Abstract
Symptoms of Armillaria root rot were observed on the herbaceous ornamental, Hemerocallis sp. (daylily), in a residential area in Walhalla, South Carolina, which was surrounded by dense, hardwood forest that also contained diseased hosts. Our objectives were to describe a natural occurrence of Armillaria root rot on daylily, a newly discovered host for the pathogen, and to characterize the Armillaria species involved. To characterize the Armillaria species collected from daylily, we used all available methods, including both traditional (sexual compatibility tests, basidiome morphology) and molecular (phylogenetic analyses of rDNA internal transcribed spacer, ITS, and intergenic spacer I, IGS-I) approaches. The presence of rhizomorphs in the topsoil of daylily beds and on the roots of symptomatic daylilies, coupled with our finding of identical ITS1 sequences among isolates originating from each of a rhizomorph, a daylily, and a neighbouring dogwood, suggests that Armillaria rhizomorphs had spread from native hosts to infect the daylilies. Basidiocarp morphology and basidiospore size best matched that documented for A. gallica. However, rDNA sequence analysis and sexual compatibility were not 100% conclusive. Phylogenetic analysis of ITS sequences revealed that the unknown Armillaria isolates were most closely related to A. calvescens and A. gallica. Analysis of IGS-I sequences was even less informative, grouping our isolates with A. cepistipes, A. gallica and A. sinapina. Sexual compatibility (mating) tests revealed that haploid isolates from daylily were compatible with three of the A. gallica tester isolates, but also one of the A. calvescens tester isolates. Our findings suggest a possible southerly distribution and expanded host range for A. gallica, and raise further questions about the Armillaria species concept as it pertains to the two closely related species, A. calvescens and A. gallica.
Résumé
Les symptômes du pourridié-agaric ont été observés sur la plante herbacée ornementale Hemerocallis sp. (belle-d'un-jour) dans un quartier résidentiel de Walhalla, en Caroline du Sud, qui était entouré par une forêt dense de feuillus qui comportait également des arbres infectés. Nos objectifs étaient de décrire une occurrence naturelle du pourridié-agaric sur la belle-d'un-jour, un hôte récemment découvert, et de caractériser l'espèce d'Armillaria impliquée dans le processus. Afin de caractériser l'espèce d'Armillaria collectée sur la belle-d'un-jour, nous avons utilisé toutes les méthodes disponibles, y compris les méthodes traditionnelles (les tests de compatibilité sexuelle, la morphologie du basidiome) et moléculaires [les analyses phylogéniques de l'espaceur transcrit interne de l'ADNr (ITS) et de l'espaceur intergénique I (IGS-I)]. La présence de rhizomorphes dans le sol des massifs de belles-d'un-jour et sur les racines des plants affichant les symptômes, associée à notre découverte de séquences ITS1 identiques dans les isolats provenant d'un rhizomorphe, d'une belle-d'un-jour et d'un cornouiller voisin, suggère que les rhizomorphes d'Armillaria s'étaient propagés des plantes indigènes hôtes aux belles-d'un-jour pour les infecter. La morphologie et la taille des basidiocarpes correspondaient le mieux à celles se rapportant à A. gallica. Toutefois, l'analyse des séquences d'ADNr et de la compatibilité sexuelle n'était pas probante. L'analyse phylogénétique des séquences de l'ITS a démontré que les isolats inconnus d'Armillaria s'apparentaient particulièrement à A. calvescenset A. gallica. Les résultats de l'analyse des séquences IGS-I étaient encore plus vagues, apparentant nos isolats à A. cepistipes, A. gallica et A. sinapina. Des tests de compatibilité sexuelle (reproduction) ont révélé que des isolats haploïdes de belle-d'un-jour étaient compatibles avec trois des isolats testeurs d'A. gallica, mais aussi avec un des isolats testeurs d'A. calvescens. Nos découvertes suggèrent, d'une part, une possible distribution méridionale et une gamme d'hôtes élargie pour A. gallica et, d'autre part, soulèvent plusieurs questions quant au concept d'espèce chez Armillaria puisqu'il se rapporte aux deux espèces proches parentes A. calvescens et A. gallica.
Introduction
Armillaria species can cause a serious root rot of trees and shrubs (Hood et al., Citation1991). Armillaria inoculum originates primarily from the residual roots of infected trees. When forests are replaced with planted hosts, the pathogen's mycelium persists saprobically in residual roots and can colonize susceptible hosts years after planting (Redfern & Filip, 1991). Past investigations of Armillaria root rot of ornamentals in the USA documented A. gallica Marxmüller & Romagn., A. mellea (Vahl:Fr.) P. Kumm. or A. tabescens (Scop.) Emel as the causal species (Motta & Korhonen, Citation1986; Baumgartner & Rizzo, Citation2001; Schnabel et al., Citation2005 a). Reports on ornamentals outside the USA have been attributed to rare introductions of A. mellea and A. gallica (Coetzee et al., Citation2001, 2005), both of which are endemic to the northern hemisphere (Guillaumin et al., Citation1991).
There are believed to be nine Armillaria species in North America, which vary in host range, geographic distribution and aggressiveness. Armillaria calvescens Bèrubè & Dessur. and A. gemina Bèrubè & Dessur. are thought to be unique to North America, while the seven other species (A. cepistipes Velen., A. gallica, A. mellea, A. nabsnona Volk & Burdsall, A. solidipes Peck (formerly known as A. ostoyae; Burdsall & Volk, Citation2008), A. sinapina Bèrubè & Dessur., and A. tabescens (which may be referred to as A. socialis; Antonin et al., Citation2006)) are also found on other continents of the northern hemisphere (Volk & Burdsall, Citation1995; Banik et al., Citation1996; Ota et al., Citation1998 a). There is one North American biological species (NABS), NABS X, that is reproductively isolated and, as yet, taxonomically undescribed (Volk & Burdsall, Citation1995).
The first report of Armillaria root rot on Hemerocallis L. (daylily; Schnabel et al., Citation2005 b) added this herbaceous ornamental, with its tuberous root crown and fleshy roots, to the already broad host range (e.g. A. mellea sensu lato with 500+ hosts; Raabe, Citation1962), which consist primarily of woody plants (Gregory et al., Citation1991). Herbaceous hosts are uncommon (Hood et al., Citation1991; Thormann et al., Citation2001). In fact, there are rare reports of Armillaria detection from the nonlignified roots of woody hosts (e.g. Bergemann & Garbelotto, Citation2006). The herbaceous hosts of Armillaria species that are best known are the achlorophyllous orchids, Galeola septentrionali and Gastrodia elata. Japanese Armillaria collections from such orchids have been identified as A. cepistipes, A. gallica, A. mellea, A. solidipes (formerly known as A. ostoyae; Burdsall & Volk, Citation2008), A. sinapina and A. tabescens, in addition to several Japanese biological species (Cha & Igarashi, Citation1995; Ota et al., Citation1998 a). Given the uncommon occurrence of Armillaria species on herbaceous hosts, we deemed it important to thoroughly investigate this observation and to conduct a complete taxonomic characterization of the species responsible for infection of daylily, to complement the preliminary observations and cursory rDNA sequence analysis previously reported (Schnabel et al., Citation2005 a).
Identification of Armillaria species requires multiple approaches, as no single test has yet to distinguish among all North American Armillaria species. The use of sexual compatibility (mating tests) between haploid tester isolates of known identity and haploid field isolates of unknown identity is the traditional approach (Korhonen, Citation1978; Anderson et al., Citation1980; Larsen et al., Citation1992). It was originally the accepted technique because it provided a definitive species-level identification, unlike basidiome morphology, and because it was the only means of identifying collections from mycelial fans, decayed wood or rhizomorphs. However, mating tests are time consuming and interpretation of results may be difficult (Fox, Citation2000). Also, haploid tester strains may degenerate over time, losing their ability to mate in vitro (Guillaumin et al., Citation1991), thereby contributing to ambiguous results. Mating tests are also insufficient for species-level identification in cases where field isolates do not mate with testers of any of the Armillaria species (Blodgett & Worrall, Citation1992; Banik et al., Citation1996), when field isolates mate with testers of two different Armillaria species (Anderson et al., Citation1980; Aguin-Casal et al., Citation2004), or when interspecific hybridization occurs (Kim et al., Citation2001). In addition, nuclear DNA content has been used as a means to separate A. gallica from closely related species (Kim et al., Citation2000).
Molecular methods that rely on PCR amplification of various regions of the rDNA offer greater resolution for Armillaria species identification, in addition to more rapid results (i.e. days compared to the two months required for a mating test), but intraspecific heterotype variation in these regions sometimes confounds identification attempts (Sicoli et al., Citation2003; Coetzee et al., Citation2005; Schnabel et al., Citation2005 a; Kim et al., Citation2006; Hanna et al., Citation2007). In addition, lack of sufficient sequence variation between certain species (e.g. A. calvescens and A. gallica; Harrington & Wingfield, Citation1995) means that not all Armillaria isolates can be distinguished. Therefore, researchers must utilize multiple approaches to identify Armillaria species, including basidiomes and basidiospore morphology, rDNA sequence comparisons, and mating tests with haploid tester isolates.
The goal of this study was to investigate a natural Armillaria infection of an herbaceous ornamental, daylily. The primary objectives of the investigation were: (i) to provide insight into Armillaria infection of an herbaceous host, and (ii) to characterize the Armillaria species responsible for inciting the infection. Information resulting from this investigation could lead to a greater understanding of Armillaria ecology at the interface of natural and agroecosystems.
Materials and methods
Declining daylily plants were encountered in beds located in a residential backyard in Walhalla, SC, which was surrounded by mature, hardwood forest. Daylily beds and an adjacent section of the surrounding, natural vegetation (combined area c. 30 × 30 m) were surveyed for symptoms and signs of Armillaria in December 2005. Potential hosts in the natural vegetation that were sampled included a declining dogwood (Cornus florida L.) and eight hardwood stumps, which were located 5 m outside of the daylily beds. Daylily crowns and roots, and Armillaria rhizomorphs and basidiomes, were collected from daylily beds. From sections of the daylily crown and the dogwood root, mycelial fans were transferred to malt extract agar (MEA; 3% malt extract, 3% glucose, 1% peptone, 1.5% agar). Rhizomorphs were surface sterilized in 10% chlorine bleach (0.6% sodium hypochlorite) for 1 min and rinsed with sterile water for 1 min. Hyphae from the rhizomorph interior were excised, while viewed under a dissecting microscope at 10×, and transferred to MEA. Five to ten tissue samples per collection were plated. One isolate each from the crown of a symptomatic daylily (designated in our collection as ‘SC.FR.04-DLC’), from rhizomorphs on roots of a symptomatic daylily (SC.FR.04-R), and from a dogwood root (SC.FR.04-DWR) were used for subsequent genetic analyses.
Basidiomes collected from daylily beds were classified using a key to North American Armillaria species (Burdsall & Volk, Citation1993). Basidiospores from two basidiomes were harvested; 10 basidiospores per basidiome were measured. Single-spore isolations were made from one basidiome using the method described previously (Schnabel et al., Citation2005 a). Briefly, basidiome gill sections were attached right side up to Petri dish lids using Vaseline. The lids were rotated four times within a period of 1 h to scatter the spores on the 2% water agar below. Plates were incubated for 2 d at 25 °C, after which hyphae from germinated spores were transferred to fresh MEA plates using a small, diamond-shaped scalpel. A total of seven single-spore cultures (SC.FR.04ss1 through SC.FR.04ss7) were maintained on MEA at 25 °C in the dark. All single-spore cultures were assumed to have originated from individual spores because of their white, fluffy mycelium, which is characteristic of haploid Armillaria cultures (Hintikka, Citation1973).
The rDNA internal transcribed spacer (ITS) of daylily SC.FR.04-DLC, rhizomorph SC.FR.04-R, dogwood SC.FR.04-DRW, and the single-spore isolates was directly amplified from mycelium of two-week-old cultures, with primers ITS1-F (Gardes & Bruns, Citation1993) and ITS4 (White et al., Citation1990). The IGS-I region of the isolate from the daylily crown (SC.FR.04-DLC) was amplified with primers LR12R and O-1 (Anderson & Stasovski, Citation1992). DNA was obtained from each culture by scraping a sterile pipette tip over an approximately 1 cm section of aerial mycelium. For PCR, the pipette tip was then dipped in a 50-μL mixture of: 5 μL Reaction Buffer (Promega, Madison, WI), 200 μM per dNTP (Amersham, Piscataway, NJ), 1 μM per primer (Invitrogen, Carslbad, CA), and 1.25 units Taq DNA Polymerase (Promega, Madison, WI). Cycling parameters for ITS amplification were one cycle of 94 °C for 2 min, followed by 30 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final cycle of 72 °C for 10 min. Cycling parameters for IGS-I amplification were 95 °C for 1 min, followed by 35 cycles of 90 °C for 30 s, 60 °C for 40 s, and 72 °C for 2 min, and a final cycle of 72 °C for 10 min. Reactions were performed in an iCycler Thermalcycler (BioRad Laboratories, Hercules, CA). PCR products were visualized on 1% agarose gels stained with ethidium bromide under UV illumination.
To avoid ambiguities in sequence chromatograms caused by frameshifts, IGS-I amplicons were cloned prior to sequencing with the TOPO TA Cloning Kit (Invitrogen, Carlsbad, CA). Eighteen randomly chosen clones from SC.FR.04-DLC were purified using the boiling mini-plasmid method (Holmes & Quigley, 1981). IGS-I amplicons and ITS amplicons were sequenced at the DNA Sequencing Facility, Clemson University, Clemson, SC. Cloned IGS-I fragments were directly sequenced from purified plasmids with M13 forward and reverse primers. ITS amplicons were purified with the QiaQuick Gel Extraction Kit (Qiagen, Valencia, CA) and sequenced using primers ITS1-F and ITS4.
ITS and IGS-I sequences from our cultures were aligned in SeqMan version 3.57 (DNASTAR Inc., Nevada City, CA) and compared with corresponding sequences from all nine North American Armillaria species and NABS X, using the nucleotide Basic Local Alignment Search Tool (BLAST). Sequences were aligned (MegAlign, DNASTAR Inc., Nevada City, CA) following the Clustal V method (Higgins & Sharp, Citation1989), and unrooted parsimonious trees were generated using DNAPARS and CONSENSE programs of Phylip 3.65 with default parameters. Trees were viewed and edited with TreeView (Page, Citation1996). Submissions to GenBank included the ITS sequence from SC.FR.04-DLC (accession number DQ469802) and IGS-I sequences of five clones from SC.FR.04-DLC (accession numbers DQ469803, DQ469804, DQ469805, DQ469806, DQ469807).
The unknown single-spore isolates, SC.FR.04ss1 through SC.FR.04ss7, were paired individually with haploid tester isolates of A. calvescens (3), A. cepistipes (3), A. gallica (5) and A. sinapina (3), as described previously for haploid–haploid mating tests with Armillaria species (Harrington et al., Citation1992). These four Armillaria species were chosen because their ITS and IGS-I sequences most closely matched those of our unknown Armillaria species. Tester isolates were provided by the Center for Forest Mycology Research (Northern Research Station USDA Forest Service, Madison, WI) and Dr Dianne Peabody (Biology Department, Stonehill College, Easton, MA). Mating tests were conducted on MEA and incubated at 25 °C in the dark. Self-pairings of our unknowns and the tester isolates served as controls. To facilitate identification, pairings were subcultured to MEA after four weeks by taking plugs of mycelium from the zone of interaction between the two haploid isolates and from the colony margins on opposite sides of the pair of agar plugs. After 10 d at 25 °C in the dark, single-spore isolates were considered compatible with a tester isolate if the subculture from the zone of interaction and at least one of the subcultures from the margins were characterized by the brown, flattened and crustose mycelium that is typical of diploid Armillaria isolates (Hintikka, Citation1973). Single-spore isolates were considered incompatible with a tester isolate if the subculture from the zone of interaction and at least one of the subcultures from the margins were characterized by the white, fluffy mycelium that is typical of haploid Armillaria isolates (Hintikka, Citation1973).
Results
More than 35 daylilies from 15 planting beds showed symptoms of impaired vigour and decline. Prior to the survey, approximately 25% of the plants in the beds were lost to Armillaria root rot. Symptomatic daylilies were visibly stunted, relative to apparently healthy plants, and were characterized by chlorotic foliage with scorched margins ( a). Of the infected plants, 10 were excavated and examined for signs of Armillaria infection. The root crowns of plants with symptomatic foliage were necrotic and had a water-soaked appearance. Cross sections through crowns revealed white mycelial fans, characteristic of Armillaria infection ( b). These fans were threaded throughout the necrotized tissues of the crown, instead of appearing in a single layer underlying the bark or epidermis as is typical for mycelial fans on the roots of trees or woody perennials. Rhizomorphs were also found attached to daylily roots ( c). Points of rhizomorph attachment occurred only on the larger roots or tuberous crown. A network of rhizomorphs spanning more than 15 m was found throughout the topsoil between and within the daylily beds ( d).
Fig. 1. Signs and symptoms of Armillaria root rot of daylily. a, Symptomatic plants were characterized by stunted, chlorotic leaves with scorched margins (bar = 25 cm). b, Necrotic crowns riddled with mycelial fans (bar = 2.5 cm). c, Rhizomorphs on the roots of symptomatic plants (bar = 2.5 cm). d, Rhizomorphs in the topsoil (bar = 2.5 cm). e, Armillaria basidiome from daylily beds that contained symptomatic plants (bar = 2.5 cm). f, Basidiospores measuring on average 6.25–7.50 μm × 5 μm (bar = 28 μm).
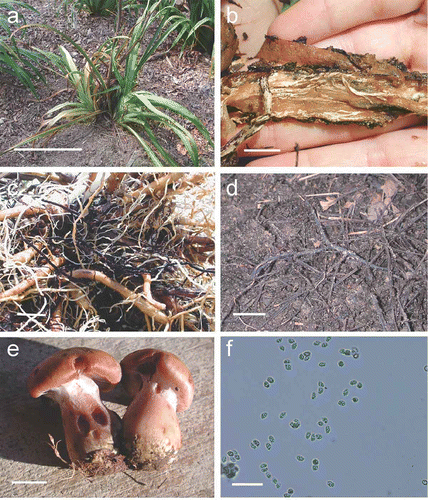
Basidiomes in the daylily beds occurred singly or in small clusters, were directly attached to rhizomorphs, and were near but not attached to plants. Mature basidiomes were characterized by a yellow-brown, scaly pileus; immature basidiomes had an arachnoid annulus and slightly swollen, pinkish-brown stipe base ( e). Using the key to North American Armillaria species (Burdsall & Volk, Citation1993), the basidiomes were identified as A. calvescens or A. gallica, which are further distinguishable only based on basidiospore size. The basidiospore size ranged from 6.25–7.50 μm in length, all with a width of 5 μm (n = 20; f). Basidiospore sizes reported for A. calvescens and A. gallica are 8.5–10 × 5–7 μm and 7.2–9.5 × 4.8–6 μm, respectively (Burdsall & Volk, Citation1993). Both the length and width of spores of the unknown Armillaria species from daylily are within the ranges reported for A. gallica spores, whereas the range of length of A. calvescens spores is higher than that of the spores we measured.
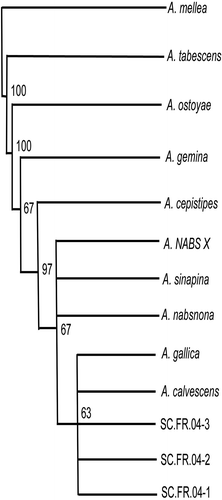
ITS sequences from the daylily crown, rhizomorph, and dogwood isolates (SC.FR.04-DLC, SC.FR.04-R and SC.FR.04-DWR, respectively), and from the seven single-spore isolates (SC.FR.04ss1 through SC.FR.04ss7) were identical to each other (data not shown). Compared to representative sequences of each of the nine North American Armillaria species and NABS X, our isolates were most similar (99% similarity) to those of A. calvescens and A. gallica (data not shown). Unrooted parsimony analysis indicated that our daylily isolates formed a monophyletic group with A. calvescens and A. gallica, supported by a 63% bootstrap value ().
Fig. 2. Parsimony analysis of ITS sequences from a daylily isolate (GenBank accession number DQ469802), nine North American Armillaria species (A. calvescens, A. cepistipes, A. gallica, A. gemina, A. mellea, A. nabsnona, A. ostoyae, A. sinapina, A. tabescens; accession numbers AY213570, AY213561, AY213579, AY213565, AY213572, AY213582, AY213556, AJ250055, AY695409, respectively), and NABS X (AY848938). Numbers at tree branches are bootstrap support values for the branching nodes.
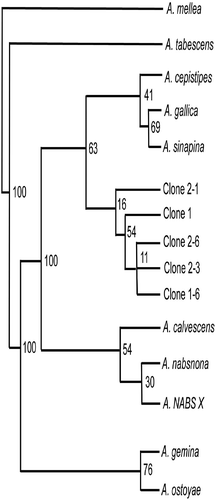
Nucleotide sequence analysis of cloned IGS-I amplicons indicated sequence heterogeneity within IGS-I of an individual Armillaria isolate from daylily (). For example, from five of 12 clones of IGS-I from SC.FR.04-DLC, variation included two insertion/deletions at bp 306 and 860, and 13 nucleotide substitutions at bp 72, 406, 424, 467, 480, 517, 522, 648, 817, 855, 856, 857 and 862 (). Unrooted parsimony analysis revealed that all five IGS-I clones from SC.FR.04-DLC clustered together. Our daylily isolates formed a sister group with A. cepistipes, A. gallica and A. sinapina that clustered apart from A. calvescens, A. nabsnona and NABS X with 100% bootstrap support ().
Fig. 3. Alignment of five, heterogeneous IGS-I sequences cloned from Armillaria isolate (GenBank accession numbers DQ469803, DQ469804, DQ469805, DQ469806, DQ469807) SC.FR.04-DLC. Dots underneath a nucleotide indicate sequence identity among clones. Dashes indicate a missing nucleotide.
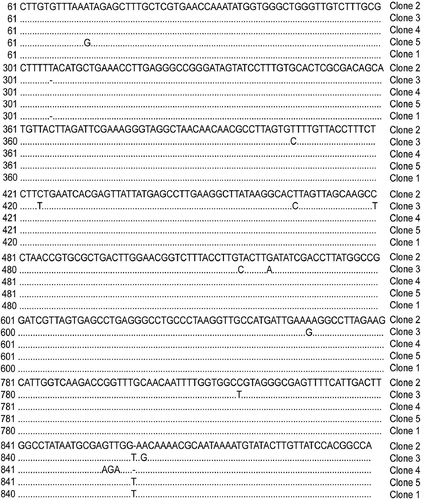
Fig. 4. Parsimony analysis of five, heterogeneous IGS-I sequences from isolate SC.FR.04-DLC (GenBank accession numbers DQ469803, DQ469804, DQ469805, DQ469806, DQ469807), nine North American Armillaria species (A. calvescens, A. cepistipes, A. gallica, A. gemina, A. mellea, A. nabsnona, A. ostoyae, A. sinapina, A. tabescens; accession numbers AY213570, AY213561, AY213579, AY213565, AY213572, AY213582, AY213556, AJ250055, AY695409, respectively) and NABS X (AY848938). Numbers at tree branches are bootstrap support values for the branching nodes.
Mating tests between single-spore isolates and haploid testers of four Armillaria species resulted in compatible reactions (i.e. two of three subcultures displayed the characteristic flat, dark and crustose morphology signifying a successful mating) with three of five A. gallica testers and one of three A. calvescens testers (). Subcultures from pairings with A. cepistipes and A. sinapina, and all of the self-pairings displayed a non-mated, fluffy morphology. In total, four of the seven single-spore isolates mated with A. gallica 442-4, and one of the single-spore isolates also mated with A. gallica MN-3 ss-1 and 01–8-2 s6. Of the four isolates that mated with A. gallica, three also mated with A. calvescens IL-7 ss-4, but with neither of the other A. calvescens testers. Of the seven single-spore isolates, three did not mate with any testers.
Table 1. Subculture morphology of sexual compatibility (mating) tests between single-basidiospore isolates from the unknown Armillaria species infecting daylily and 14 testers of four Armillaria species
Discussion
Prior to this study, investigation of Armillaria root rot of planted hosts in the southeastern USA was restricted to peach (Prunus persica (L.) Batsch), and was originally thought to be caused by A. tabescens and, to a lesser degree, A. mellea (Petersen, Citation1960). A more extensive study confirmed A. tabescens as the primary cause of Armillaria root rot of not only peach, but also ornamentals (Schnabel et al., Citation2005 a). Our finding of identical ITS sequences among isolates from different sources of the pathogen (basidiospores, mycelial fans and rhizomorphs) and different hosts (daylily and dogwood) suggests that a single Armillaria genotype can infect other herbaceous hosts planted near infected hardwood hosts, such as dogwood. This species was neither A. mellea nor A. tabescens. Another unique feature of this case study was the presence of abundant rhizomorph networks spanning the daylily beds. Rhizomorphs have rarely been observed in the southeastern USA, where contact between infected root fragments is thought to be the predominant means of Armillaria spread in peach orchards (Savage et al., Citation1953; Kable, Citation1974).
A consensus of basidiome morphology, basidiospore size, phylogenetic analyses of two rDNA regions, and the results of mating tests with haploid tester isolates of known North American Armillaria species suggest that A. gallica is most likely the cause of Armillaria root rot on daylily. Analyses of IGS-I revealed similarity between our daylily isolates and three Armillaria species (A. cepistipes, A. gallica and A. sinapina). Basidiocarp morphology and parsimony analysis of ITS narrowed the identity to A. calvescens or A. gallica. Identity was further narrowed to A. gallica based on basidiospore morphology (6.25–7.50 × 5 μm), which is more similar to what is reported for A. gallica (7.2–9.5 × 4.8–6 μm) than for A. calvescens (8.5–10 × 5–7 μm; http://botit.botany.wisc.edu/toms_fungi/armkey.html). Armillaria gallica identity was also confirmed, in part, by the apparent mating of our unknown single-spore isolates in mating tests with A. gallica haploid testers.
The mating tests were not 100% conclusive, however, in that three single-spore isolates from daylily were compatible with testers of both A. gallica and A. calvescens. Armillaria calvescens and A. gallica are thought to be closely related, as evidenced by comparisons of IGS-I (Anderson & Stasovski, Citation1992), RFLPs of mtDNA (Smith & Anderson, Citation1989), anonymous nucleotide sequences (Piercey-Normore et al., Citation1998), and AFLPs (Kim et al., Citation2006), which group these two species together and apart from other Armillaria species. In fact, the most widely used diagnostic technique for Armillaria, which is based on AluI restriction patterns of IGS-I (Harrington & Wingfield, Citation1995), does not distinguish A. calvescens from North American A. gallica. Previous attempts to identify field isolates from the northeastern USA using this technique do not resolve identifications of A. calvescens or A. gallica (Frontz et al., Citation1998). Further complication in distinguishing A. calvescens from A. gallica comes from the fact that they also share overlapping ranges in eastern North America. Armillaria calvescens has been reported from southeastern Canada to Pennsylvania (Anderson & Ullrich, Citation1979; Banik et al., Citation1995) and A. gallica from southeastern Canada to Virginia (Anderson & Ullrich, Citation1979; Dumas, Citation1988; Blodgett & Worrall, Citation1992; Banik et al., Citation1995; Frontz et al., Citation1998). Another possible explanation for the compatibility between our haploid field isolates and testers of both A. calvescens and A. gallica is based on interspecific hybridization (Kim et al., Citation2001).
Another explanation for compatible matings between our daylily isolates and testers of both A. gallica and A. calvescens is that these closely related species (Anderson & Stasovski, Citation1992) are partially interfertile, a phenomenon also documented for other North American Armillaria species (Anderson et al., Citation1980; Morrison et al., Citation1985). Based on previous reports of interfertility between haploid testers of known species (Anderson & Stasovski, Citation1992), coupled with the fact that field isolates have sometimes been shown to be compatible with more than one Armillaria species (Anderson et al., Citation1980; Aguin-Casal et al., Citation2004), it seems that our finding of compatibility between our haploid field isolates and testers of both A. calvescens and A. gallica is not out of the ordinary. Researchers who previously encountered interfertility relied, in part, on the percentage of compatible matings as the best possible means of making a decision on species identity (Banik & Burdsall, Citation1998). The fact that haploid isolates of both A. calvescens and A. gallica tend to be less fluffy than those of other Armillaria species makes it more difficult to rate matings with these two species than with other Armillaria species (Korhonen, Citation1978). Therefore, it is possible that our subculture ratings were inaccurate.
Phylogenetic analyses of IGS-I indicated no close relationship between our daylily isolates and other North American Armillaria species. The closest matches were A. cepistipes, A. gallica and A. sinapina, but with weak bootstrap support. ITS provided slightly higher resolution, but narrowed the identity of the daylily isolates to A. calvescens and A. gallica. The IGS-I and ITS may not be reliable for distinguishing A. calvescens, A. cepistipes, A. gallica and A. sinapina (Kim et al., Citation2006). It is likely that the high diversity in IGS-I among our isolates contributed to the lack of resolution. IGS regions are part of the tandemly repeated rDNA multigene family (Long & Dawid, Citation1980). Although intraspecies heterogeneity in IGS of most eukaryotes is reportedly low (Elder & Turner, Citation1995), heterogeneity has been reported in IGS-I, in addition to ITS, in multiple Armillaria species (Long & Dawid Citation1980; Elder & Turner, Citation1995; Bryson et al., Citation2003; Coetzee et al., Citation2005) and in other fungi (Morton et al., Citation1995). Our finding of heterogeneity in IGS-I in five of twelve cloned amplicons from a single daylily isolate support these reports. The fact that IGS-I exhibits greater variability than ITS and is heterogeneous in some Armillaria species makes this region more cumbersome for species-level identification.
The presence of rhizomorphs in the topsoil of daylily beds and on the roots of symptomatic daylilies, coupled with our finding of identical ITS sequences among daylily isolates and the dogwood isolate, suggest that rhizomorphs of A. gallica spread from resident hosts to the daylilies. Armillaria species grow vegetatively from a single point of origin to colonize adjacent root systems (Kile, Citation1983; Rizzo & Harrington, Citation1993; Morton et al., Citation1995; Rizzo et al., Citation1998; Baumgartner & Rizzo, Citation2001; Coetzee et al., Citation2001; Sicoli et al., Citation2003). Indeed, single A. gallica genotypes (individuals) have been found to cover sections of forest greater than 15 ha (Smith et al., Citation1992). It is possible that logging of forest trees fuelled the expansion of A. gallica at our study site. Armillaria gallica is reportedly a pathogen of weakened forest trees in North America (Kile et al., Citation1991; Baumgartner & Rizzo, Citation2001) and Europe (Rishbeth, Citation1985; Guillaumin et al., Citation1989). Based on the well documented relationship between logging operations and Armillaria root rot (Kile et al., Citation1991), it is possible that the increase in woody host substrate made available by logging allowed A. gallica to overtake healthy daylilies at our study site, despite its rare occurrence on herbaceous hosts.
The eastern North American range of A. gallica was previously reported to extend from southeastern Canada to Virginia (Anderson & Ullrich, Citation1979; Rishbeth, Citation1985; Dumas, Citation1988; Kile et al., Citation1991; Blodgett & Worrall, Citation1992; Banik et al., Citation1995). Our finding of A. gallica in South Carolina further extends the southeastern distribution of this species. Based on reports of A. gallica from western North America (Morrison et al., Citation1985; Volk et al., Citation1996), the western North American range of A. gallica is relatively continuous from British Columbia to Baja California, Mexico.
Armillaria infection of daylily has not been previously investigated, but is consistent with reports of other herbaceous hosts, most of which have thick, fleshy roots. For example, mycelial fans and rhizomorphs of A. ostoyae have been found on rhizomes of Epilobium angustifolium L. (Klein-Gebbinck et al., Citation1991). Several Armillaria species including A. gallica have been identified from achlorophyllous, mycoheterotrophic orchids (Ota et al., Citation1998 b; Cha & Igarashi, Citation1995). Armillaria is thought to serve as the mycobiont in a complex symbiotic relationship in which the Armillaria mycelium is consumed by the orchid (Kusano, Citation1911). All other reports of herbaceous infection describe a typical, pathogenic symbiosis on herbaceous hosts from the field (Thomas, Citation1934; Raabe, Citation1958; Shaw III et al., Citation1976; Gregory, Citation1985; Klein-Gebbinck et al., Citation1991; Mwenje et al., Citation1998; Raziq & Fox, Citation2005). It is important to note that herbaceous hosts infected with Armillaria in the field, including our findings on daylily, are always proximal to woody substrate (Klein-Gebbinck et al., Citation1991), suggesting that woody hosts serve as the source of inoculum. It is, therefore, likely that a woody substrate is needed for sufficient nutrition or for persistence of the pathogen from one growing season to the next.
Acknowledgements
We thank S.E. Bergemann (University of California, Berkeley) and M.E. Smith (University of California, Davis) for comments on this manuscript.
Notes
†Technical contribution number 5250 of the Clemson University Experiment Station. This material is based upon work supported by USDA-CSREES under project number SC-1000642.
References
- Aguin-Casal , O. , Sainz-Oses , M.J. and Mansilla-Wazquez , J.P. 2004 . Armillaria species infesting vineyards in northwestern Spain . Eur. J. Plant Pathol. , 110 : 683 – 687 .
- Anderson , J.B. , Korhonen , K. and Ullrich , R.C. 1980 . Relationships between European and North-American biological species of Armillaria mellea . Exp. Mycol. , 4 : 87 – 95 .
- Anderson , J.B. and Stasovski , E. 1992 . Molecular phylogeny of northern hemisphere species of Armillaria . Mycologia , 84 : 505 – 516 .
- Anderson , J.B. and Ullrich , R.C. 1979 . Biological species of Armillaria mellea in North America . Mycologia , 71 : 402 – 414 .
- Antonin , V. , Jankovsky , L. , Lochman , J. and Tomsovsky , M. 2006 . Armillaria socialis – morphological-anatomical and ecological characteristics, pathology, distribution in the Czech Republic and Europe and remarks on its genetic variation . Czech Mycol. , 58 : 209 – 224 .
- Banik , M.T. and Burdsall , H.H. 1998 . Assessment of compatibility among Armillaria cepistipes, A. sinapina, and North American biological species X and XI, using culture morphology and molecular biology . Mycologia , 90 : 798 – 805 .
- Banik , M.T. , Paul , J.A. and Burdsall , H.H. 1995 . Identification of Armillaria species from Wisconsin and adjacent areas . Mycologia , 87 : 707 – 712 .
- Banik , M.T. , Volk , T.J. and Burdsall , H.H. 1996 . Armillaria species of the Olympic Peninsula of Washington state, including confirmation of North American biological species XI . Mycologia , 88 : 492 – 496 .
- Baumgartner , K. and Rizzo , D.M. 2001 . Distribution of Armillaria species in California . Mycologia , 93 : 821 – 830 .
- Bergemann , S.E. and Garbelotto , M. 2006 . High diversity of fungi recovered from the roots of mature tan oak (Lithocarpus densiflorus) in northern California . Can. J. Bot. , 84 : 1380 – 1394 .
- Blodgett , J.T. and Worrall , J.J. 1992 . Distributions and hosts of Armillaria species in New York . Plant Dis. , 76 : 166 – 170 .
- Bryson , P.K. , Simpson , C.R. and Schnabel , G. 2003 . Nucleotide sequence analysis of the ribosomal 5.8S and the internal transcribed spacer regions 1 and 2 from North American Armillaria species . Phytopathology , 93 : S12
- Burdsall , H.H. and Volk , T.J. 1993 . The state of taxonomy of the genus Armillaria . McIlvainea , 11 : 4 – 11 .
- Burdsall , H.H. and Volk , T.J. 2008 . Armillaria solidipes, an older name for the fungus called Armillaria ostoyae . North Am. Fungi , 3 : 261 – 267 .
- Cha , J.Y. and Igarashi , T. 1995 . Armillaria species associated with Gastrodia elata in Japan . Eur. J. Forest Pathol. , 25 : 319 – 326 .
- Coetzee , M.P.A. , Wingfield , B.D. , Harrington , T.C. , Steimel , J. , Coutinho , T.A. and Wingfield , M.J. 2001 . The root rot fungus Armillaria mellea introduced into South Africa by early Dutch settlers . Mol. Ecol. , 54 : 36 – 45 .
- Coetzee , M.P.A. , Wingfield , B.D. , Kirisits , T. , Chhetri , B.D. , Bloomer , P. and Wingfield , M.J. 2005 . Identification of Armillaria isolates from Bhutan based on DNA sequence comparisons . Plant Pathol. , 54 : 36 – 45 .
- Dumas , M.T. 1988 . Biological species of Armillaria in the mixed wood forest of northern Ontario . Can. J. Forest Res. , 18 : 872 – 874 .
- Elder , J.F.J. and Turner , B.J. 1995 . Concerted evolution of repetitive DNA sequences in eukaryotes . Quart. Rev. Biol. , 70 : 297 – 230 .
- Fox , R.T.V. 2000 . “ Biology and life cycle ” . In Armillaria Root Rot: Biology and Control of Honey Fungus , Edited by: Fox , R.T.V. 3 – 44 . Andover : Intercept Limited .
- Frontz , T.M. , Davis , D.D. , Bunyard , B.A. and Royse , D.J. 1998 . Identification of Armillaria species isolated from bigtooth aspen based on rDNA RFLP analysis . Can. J. For. Res. , 28 : 141 – 149 .
- Gardes , M. and Bruns , T.D. 1993 . ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rust . Mol. Ecol. , 2 : 113 – 118 .
- Gregory , S.C. 1985 . The use of potato tubers in pathogenicity studies of Armillaria isolates . Plant Pathol. , 34 : 41 – 48 .
- Gregory , S.T. , Rishbeth , J. and Shaw , C.G. III . 1991 . “ Pathogenicity and virulence ” . In Armillaria ROOT DISEASE. Agriculture Handbook No. 691 , Edited by: Shaw , C.G and Kile , G.A. 76 – 87 . Washington, DC : United States Department of Agriculture Forest Service .
- Guillaumin , J.J. , Anderson , J.B. and Korhonen , K. 1991 . “ Life cycle, interfertility and biological species ” . In Armillaria Root Disease. Agriculture Handbook No. 691 , Edited by: Shaw , C.G and Kile , G.A. 10 – 20 . Washington, DC : United States Department of Agriculture Forest Service .
- Guillaumin , J.J. , Mohammed , C. and Berthelay , S. Armillaria species in the northern temperate hemisphere. In D. Morrison (ed.) . Seventh International Conference on Root & Butt Rots . Victoria, British Columbia. pp. 27 – 43 .
- Hanna , J.W. , Klopfenstein , N.B. , Kim , M.S. , McDonald , G.I. and Moore , J.A. 2007 . Phylogeographic patterns of Armillaria ostoyae in the western United States . For. Pathol. , 37 : 192 – 216 .
- Harrington , T.C. and Wingfield , B.D. 1995 . A PCR based identification method for species of Armillaria . Mycologia , 87 : 280 – 288 .
- Harrington , T.C. , Worrall , J.J. and Baker , F.A. 1992 . “ Armillaria ” . In Methods for Research on Soilborne Phytopathogenic Fungi , Edited by: Singleton , L.L. , Mihail , J.D. and Rush , C.M. 81 – 85 . St. Paul, MN : American Phytopathological Society .
- Higgins , D.G. and Sharp , P.M. 1989 . Fast and sensitive multiple sequence alignments on a microcomputer . Comp. Appl. Biosci. , 2 : 151 – 153 .
- Hintikka , V. 1973 . A note on the polarity of Armillariella mellea . Karstenia , 13 : 32 – 39 .
- Hood , D.S. and Quigley , M. 1981 . A rapid boiling method for the preparation of bacterial plasmids . Anal. Biochem. , 114 : 193 – 197 .
- Hood , I.A. , Redfern , D.B. and Kile , G.A. 1991 . “ Armillaria in planted hosts ” . In Armillaria Root Disease. Agriculture Handbook No. 691 , Edited by: Shaw , C.G. and Kile , G.A. 122 – 149 . Washington, DC : United States Department of Agriculture Forest Service .
- Kable , P.F. 1974 . Spread of Armillariella sp. in a peach orchard . Trans. Brit. Mycol. Soc. , 62 : 89 – 98 .
- Kile , G.A. 1983 . Identification of genotypes and the clonal development of Armillaria luteobubalina Watling and Kile in eucalypt forests . Aust. J. Bot. , 31 : 657 – 671 .
- Kile , G.A. , McDonald , G.I. and Byler , J.W. 1991 . “ Ecology and disease in natural forests ” . In Armillaria Root Disease. Agriculture Handbook No. 691 , Edited by: Shaw , C.G. and Kile , G.A. 102 – 121 . Washington, DC : United States Department of Agriculture Forest Service .
- Kim , M.S. , Klopfenstein , N.B. , Hanna , J.W. and McDonald , G.I. 2006 . Characterization of North American Armillaria species: genetic relationships determined by ribosomal DNA sequences and AFLP markers . For. Pathol. , 36 : 145 – 164 .
- Kim , M.S. , Klopfenstein , N.B. , McDonald , G.I. , Arumuganathan , K. and Vidaver , L.K. 2000 . Characterization of North American Armillaria species by nuclear DNA content and RFLP analysis . Mycologia , 92 : 874 – 883 .
- Kim , M.S. , Klopfenstein , N.B. , McDonald , G.I. , Arumuganathan , K. and Vidaver , A.K. 2001 . Use of flow cytometry, fluorescence microscopy, and PCR-based techniques to assess intraspecific and interspecific matings of Armillaria species . Mycol. Res. , 105 : 153 – 163 .
- Klein-Gebbinck , H.W. , Blenis , P.V. and Hiratsuka , Y. 1991 . Spread of Armillaria ostoyae in juvenile lodgepole pine stands in West Central Alberta . Can. J. For. Res. , 21 : 20 – 24 .
- Korhonen , K. 1978 . Interfertility and clonal size in the Armillaria mellea complex . Karstenia , 18 : 31 – 42 .
- Kusano , S. 1911 . Gastrodia elata and its symbiotic association with Armillaria mellea . J. College Agric. , 4 : 1 – 65 .
- Larsen , M.J. , Banik , M.T. and Burdsall , H.H. Jr. 1992 . Clamp connections in North American Armillaria species: occurrence and potential application for delimiting species . Mycologia , 84 : 274 – 218 .
- Long , E.O. and Dawid , I.B. 1980 . Repeated genes in eukaryotes . Annu. Rev. Biochem. , 49 : 727 – 764 .
- Morrison , D.J. , Chu , D. and Johnson , A.L.S. 1985 . Species of Armillaria in British Columbia . Can. J. Plant Pathol. , 7 : 242 – 246 .
- Morton , A. , Tabrett , A. , Carder , J. and Barbara , D. 1995 . Sub-repeat sequences in the ribosomal RNA intergenic regions of Verticillium albo-atrum and V. dahliae . Mycol. Res. , 99 : 257 – 266 .
- Motta , J. and Korhonen , K. 1986 . A note on Armillaria mellea and Armillaria bulbosa from the middle Atlantic states . Mycologia , 78 : 471 – 474 .
- Mwenje , E. , Ride , J.P. and Pearce , R.B. 1998 . Distribution of Zimbabwean Armillaria groups and their pathogenicity on cassava . Plant Pathol. , 47 : 623 – 634 .
- Ota , Y. , Fukuda , K. and Suzuki , K. 1998a . The nonheterothallic life cycle of Japanese Armillaria mellea . Mycologia , 90 : 396 – 405 .
- Ota , Y. , Matsushita , N. , Nagasawa , E. , Terashita , T. , Fukuda , K. and Suzuki , K. 1998b . Biological species of Armillaria in Japan . Plant Dis. , 82 : 537 – 543 .
- Page , R.D.M. 1996 . TREEVIEW: an application to display phylogenetic trees on personal computers . Comp. Appl. Biosci. , 12 : 357 – 358 .
- Petersen , D.H. 1960 . Hymenomycetous fungi except Polyporus associated with wood decay of living peach trees in South Carolina . Plant Dis. Rep. , 44 : 891 – 893 .
- Piercey-Normore , M.D. , Egger , K.N. and Berube , J.A. 1998 . Molecular phylogeny and evolutionary divergence of North American biological species of Armillaria . Mol. Phylogenet. Evol. , 10 : 49 – 66 .
- Raabe , R.D. 1958 . Some previously unreported non-woody hosts of Armillaria mellea in California . Plant Dis. Rep. , 42 : 1025
- Raabe , R.D. 1962 . Host list of the root rot fungus Armillaria mellea . Hilgardia , 33 : 25 – 88 .
- Raziq , F. and Fox , R.T.V. 2005 . Combinations of fungal antagonists for biological control of Armillaria root rot of strawberry plants . Biol. Agric. Hortic. , 23 : 45 – 57 .
- Redfern , D.B. and Filip , G.M. 1990 . “ Inoculum and infection ” . In Armillana Root Disease. Agriculture Handbook No. 691 , Edited by: Shaw , C.G. III and Kile , G.A. 48 – 61 . Washington, DC : United States Department of Agriculture Forest Service .
- Rishbeth , J. 1985 . Infection cycle of Armillaria and host response . Eur. J. For. Pathol. , 15 : 332 – 341 .
- Rizzo , D.M. and Harrington , T.C. 1993 . Delineation and biology of clones of Armillaria ostoyae, A. gemina and A. calvescens . Mycologia , 85 : 164 – 174 .
- Rizzo , D.M. , Whiting , E.C. and Elkins , R.B. 1998 . Spatial distribution of Armillaria mellea in pear orchards . Plant Dis. , 82 : 1226 – 1231 .
- Savage , E.F. , Weinberger , J.H. , Luttrell , E.S. and Rhoads , A.S. 1953 . Clitocybe root rot – a disease of economic importance in Georgia peach orchards . Plant Dis. Rep. , 37 : 269 – 270 .
- Schnabel , G. , Ash , J.S. and Bryson , K.P. 2005a . Identification and characterization of Armillaria tabescens from the southeastern United States . Mycol. Res. , 109 : 1208 – 1222 .
- Schnabel , G. , Bussey , K.E. and Bryson , P.K. 2005b . First report of Armillaria gallica causing Armillaria root rot in daylily in South Carolina . Plant Dis. , 89 : 683
- Shaw III , C.G. , Sijnja , D. and MacKenzie , M. 1976 . Toetoe (Cortaderia fulvida): a new graminaceous host for Armillaria root rot . N. Z. J. For. , 21 : 265 – 268 .
- Sicoli , G. , Fatehi , J. and Stenlid , J. 2003 . Development of species-specific PCR primers on rDNA for the identification of European Armillaria species . For. Pathol. , 33 : 287 – 297 .
- Smith , M.L. and Anderson , J.B. 1989 . Restriction fragment length polymorphisms in mitochondrial DNAs of Armillaria: identification of North American biological species . Mycol. Res. , 93 : 247 – 256 .
- Smith , M.L. , Bruhn , J.N. and Anderson , J.B. 1992 . The fungus Armillaria bulbosa is among the largest and oldest living organisms . Nature , 356 : 428 – 431 .
- Thomas , H.E. 1934 . Studies on Armillaria mellea (Vahl) Quel., infection parasitism and host resistance . J. Agr. Res. , 48 : 187 – 218 .
- Thormann , M.N. , Myrholm , C.L. and Mallett , K.I. 2001 . Armillaria sinapina in herbaceous plant material from a peatland in Alberta, Canada . Can. J. Bot. , 79 : 643 – 647 .
- Volk , T.J. and Burdsall , H.H. 1995 . Nomenclatural study of Armillaria and Armillariella species . Fungiflora , 8 : 121
- Volk , T.J. , Burdsall , H.H. and Banik , M.T. 1996 . Armillaria nabsnona, a new species from western North America . Mycologia , 88 : 484 – 491 .
- White , T.J. , Bruns , T. , Lee , S.W. and Taylor , G. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: A Guide to Methods and Applications , Edited by: White , T.J. , Innis , M. Gelfand , D.H. 315 – 324 . San Diego, CA : Academic Press .