK-supplemented media was also determined in order to ensure that the addition of CK did not cause drastic changes in pH. Autoclaved media was allowed to cool to 60 oC prior to supplementing with CK solutions or water/NaOH (for controls) before pouring into 100 mm Petri dishes. Medium containing adenine was prepared by dissolving in 1N HCl and incubating at 50 °C in a water bath until completely dissolved, while adenine hemisulfate was dissolved in distilled H2O, together with appropriate HCl controls.
Fungal agar blocks of 4 mm size containing both spores and mycelia were placed in the middle of the Petri dishes containing the CKs, adenine and adenine hemisulfate supplements or their respective controls. Dishes with A. brassicae were incubated in the dark, whereas those with L. maculans were kept under white light with a 12 h photoperiod for 15 days at room temperature to monitor fungal growth. The diameter of fungal colonies was measured after 15 days. The entire experiment was repeated at least three times.
Histological studies
Hormone and non-hormone-treated pathogen-inoculated leaves as well as un-inoculated (mock-infected) controls were cut into small (5 × 10 mm) pieces, and fixed in FAA (formalin, acetic acid and ethyl alcohol; Yeung & Saxena, Citation2005) under vacuum at room temperature overnight. Following fixation, they were subsequently dehydrated in a series of graded ethanol/water solutions, changed to toluene and later infiltrated with Paraplast® using a Fisher Histomatic Tissue Processor (Model 166; Pittsburgh, PA, USA). Paradermal sections (7μm thick) were prepared using an AO Rotary microtome (Spencer 820; Buffalo, NY, USA), affixed to glass slides, de-paraffinated with toluene, rehydrated to 50% ethanol and stained with Aniline blue in Lacto-phenol for 10 min (Larone, Citation1995). After this, stained sections were rinsed thrice with water (3 min each) and counterstained with acidified Eosin Y for 1 min (Dougherty, Citation1981), dehydrated in ethanol followed by toluene and mounted with DPX® (Electron Microscopy Sciences, Hatfield, PA, USA) mounting medium. The sections were viewed with a Leica DM RXA microscope (Leica Microsystems, Wetzlar, Germany), analyzed using Macrofire™ software (Optronics, Goleta, CA, USA) and photographed with an Optronics digital camera.
Statistical analysis
Statistical analysis of disease score results was performed using analysis of variance (ANOVA) with the mixed model procedure of SAS version 9.1 (Statistical Analysis system; SAS Institute Inc., Cary, NC, USA).
Results
Effects of CKs on plant–pathogen interaction
The effects of BAP were investigated using both detached leaf and whole plant systems. When detached leaves were treated with various concentrations of BAP and challenged with the pathogen, a significant reduction in the lesion size caused by L. maculans on B. napus was observed at all concentrations when compared with the untreated controls ( a), with the highest reduction being observed at 0.5 mM BAP. Although a statistically significant effect on disease severity was not observed with increasing concentration of BAP, fewer disease symptoms were observed on those leaves treated with higher concentrations ( a).
Fig. 1. Disease symptoms induced by L. maculans and A. brassicae isolates on detached leaves of Brassica plants. a, Effects of various concentrations of BAP treatments on the disease severity score (%) induced by L. maculans on B. napus or b, by A. brassicae isolate UAMH 7476, and c, Ontario on B. napus and B. carinata. Data were analyzed by ANOVA (P < 0.05) and different letters on the histogram (capital letters for ‘Westar’; lower case for ‘Carinata’ plants) indicate significant (P < 0.05) differences for each plant line under different concentrations of hormone treatments.
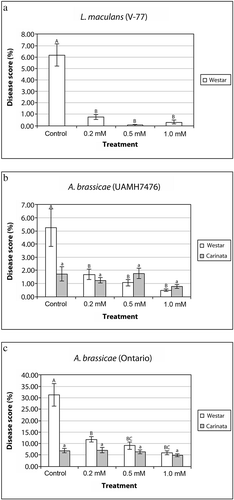
Similarly, BAP-treated B. napus leaves infected with both A. brassicae isolates (UAMH 7476 and Ontario isolates) also demonstrated a reduction of visual symptoms ( b) and a significant (P < 0.05) reduction in severity ( b and c). No significant effects of concentration of BAP were observed on severity of disease caused by this pathogen ( b and c). Because B. carinata has been reported to exhibit a degree of tolerance to A. brassicae (Bansal et al., Citation1990), we wanted to investigate whether BAP treatment could further increase the tolerance of this species to both isolates of A. brassicae. The results from detached B. carinata leaf experiments are shown in b and c. This species was more tolerant to A. brassicae when compared to B. napus; however, the application of BAP did not significantly affect disease symptoms or severity.
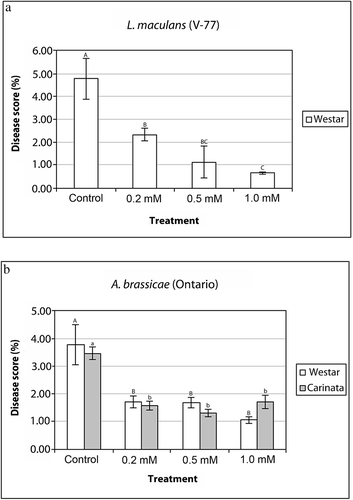
In the whole-plant experiments, a similar significant reduction in disease symptoms caused by L. maculans was found in BAP-sprayed B. napus plants when compared with the control ( a). In addition, higher concentrations of BAP (i.e. 0.5 and 1.0 mM) showed significant disease reduction as compared to 0.2 mM. In A. brassicae, we tested only the Ontario isolate in these whole-plant experiments because this isolate was more virulent than UAMH 7476. Our observations showed significant differences in disease symptoms between susceptible B. napus and moderately tolerant B. carinata but no significant differences with the three concentrations of BAP tested ( b).
Fig. 2. Severity of disease symptoms induced by L. maculans and A. brassicae (Ontario isolate) on whole leaves of Brassica plants. Effects of various concentrations of BAP treatments on the disease severity score (%) induced by a, L. maculans on B. napus, and b, by A. brassicae on B. napus and B. carinata. Data were analyzed by ANOVA (P < 0.05) and different letters on the histogram (capital letters for ‘Westar’; lower case for ‘Carinata’ plants) indicate significant (P < 0.05) differences for each plant line under different concentrations of hormonal treatments.
Effect of CKs on fungal growth
In L. maculans, 0.2 mM BAP significantly (P < 0.05) reduced fungal growth when compared with the control (). At higher concentrations (0.5 and 1.0 mM), a further significant reduction in colony diameter was observed. To determine whether the effects were unique to BAP, we also tested the ability of kinetin to inhibit the growth of L. maculans. We observed that kinetin did not significantly (P < 0.05) reduce fungal growth () when compared with the controls.
Table 1. Effect of cytokinins and other additives on colony growth of three phytopathogenic fungi
In A. brassicae, both CKs significantly (P < 0.05) reduced fungal colony diameter in a dose-dependent manner (). Compared with L. maculans, BAP showed a more drastic growth inhibition of A. brassicae with no growth observed at a concentration of 1.0 mM (). In addition, kinetin also inhibited the growth of both isolates of A. brassicae to a greater degree compared with L. maculans ().
Since one of the differences between BAP and kinetin is the presence or absence of a benzene ring, we hypothesized that the benzene ring in BAP may be responsible for the higher inhibitory activity of BAP. We therefore tested the ability of adenine and adenine hemisulfate (known to have CK-like activity; Chandra et al., Citation2003) for their ability to inhibit fungal growth. Our results () indicated that neither compound significantly affected the growth of either pathogen, suggesting that the benzene ring structure in BAP may be responsible for the higher inhibitory activity.
Histological characterization
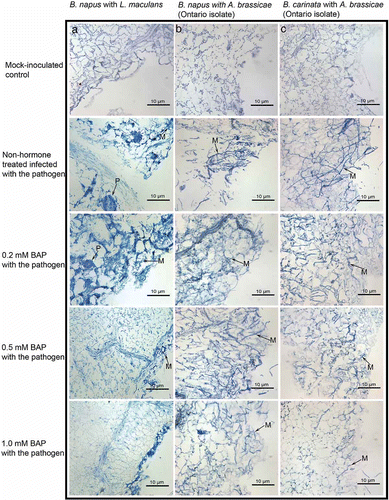
The effects on BAP treatment on the host–pathogen interaction of B. napus and B. carinata were investigated using light microscopy. Whole plant leaves were used for histological studies. Mock-inoculated controls revealed dead and injured cells at the wounding site in both species (). In the case of untreated B. napus inoculated with L. maculans, dead and lysed plant cells with extensive fungal mycelial growth and the presence of pycnidia were observed (). Similar results were obtained in 0.2 mM BAP-treated B. napus inoculated with L. maculans, whereas a reduction in mycelial growth and the absence of pycnidial bodies was seen at 0.5 mM BAP and no fungal growth could be seen at 1.0 mM BAP (). With A. brassicae, fungal mycelial growth was observed in both hormone-treated and untreated samples although growth was reduced at 1.0 mM BAP concentration in both species of Brassica. Furthermore, the reduction in fungal growth was more evident in B. carinata as compared with B. napus at this concentration.
Fig. 3. Histological studies of responses of hormone-treated and untreated leaves of whole plants. Panels in (a) illustrate the effects of various concentrations of BAP on the interaction of B. napus (‘Westar’) leaves with L. maculans; with A. brassicae (Ontario isolate), (b); and B. carinata with A. brassicae (Ontario isolate), (c). The arrows with M indicate fungal mycelia, and P indicate pycnidia.
Discussion
Cytokinins such as kinetin and BAP are known to delay senescence by preventing degradation of chlorophyll and photosynthetic proteins, and are more effective than zeatin and 2ip (N6-2-Isopentenyladenine) in this regard (Hamzi & Skoog, Citation1964; Yamada et al., Citation1964; Tetley & Thimann, Citation1974; Badenoch-Jones et al., Citation1996). Injured and senescing plants are more susceptible to pathogen attack (Conn et al., Citation1990; Barna & Gyorgyi, Citation1992) and therefore, exogenous application of CKs may aid in the delay and reduction of disease. For example, transgenic tobacco lines with higher CK levels were observed to be more tolerant to tobacco necrosis virus (TNV; Pogany et al., Citation2004). Additionally, a combination of BAP and the auxin α-naphthaleneacetic acid (NAA) has been reported to inhibit the growth of Phaeolus schweinitzii (Fr.) Pat. (Hrib et al., Citation1993). CK also had a suppressive effect on the wildfire disease of tobacco caused by the bacterium Pseudomonas tabaci (Van Hall) (Lovrekovich & Farkas, Citation1963). In another study, CKs were observed to induce resistance in Phaseolus vulgaris L. to the white clover mosaic potexvirus (Clarke et al., Citation1998) and they also affected the growth of the fungus Erysiphe cichoracearum DC on leaf discs of tobacco (Cole & Fernandes, Citation1970). CKs have also been reported to enhance the resistance of barley to the fungal pathogen Erysiphe graminis f. sp. hordei, whereas ABA increased the susceptibility of the host towards the pathogen (Edwards, Citation1983). As mentioned earlier, A. brassicae synthesizes ABA (Dahiya et al., Citation1988) and CKs are well recognized to have an ABA-antagonistic effect, which could counteract the effects of ABA and prevent disease induction and progression. Results from this study demonstrate that the CK BAP can reduce symptoms by both L. maculans and A. brassicae. Furthermore, our results also demonstrate that BAP inhibits the in vitro growth of both pathogens with a higher inhibitory effect against L. maculans. To the best of our knowledge, even though CKs have been implicated in other host–pathogen interactions, this is the first direct demonstration of a protective role of BAP against these two pathogens.
BAP and kinetin have been reported to inhibit mycelial growth and sexual reproduction e.g. production of oogonia in Saprolegnia australis (Elliott), whereas adenine, purines and hypoxanthine stimulated oogonial production (Elliott, Citation1967). Interestingly, it was also observed that although both kinetin and BAP caused similar responses in S. australis, BAP caused greater inhibition of growth and sexual reproduction at a lower concentration compared with kinetin (Elliott, Citation1967). Our histological observations indicated that higher concentrations of BAP reduced mycelial growth of L. maculans and also inhibited the formation of pycnidia. The reduction in pycnidia formation at 0.5 mM BAP and their absence at higher concentrations could be similar to the reduction of oogonia production in Saprolegnia due to cytokinin treatment (Elliott, Citation1967). Thus, a reduction in pycnidia formation by BAP treatment could reduce secondary inoculum within the plant tissue.
We also observed that BAP was better at inhibiting fungal growth when compared with kinetin, which may be due to the structural differences between the two types of CKs used in these studies. The presence of the benzyl group in BAP seems to be responsible for the better activity of this compound compared with kinetin. Compounds containing a benzyl group in their structures have been reported to be inhibitory to fungal growth; for example, cinnamic acid reduced the growth of Neurospora crassa (Shear & B.O. Dodge) by approximately 94% after 24 h incubation (Said et al., Citation2004; Neves et al., Citation2005). This hypothesis needs to be investigated further through the synthesis of appropriate compounds and testing their inhibitory activity.
Although BAP showed direct inhibitory effects on the growth of the pathogens in vivo and in vitro, there can be additional effects on the plant which would also affect pathogen establishment and growth. Indeed, it is well known that CKs also activate numerous plant defence response genes (Memelink et al., Citation1987; Smigocki et al., Citation1993; Schäfer et al., Citation2000). Our efforts are now directed towards understanding the roles of specific CK-inducible defence genes in relation to these two pathosystems.
Acknowledgements
Financial support from Alberta Crop Industry Development Fund (ACIDF) and Alberta Agricultural Research Institute is gratefully acknowledged. We also thank Shirley Brezden for assistance with fungal cultures and the microscopy unit at the Department of Biological Sciences, University of Alberta.
References
- Arditti , J. , Flick , B. and Jeffrey , D. 1971 . Post-pollination phenomena in orchid flowers . New Phytol. , 70 : 333 – 341 .
- Ayer , W.A. and Rodriguez , L.M.P. 1987 . Metabolites produced by Alternaria brassicae, the blackspot pathogen of canola. Part 1, the phytotoxic components . J. Nat. Prod. , 50 : 400 – 407 .
- Badenoch-Jones , J. , Parker , C.W. , Letham , D.S. and Singh , S. 1996 . Effect of cytokinins supplied via the xylem at multiples of endogenous concentrations on transpiration and senescence in de-rooted seedlings of oat and wheat . Plant Cell Environ. , 19 : 504 – 516 .
- Bains , P.S. and Tewari , J.P. 1987 . Purification, chemical characterization and host specificity of the toxin produced by Alternaria brassicae . Physiol. Mol. Plant Pathol. , 30 : 259 – 271 .
- Bansal , V.K. , Seguin-Swartz , G. , Rakow , G.F.W. and Petrie , G.A. 1990 . Reaction of Brassica species to infection by Alternaria brassicae . Can. J. Plant Sci. , 70 : 1159 – 1162 .
- Barna , B. and Gyorgyi , B. 1992 . Resistance of young versus old tobacco leaves to necrotrophs, fusaric acid, cell wall-degrading enzymes and autolysis of membrane lipids . Physiol. Mol. Plant Pathol. , 40 : 247 – 257 .
- Chandra , A. , Gupta , V. , Burma , P. and Pental , D. 2003 . Patterns of morphogenesis from cotyledon explants of pigeon pea . In Vitro Cell. Develop. Biol. Plant. , 39 : 514 – 519 .
- Clarke , S.F. , Burritt , D.J. , Jameson , P.E. and Guy , P.L. 1998 . Influence of plant hormones on virus replication and pathogenesis-related proteins in Phaseolus vulgaris L. infected with white clover mosaic potexvirus . Physiol. Mol. Plant Pathol. , 53 : 195 – 207 .
- Cole , J.S. and Fernandes , D.L. 1970 . Changes in the resistance of tobacco leaf to Erysiphe cichoracearum DC. Induced by topping, cytokinins and antibiotics . Ann. Appl. Biol. , 66 : 239 – 243 .
- Conn , K.L. , Tewari , J.P. and Awasthi , R.P. 1990 . A disease assessment key for Alternaria blackspot in rapeseed and mustard . Can. Plant Dis. Sur. , 70 : 19 – 22 .
- Dahiya , J.S. , Tewari , J.P. and Woods , D.L. 1988 . Abscisic acid from Alternaria brassicae . Phytochemistry , 27 : 2983 – 2984 .
- Degenhardt , K.J. , Skoropad , W.P. and Kondra , Z.P. 1974 . Effects of Alternaria blackspot on yield, oil content and protein content of rapeseed . Can. J. Plant Sci. , 54 : 795 – 799 .
- Dekhuijzen , H.M. 1976 . “ Endogenous cytokinins in healthy and diseased plant ” . In Physiological Plant Pathology , Edited by: Heitefuss , R. and Williams , P.H. 526 – 559 . Berlin : Springer-Verlag .
- Dekker , J.W. 1963 . Effect of kinetin on powdery mildew . Nature , 197 : 1027 – 1028 .
- Dougherty , W.J. 1981 . “ Preparation of semi-thin sections of tissues embedded in water-soluble methacrylate for light microscopy ” . In Staining Procedures , 4th , Edited by: Clark , G. 27 – 38 . Baltimore, MD : Williams & Wilkins .
- Edwards , H.H. 1983 . Effect of kinetin, abscisic acid and cations on host-parasite relations of barley inoculated with Erisiphe graminis f. sp. hordei . Phytopathol. Z. , 107 : 22 – 30 .
- El-Antalby , H.M.M. , Wareing , P.F. and Hilmian , J. 1967 . Some physiological responses to D, L-abscisin (dormin) . Planta , 73 : 47 – 90 .
- Elliott , R.F. 1967 . Effects of kinetin and related compounds on growth and sexual reproduction of Saprolegnia australis . Planta , 77 : 164 – 175 .
- Greene , E.M. 1980 . Cytokinin production by micro-organisms . Bot. Rev. , 46 : 25 – 74 .
- Hammond , K.E. and Lewis , B.G. 1987 . The establishment of systemic infection in leaves of oilseed rape by Leptosphaeria maculans . Plant Pathol. , 36 : 135 – 147 .
- Hammond , K.E. , Lewis , B.G. and Musa , T.M. 1985 . A systemic pathway in the infection of oilseed rape plants by Leptosphaeria maculans . Plant Pathol. , 34 : 557 – 565 .
- Hamzi , Q.H. and Skoog , F. 1964 . Kinetin-like growth promoting activity of 1-benzyl-6-aminopurine and 1-(γ, γ-dimethylallyl)-6-aminopurine . Proc. Nat. Acad. Sci. USA , 51 : 76 – 83 .
- Hrib , J. , Vookova , B. and Flak , P. 1993 . Effect of auxin, cytokinin and glutamine on mycelial growth of Phaeolus schweinitzii . Eur. J. For. Pathol. , 23 : 269 – 275 .
- Larone , D.H. 1995 . Medically Important Fungi – A Guide to Identification , 3rd , Washington, DC : American Society for Microbiology Press .
- Li , H. , Sivasithamparam , K. , Barbetti , M.J. and Kuo , J. 2004 . Germination and invasion by pycnidiospores and ascospores of Leptosphaeria maculans on spring-type Brassica napus canola varieties with varying susceptibility to blackleg . J. Gen. Plant Pathol. , 70 : 261 – 269 .
- Lovrekovich , L. and Farkas , G.L. 1963 . Kinetin as an antagonist to the toxic effect of Pseudomonas tabaci . Nature , 198 : 710
- Memelink , J. , Hoge , J.H.C. and Schilperoort , R.A. 1987 . Cytokinin stress changes the developmental regulation of several defence-related genes in tobacco . EMBO J. , 6 : 3579 – 3583 .
- Neves , F.M. , Kawano , C.Y. and Said , S. 2005 . Effect of benzene compounds from plants on the growth and hyphal morphology in Neurospora crassa . Braz. J. Microbiol. , 36 : 190 – 195 .
- Pogany , M. , Koehl , J. , Heiser , I. , Elstner , E.F. and Barna , B. 2004 . Juvenility of tobacco induced by cytokinin gene introduction decreases susceptibility to tobacco necrosis virus and confers tolerance to oxidative stress . Physiol. Mol. Plant Pathol. , 65 : 39 – 47 .
- Rimmer , S.R. and van den Berg , C.G.J. 1992 . Resistance of oilseed Brassica spp. to blackleg caused by Leptosphaeria maculans. Can . J. Plant Pathol. , 14 : 56 – 66 .
- Rudnicki , R.J.M. and Pieniazek , J. 1968 . Accumulation of abscisic acid during ripeneing of pears (Clappis favoiurite) in various storage conditions . Bulletin de l'Académie Polonaise des Sci. , 16 : 509 – 512 .
- Sacristan , M.D. and Gerdemann , M. 1986 . Different behaviour of Brassica juncea and Brassica carinata as sources of Phoma lingam resistance in experiments of interspecific transfer to Brassica napus . Plant Breed. , 97 : 304 – 314 .
- Said , S. , Neves , F.M. and Griths , A.J.F. 2004 . Cinnamic acid inhibits the growth of the fungus Neurospora crassa, but is eliminated as acetophenone . Int. Biodeter. Biodegr. , 54 : 1 – 6 .
- Schäfer , S. , Krolzik , S. , Romanov , G.A. and Schmülling , T. 2000 . Cytokinin-regulated transcripts in tobacco cell culture . Plant Growth Reg. , 32 : 307 – 313 .
- Smart , C.M. 1994 . Gene expression during leaf senescence . New Phytol. , 126 : 419 – 448 .
- Smigocki , A. Jr. , Neal , J.W. , McCanna , I. and Douglass , L. 1993 . Cytokinin-mediated insect resistance in Nicotiana plants transformed with the ipt gene . Plant Mol. Biol. , 23 : 325 – 335 .
- Sprague , S.J. , Marcroft , S.J. , Hayden , H.L. and Howlett , B.J. 2006 . Major gene resistance to blackleg in Brassica napus overcome within three years of production in southeastern Australia . Plant Dis. , 90 : 190 – 198 .
- Stavely , J.R. and Slana , L.J. 1971 . Relation of leaf age to the reaction of tobacco to Alternaria alternata . Phytopathology , 61 : 73 – 8 .
- Tetley , R.M. and Thimann , K.V. 1974 . The metabolism of oat leaves during senescence. I. Respiration, carbohydrate metabolism and the action of cytokinins . Plant Physiol. , 54 : 294 – 303 .
- Tewari , J.P. and Conn , K.L. 1988 . Incidence of the blackspot of canola caused by Alternaria brassicae (Berk.) Sacc . Can. Plant Dis. Surv. , 68 : 103
- Verma , P.R. and Saharan , G.S. 1994 . Monograph on Alternaria Diseases of Crucifers. Technical Bulletin 1994–6E , Saskatoon, , Canada : Agriculture and Agri-Food Canada .
- West , J.S. , Kharbanda , P.D. , Barbetti , M.J. and Fitt , B D.L. 2001 . Epidemiology and management of Leptosphaeria maculans (Phoma stem canker) on oilseed rape in Australia, Canada and Europe . Plant Pathol. , 50 : 10 – 27 .
- Yamada , N. , Suge , H. , Nakamura , H. and Tazima , K. 1964 . Chemical control of plant growth and development. V. Effect of kinetin and other chemicals on degradation of chlorophyll in rice plant . Proc. Crop Sci. Soc. Japan. , 32 : 254 – 258 .
- Yeung , E.C. and Saxena , P.K. 2005 . “ Histological techniques ” . In Protocol for Somatic Embryogenesis in Woody Plants , Edited by: Jain , S.M. and Gupta , P.K. 517 – 537 . Dordrecht : Springer .