Abstract
Mycosphaerella blight (Mycosphaerella pinodes) is the most important disease of field pea (Pisum sativum) in western Canada, occurring in almost every field each year. The objective of the study was to assess the impact of seed infection on epidemics of mycosphaerella blight. In preparation for the trial, seed lots of a susceptible and partially resistant cultivar with different levels of seed infection with M. pinodes were produced at a single site with uniform handling and storage conditions. Field trials were conducted in 2005 and 2006 at three sites in western Canada where levels of external inoculum were high (Vegreville, AB, Saskatoon, SK, Morden, MB), and one site (Bradford, ON) with little external inoculum. For each cultivar, the seed infection treatments were: (i) high seed infection (26–47% of seed infected with M. pinodes); (ii) intermediate seed infection (6–15% of seed infected); (iii) fungicide – intermediate seed lot treated with a fungicide to minimize seed transmission; and (iv) low seed infection (0–2% of seed infected). Seedling establishment was higher in the low seed infection treatment than the other treatments in four of eight station years, but there were no consistent differences among the other treatments. There were small differences in blight severity among treatments in several station years, but no differences were observed at Bradford, the site with minimal external inoculum. We conclude that seed infection with M. pinodes does not contribute substantially to above-ground symptoms in the year of planting. However, very high levels of seed infection reduced seedling establishment, so continued seed testing for germination is recommended.
Résumé
L'anthracnose (Mycosphaerella pinodes) est la plus importante maladie du pois des champs (Pisum sativum) dans l'Ouest canadien, apparaissant dans presque tous les champs chaque année. Le but de l'étude était d'évaluer les effets de l'infection des semences sur les épidémies d'anthracnose. Pour préparer l'essai, des lots de semences de cultivars réceptifs et partiellement résistants, infectés à divers degrés par M. pinodes, ont été produits sur un site unique, dans des conditions uniformes de manipulation et d'entreposage. Les essais en champs ont été menés en 2005 et 2006 à trois sites de l'Ouest canadien où les quantités d'inoculum externe étaient élevées (Vegreville, AB; Saskatoon, SK; Morden, MB) et à un site où le taux d'inoculum externe était faible (Bradford, ON). Pour chaque cultivar, les traitements de l'infection des semences étaient: (i) degré élevé d'infection (26 à 47 % des semences infectées par M. pinodes); (ii) degré modéré d'infection (6 à 15 % de semences infectées); (iii) fongicide — lot infecté modérément, traité avec un fongicide pour minimiser la transmission par la semence; et (iv) faible degré d'infection (0 à 2 % de semences infectées). Durant quatre années-stations sur huit, le taux d'établissement des semis a été plus élevé chez les semences affichant un faible degré d'infection que chez les autres traitements, mais il n'y avait pas de différences uniformes. Il y avait de faibles différences quant à la gravité de l'anthracnose parmi les traitements au cours des diverses années-stations, mais aucune différence n'a été observée à Bradford, le site qui affichait le moins d'inoculum externe. Nous concluons que l'infection des semences par M. pinodes ne contribue pas fortement aux symptômes apparaissant au-dessus du sol durant l'année de la mise en terre. Toutefois, des degrés très élevés d'infection des semences ont réduit l'établissement des semis, c'est pourquoi nous recommandons de poursuivre les tests de germination.
Introduction
Mycosphaerella blight, caused primarily by Mycosphaerella pinodes (Berk. & Blox.) Vestergr., is an important constraint to the production of field pea (Pisum sativum L.) in western Canada. Mycosphaerella blight is present in almost all pea fields each year (Xue, Citation2003) and yield losses caused by disease can be high (Wang et al., Citation2006). In contrast, most seed lots produced in western Canada have little or no visible discolouration or shrivelling associated with mycosphaerella blight and less that 5% infection with M. pinodes (Morrall et al., Citation2006, Citation2007). Management recommendations for mycosphaerella blight focus on crop rotation and use of quality seed with high germination and low levels of seed infection because no strong sources of resistance to blight have been identified (Zhang et al., Citation2006, Citation2007; Zhang & Gossen, Citation2007) and the impact of fungicide application on seed yield is inconsistent (Gossen et al., Citation2001, Citation2008).
Genetic resistance to seed infection by pathogens in the mycosphaerella blight complex has been identified (Skolko et al., Citation1954), and has been put forward as a potential way to reduce the impact of blight by reducing levels of seed-borne inoculum (Xue et al., Citation1996). In addition to a role in foliar disease epidemics, seed infection with M. pinodes can reduce seedling establishment (Hwang et al., Citation1991; Xue et al., Citation1996; Moussart et al., Citation1998). However, pea crops can compensate for substantial losses in seedling density, and large reductions in stand density are required to affect yield (Hwang et al., Citation2001, Citation2006a , Citation2007).
Our hypothesis was that the frequency and impact of seed-to-seedling transmission of M. pinodes on subsequent epidemics of mycosphaerella blight in western Canada is low, based on two lines of reasoning. First, lesions of M. pinodes on seedlings from infected seed develop initially on the hypocotyl and base of the epicotyl, where they rapidly girdle and kill seedlings (Maude, Citation1966). This pattern of development has been interpreted as evidence that seed infection has a major impact on development of blight epidemics (Skolko et al., Citation1954; Maude, Citation1966; Xue et al., Citation1996). However, rapid death of pea seedlings frequently traps the pathogen below the soil surface, limiting opportunities for splash dispersal of asexual conidia (Moussart et al., Citation1998). The above-ground portions of dead seedlings might subsequently be colonized by the pathogen and become a source of inoculum, but that process takes time and occurs infrequently (e.g. in only 5–10% of infected seedlings) (Maude, Citation1966). As a result, we expect the impact of this process on subsequent epidemics to be low. The second line of reasoning is that air-borne ascospores originating from infected pea crop residue are likely an important source of primary inoculum in the region. The pathogen survives for (at most) only a few cropping seasons in infected stubble (Sheridan, Citation1973; Zhang et al., Citation2005a), so relatively short rotations should reduce or remove the pathogen from the field. However, blight symptoms occur in every field each year with little or no difference associated with crop rotation or tillage history (Gossen, unpublished; Bailey et al., Citation2001).
The impact of seed-borne inoculum of M. pinodes on subsequent blight development and seed yield has been examined previously (Hwang et al., Citation1991; Bretag et al., Citation1995; Moussart et al., Citation1998). These studies have failed to show a significant relationship between level of seed infection and severity of mycosphaerella blight. Unfortunately, high levels of background inoculum and a small number of sites per trial did not allow for separation of mycosphaerella blight disease resulting from background (overwintering) inoculum within and adjacent to the study area from disease resulting directly from seed infection. Also, these studies were compromised by use of seed lots from several locations, which potentially confounded the impact of seed infection with the environmental and agronomic conditions during seed production and storage of each seed lot. Seed harvest, storage and handling conditions can cause dramatic reductions in seedling vigour and establishment without having a measurable impact on germination in standard germination tests (Duczmal & Minicka, Citation1989; Hwang et al., Citation2001).
To examine our hypothesis concerning the impact of seed infection on subsequent epidemics of mycosphaerella blight, seed lots of a susceptible and partially resistant cultivar were produced at a single site. They were managed in the same way (seeding date, weed control, harvest date and equipment, storage), so that they differed only in the level of seed infection. These seed lots were then used in studies conducted across a wide range of environments to assess the impact of seed infection on blight severity and yield. Sites were selected to represent locations where overwintering inoculum was expected to be abundant and where it was absent.
Materials and methods
Production of seed lots
In preparation for the trial, seed of two field pea cultivars was produced at Saskatoon in 2003 – ‘Keoma’ is highly susceptible to M. pinodes and ‘Carneval’ is partially resistant (Xue, Citation2003; Hwang et al., Citation2006b ). For each cultivar, there were two 20 m × 24 m plots in each of four replicates; one plot was inoculated with pea crop residue heavily infected with M. pinodes and the other was protected with a single foliar application of chlorothalonil fungicide (Bravo 500 FL at 1 kg a.i. ha−1, Syngenta Crop Protection Canada Inc., Guelph, ON) at early flower. The intent was to develop seed lots that had been produced under identical conditions, with uniform seed source, dates of seeding and harvest, environmental and storage conditions, but that differed as much as possible in incidence of infection with M. pinodes.
The proportion of seed infected with M. pinodes was assessed on two replicates per seed lot. One hundred seeds per replicate plot were surface sterilized for 2 min in 0.6% NaOCl, air-dried, and placed (five seeds/dish) on 9 cm diameter Petri dishes of commercial potato dextrose agar plus streptomycin (Fisher Scientific, Edmonton, AB) at 0.1 mg mL−1. Dishes were incubated on a laboratory benchtop at about 22 °C and ambient light for seven days, and then the incidence of M. pinodes was counted.
Dry conditions during pod set and seed maturation severely restricted transmission of M. pinodes to seed in 2003. As a result, this preliminary test was repeated in 2004. Conditions late in the growing season of 2004 were cool and wet, which is highly conducive to the development of epidemics of mycosphaerella blight.
Main trial
The seed lots produced in 2003 and 2004 were utilized in the main study, which was conducted at four sites in 2005 and 2006. These sites were selected to provide the widest possible range in environment, climate and pathogen population. At three of the four sites, Vegreville, AB, Saskatoon, SK, and Morden, MB, air-borne ascospores of M. pinodes was known to be present in most years due to a long history of production of field pea crops. At Bradford, ON, inoculum was expected to be absent or occur at very low levels because there was no history of commercial field pea production in the area. The experimental design was a factorial randomized complete block with four replications. The intent was to assess as wide a range of seed infection levels as possible. The treatments were seed lots of ‘Keoma’ and ‘Carneval’ produced at Saskatoon. Four seed infection treatments were examined: (i) heavily infected – seed from the inoculated blocks in 2004, with 47% seed infection with M. pinodes in ‘Keoma’ and 26% infection in ‘Carneval’; (ii) intermediate infection – seed from the fungicide-treated blocks in 2004, with 15% seed infection with M. pinodes in ‘Keoma’ and 6% infection in ‘Carneval’ (note that these rates of seed infection, although substantially lower than the levels in the heavily infected treatment, are higher than in most lots produced in the region in most years); (iii) intermediate seed treated with carbathiin + thiabendazole (Crown® at 6 mL kg−1 of seed, a.i. carbathiin (9.2%) + thiabendazole (5.8%), Chemtura Co./Cie, Elmira, ON); and (iv) low infection – seed from 2003, with 0% seed infection with M. pinodes in ‘Keoma’ and 2% infection in ‘Carneval’. Seed from 2003 was used as a healthy control for seed-to-seedling transmission of the pathogen. However, it does not represent a true control for seedling establishment because it was produced under different conditions than the seed lots from 2004. Crown® fungicide was chosen because it is registered in Canada to reduce seed transmission of similar pathogens on other pulse crops, e.g. Ascochyta lentis on lentil (Morrall, Citation1997) and A. rabiei on chickpea (Chang et al., Citation1997; Ahmed et al., Citation2006). Crown® also has some activity against root rot pathogens common in western Canada.
Trials at Saskatoon were conducted on a heavy clay-loam soil at the AAFC Research Farm each year. Trials at Vegreville were conducted on a sandy-loam soil at the Alberta Research Council Farm. Trials at Morden were conducted at the AAFC Research Station on a clay-loam soil. Trials at Bradford were conducted on a sandy-loam mineral soil near the Muck Crops Research Station of the University of Guelph. Each of these fields had at least two years of non-host crops since the last field pea crop, and so was expected to be almost completely free of inoculum from crop residue present within the plot area (Zhang et al., Citation2005b ). Each plot consisted of four 6 m long rows (5 m at Morden) seeded at 85 seed m−2 using a double-disc drill seeder, except at Bradford where plots were seeded at a higher rate using a push seeder. Spacing between rows was 0.3 m at Saskatoon and Bradford, and 0.2 m at Vegreville and Morden. Spacing between plots was 1.2 m (0.6 at Morden). In 2005, the site at Morden was abandoned due to extended flooding just after seedling emergence
The basic outline of the study was the same for all sites. Seedling establishment, rated as counts of the number of seedlings in two adjacent 1 m long lengths of row at two sites per plot, was assessed about three weeks after planting. Mycosphaerella blight severity on foliage (0–9 scale, Xue et al., Citation1996) was assessed on 10 plants per plot at 10–20 day intervals, starting at early flowering. Severity on stems was also assessed during pod filling in three station-years using a 0–9 rating scale (Wang et al., Citation2006) to assess 10 plants per plot. Lodging was assessed prior to crop maturity on a 0–9 scale (Wang et al., Citation2006) in four station-years. When the seed was mature, the plots were harvested with a combine harvester, except at Bradford where 1 m2 areas were picked by hand, and the seed was cleaned, air-dried and weighed. The dates of critical operations for each station-year are summarized in .
Table 1. Dates of important operations at the four sites in 2005 and 2006
In 2006, a second site (Site 2) was established at Morden to replace the trial lost to flooding in 2005. It was located 5 km east of the AAFC Research Station at Morden, MB on a sandy-loam soil. Site 1 was irrigated several times during pod filling in an attempt to increase disease pressure under dry conditions. In 2005 and 2006 at Bradford, only trace levels of mycosphaerella blight on foliage developed during the growing season, so no assessments of severity were made.
Analysis of variance (General Linear Model Procedure of SAS version 9.1) was used to analyse each response variable separately at each site, and means were separated based on the Waller–Duncan k-ratio t test. Data were generally not combined across locations because there was a site × year × treatment interaction for many of the variables. The area under the disease progress curve for foliar blight was calculated for each station-year, but showed the same pattern of response as the disease rating data from individual dates and so will not be discussed further. The only response variable that was analysed across station-years was lodging. It was assessed using a mixed model analysis of variance with cultivar and seed infection level as fixed factors, and year and replication as random factors. Differences are significant at P ≤ 0.05 unless otherwise stated.
Results
Production of seed lots
In 2003, development of mycosphaerella blight was slow due to hot, dry weather. No disease was present at flowering, so the fungicide treatment was not applied. Seed yield was low (mean = 1.3 Mg ha−1) and did not differ between the cultivars. The mean incidence of M. pinodes in the seed was also low (0% for ‘Keoma’, 2% for ‘Carneval’).
At the first rating date in 2004 (6 August), severity was low (mean = 2.4) and there were no differences among treatments. By 16 August, severity was still low (mean = 3.2), but fungicide application had reduced severity slightly (data not shown). At the final rating date on 27 August, severity had increased substantially and there were differences between the fungicide-treated and non-sprayed treatments for each cultivar (‘Keoma’ non-sprayed = 6.2, sprayed = 4.8; ‘Carneval’ non-sprayed = 5.4, sprayed = 4.2; standard error = 0.3).
Seed harvested from the inoculated blocks was heavily infected with M. pinodes, while seed from the fungicide-treated blocks had lower levels of infection (‘Keoma’ non-sprayed = 37%; sprayed = 22 %, ‘Carneval’ non-sprayed = 27%, sprayed = 17%; standard error = 2.7).
Seedling establishment
The trial at Morden in 2005 was flooded after seeding and had to be abandoned. Across all of the other sites, seedling establishment exhibited a consistent pattern of response (). Establishment in the low infection treatment was generally higher than the other treatments. Where differences occurred (10 of 16 cultivar × site combinations), establishment in the intermediate treatment was slightly higher than for the high infection treatment, but seed treatment with Crown® fungicide did not increase establishment.
Table 2. Impact of seed infection on seedling establishment (seedlings m−2) of two field pea cultivars across all four sites in 2005 and 2006
Blight severity and seed yield
In 2005 at Saskatoon, weather conditions in July were relatively cool and wet (), which is favourable for epidemics of mycosphaerella blight. There were no differences in mycosphaerella blight severity among the treatments at the two early rating dates. At the third and fourth dates, severity on foliage was higher in the high infection treatment than the other treatments (). Seed yield was higher in the low infection treatment than in the other seed infection treatments for both cultivars, but there were no differences between the high and intermediate infection treatments (). Weather conditions at Vegreville in 2005 were not as favourable for blight development as at Saskatoon (), so levels of mycosphaerella blight at the end of the season were low (). There were no differences in blight severity among the treatments at four of five rating dates, and no consistent pattern of response at the remaining date (data not shown). There were no differences in seed yield among seed infection treatments for ‘Keoma’, but yield in the low infection treatment was slightly higher than the other seed infection treatments for ‘Carneval’ (). At Bradford in 2005, weather conditions were hot and relatively dry (). Only trace levels of mycosphaerella blight were observed in the study throughout the growing season (data not shown).
Fig. 1. Disease progress in station-years where seed infection treatment (H = high infection, I = intermediate, C = I + Crown fungicide, L = low infection) for two field pea cultivars had an impact on severity of mycosphaerella blight. Capped lines represent standard errors.
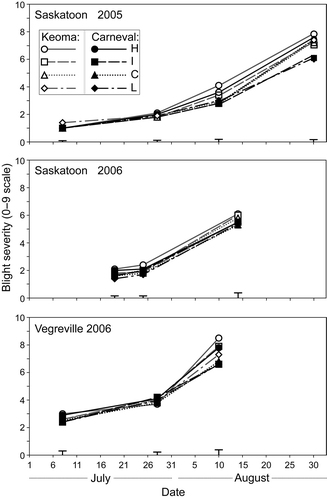
Fig. 2. Mean disease progress on two field pea cultivars in three station-years where seed infection treatment did not affect the severity of mycosphaerella blight. Standard errors were smaller than the symbols in the graphic and are not presented. Note: In addition, only trace levels of blight developed at Bradford in either year.
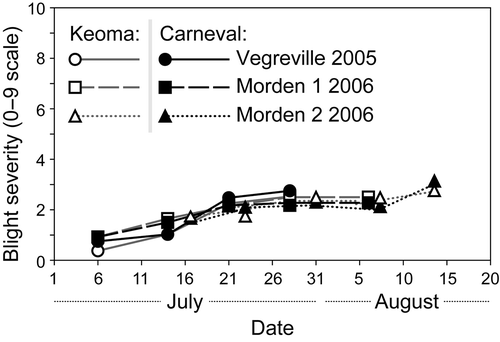
Table 3. Monthly mean temperature and precipitation for the test sites in 2005 and 2006 compared with the long-term (30-year) mean (LTM) values
Table 4. Impact of seed infection on seed yield (Mg/ha) of two field pea cultivars across all four sites in 2005 and 2006
In 2006 at Saskatoon, blight was slightly more severe in the high infection treatment than the low infection treatment for both cultivars at the two early rating dates (). However, these small differences had disappeared by the final rating date. Seed infection treatment did not affect seedling establishment or seed yield ( and ).
Conditions were conducive for development of mycosphaerella blight at Vegreville in 2006 () and blight severity at maturity was high. There were no differences in blight severity at the first two ratings dates, but severity was higher in the high infection level treatment at the final rating date than the other treatments (). There were no differences in seed yield among treatments (). At Morden in 2006, weather conditions were relatively dry () and blight severity was low throughout the season at both sites (). There were no differences in severity at any date. Treatment did not affect seed weight (data not shown) or seed yield () at either site.
At Bradford in 2006, weather conditions were hot and dry throughout the growing season (). There were no differences in the incidence of seedling blight (counted at the same time as seedling emergence, data not shown). Symptoms of mycosphaerella blight developed late in the growing season at trace levels in both years, but there were no differences in blight severity among treatments (data not shown). There were no differences in seed yield for ‘Carneval’, but yield of ‘Keoma’ was lower for the high infection treatment than for the low infection and intermediate plus fungicide treatments ().
Severity on stems was assessed at the end of the growing season at several sites. At Saskatoon in 2005 and Vegreville in 2006, severity on stems was higher on the high infection treatment than the low infection treatment. At Vegreville in 2005 and Saskatoon in 2006, there were no differences in blight severity on foliage or stems associated with seed infection treatment. At all four sites, severity on stems showed the same pattern of response as on foliage at the end of the season (data not shown).
Lodging was assessed prior to harvest in four station-years (). Across all sites, cultivar, station-year, and their interaction had a substantial effect, with ‘Carnival’ showing less lodging than ‘Keoma’ (P ≤ 0.001). There was also a relatively small interaction between seed infection and station-year (P ≤ 0.01). When the data were assessed on individual station-years, there were no differences among treatments at Vegreville in 2005 or Morden Site 1 in 2006. Lodging was more severe in the high infection than low infection treatment at Saskatoon in 2006, but was less severe in the high infection treatment at Morden Site 2 in 2006. Differences in lodging could affect ease of harvest and subsequent seed quality in commercial operations, but there was no obvious pattern of relationship with seed quality in the present study.
Table 5. Impact of seed infection on lodging (0–9 scale, where 0 = no lodging and 9 = completely lodged) in two field pea cultivars at Vegreville in 2005, Saskatoon in 2006, and two sites at Morden in 2006
Discussion
The incidence of seed infection with M. pinodes in commercial fields in Saskatchewan is generally less than 5%, and almost always lower than 10% (for recent examples, see Morrall et al., Citation2006, Citation2007). The incidence of infection with M. pinodes in the seed lots produced in the preliminary study was relatively low (mean 1%) in 2003 and high (mean > 20%) in 2004. Use of seed from 2003 and 2004 (produced and stored under uniform conditions) in the main trial permitted assessment of the impact of seed infection on subsequent foliar severity of mycosphaerella blight across a wide range of seed infection incidence (1–47%). The study was carried out across four widely separated sites, which provided a range of weather conditions, soil types, and even levels of external inoculum. Use of a wide range of infection, uniform seed lots, and repetition across geographically separated sites represent important improvements in methodology over previous studies (Hwang et al., Citation1991; Bretag et al., Citation1995; Moussart et al., Citation1998).
Most commercial producers would not consider planting a poor seed lot like the high infection treatment, which contained many discoloured and shrivelled seeds and had a very high incidence of infection with M. pinodes. However, they would not know that the intermediate treatment, which had a high level of infection (15% seed infection with M. pinodes in ‘Keoma’ and 6% in ‘Carneval’) but no visible symptoms, was heavily infected without a seed test. A seed lot with no visible symptoms and little or no seed infection, produced at the same site the previous year, was included as a low disease control. Although it does not constitute a true control for seed establishment because it was produced under different weather conditions than the seed lots in 2004, it was included to assess the benefit of blight-free seed.
At the sites in western Canada, differences in mycosphaerella blight severity during the growing season were small in three station-years and there were no differences among treatments in the remaining three station-years. At each assessment date for all of the sites, there was little or no difference between the two cultivars and no difference between the low infection treatment and the intermediate seed lot with or without seed treatment. Conditions were favourable for blight development at all three station-years where small differences in severity were noted.
The site at Bradford is the only one where air-borne inoculum from nearby fields was not expected to be present during the growing season. As a result, the site at Bradford was particularly important because it permitted direct examination of the impact of seed-to-seedling transmission of M. pinodes at a site where seed transmission was the only inoculum available to initiate a mycosphaerella blight epidemic. Only trace levels of disease were observed at Bradford in either year, and there were no differences in mycosphaerella blight levels among treatments. Also, there were no differences among treatments in the incidence of dead/dying seedlings (data not shown), which was assessed when the emergence counts were taken. The observation that almost no disease developed at Bradford provides the clearest and most unequivocal indication possible that infected seed is not an important source of initial inoculum in areas where the pathogen is endemic.
Weather conditions at Bradford were not particularly conducive for the development of epidemics of mycosphaerella blight, but were within the range of conditions observed at the other sites () where blight developed in every plot. Also, the mycosphaerella blight complex was an important constraint to pea production in Ontario in the 1950s (Skolko et al., Citation1954; Wallen, Citation1965) before production shifted to other regions of the country. Based on consideration of the weather data at Bradford together with historical reports of severe leaf spot on pea caused by M. pinodes in the region, we are confident that M. pinodes would have caused disease in this trial if inoculum had been present. However, a low level of seed-to-seedling transmission might have been masked by dry conditions at that site over both years of the study.
In contrast, seed infection treatment consistently affected seedling establishment. In seven of eight station-years, establishment from the high-infection seed lot was lower than from the low infection seed lot. Establishment was higher in the intermediate seed than the high-infection seed lot in only three of eight station-years, even though the intermediate seed lot had no visibly damaged seed and the high treatment contained substantial quantities of shrivelled and discoloured seed. In contrast, establishment was higher in the low infection treatment than the intermediate treatment in six of eight station-years, even though the two lots were visually very similar and they had been harvested and maintained under uniform conditions. Seed treatment with Crown® improved establishment relative to the intermediate seed lot in only 2 of 16 station-year × cultivar combinations. We conclude that treatment with Crown did not have an important impact on establishment in this study.
A pea crop has a remarkable ability to compensate for gaps in the stand (Hwang et al., Citation2001, Citation2006a , Citation2007). Despite the large differences in seedling establishment, it was not possible to distinguish visually among the treatments at maturity based on canopy density. However, pea is a relatively weak weed competitor, and weed control in these trials included both herbicide application and hand roguing as required. Yield in the thin stands of the high infection treatment would likely have been severely reduced by weed competition in most commercial situations.
Despite the consistent differences in seedling establishment and occasional differences in mycosphaerella blight severity, seed yield in the low infection treatment was higher than the high infection treatment in only three of eight station-years for even one of the cultivars. Yield in the intermediate treatment did not differ from that of the high infection treatment in any year, and was lower than the low infection treatment in only two of eight station years. There was no clear association between levels of mycosphaerella blight and yield; yield was reduced in one station-year when there was a difference in blight severity among the treatments (Saskatoon 2005), and in two station-years (Vegreville 2005, Bradford 2006) when disease levels were low and there were no differences in severity. Seed treatment with Crown® did not affect seed yield at any site. These results support previous reports (Hwang et al., Citation1991; Bretag et al., Citation1995; Moussart et al., Citation1998) that seed infection reduced seedling establishment but does not generally affect blight severity or yield.
In addition, the results of the current study allow us to make inferences on the relative importance of overwintering inoculum compared with seed-borne inoculum for epidemic development of mycosphaerella blight in western Canada. At Bradford, where there was little or no overwintering inoculum in the area, only trace levels of disease developed in both years. The lesions that were observed at this site developed late in the season at the top of the canopy, likely from air-borne ascospores released from fresh peas grown in home gardens in the area. At the other three sites, the initial symptoms developed near the base of the foliage after closure of the canopy, which generally occurred during or after flowering. This pattern of development could have been caused by splash-dispersed conidia within the plot, seed-borne inoculum, or air-borne ascospores. However, the sites had been chosen based on relatively long rotations without field pea to eliminate inoculum on pea residue in the study area (Zhang et al., Citation2005a ). Therefore, we believe that high levels of splash-dispersed conidia are an unlikely source of this initial inoculum. Similarly, several studies indicate that the frequency of seed-to-seedling transmission of M. pinodes is low (Hwang et al., Citation1991; Bretag et al., Citation1995; Moussart et al., Citation1998), and these observations were confirmed in the current study. As a result, seed-borne inoculum is also considered to be an unlikely source of initial inoculum. In contrast, air-borne ascospores are known to be an effective source of inoculum in this host–pathogen system and are capable of initiating epidemics at a distance from the inoculum source (Roger & Tivoli, Citation1996). Therefore, we conclude that air-borne ascospores were likely the main source of initial inoculum at these sites.
The results of the current study, taken together with similar findings in previous studies (Hwang et al., Citation1991; Bretag et al., Citation1995; Moussart et al., Citation1998), support our hypothesis that the frequency of seed-to-seedling (above-ground) transmission of M. pinodes is low. Also, we infer from these observations that infected seed is much less important as a source of inoculum for initiating epidemics of mycosphaerella blight in regions of high pea production than inoculum on overwintering crop residue. This provides a strong indication that breeding for resistance to seed infection, as advocated by a number of authors (Skolko et al., Citation1954; Maude, Citation1966; Xue et al., Citation1996), will have little or no impact on epidemics of mycosphaerella blight in regions where the disease is already endemic.
In conclusion, high levels of seed infection had little impact on subsequent epidemics of mycosphaerella blight, and even less impact on seed yield. We inferred from these data that overwintering inoculum of M. pinodes on crop residue is much more important in epidemic development of mycosphaerella blight than seed-borne inoculum in western Canada, so breeding for resistance to seed infection would have little or no impact on epidemics of mycosphaerella blight. However, the incidence of seed infection did have a large and consistent impact on seedling establishment, which could in turn have an impact on important factors such as weed competition in commercial fields. These results indicate that assessment of seed germination and vigour are more important for most producers than tests for M. pinodes on pea seed. However, even very low levels of seed transmission could be important where there is potential to introduce the pathogen into an area where it occurs infrequently or not at all.
Acknowledgements
We thank K. Bassendowski, W.C. Penner, J. Schroeder, D.B. Stoesz, G. Turnbull, and K. Vander Kooi for technical assistance. Also, thanks to the Saskatchewan Pulse Development Fund and the Saskatchewan Agriculture Development Fund for partial funding of the project.
References
- Ahmed , H.U. , Chang , K.F. , Hwang , S.F. , Gossen , B.D. , Howard , R.J. and Warkentin , T.D. 2006 . Components of disease resistance in desi and kabuli chickpea varieties against ascochyta blight . Plant Pathol. J. , 5 : 336 – 342 .
- Bailey , K.L. , Gossen , B.D. , Lafond , G.P , Watson , P.R. and Derksen , D.A. 2001 . Effect of tillage and crop rotation on root and foliar diseases of wheat and pea in Saskatchewan from 1991–1998: univariate and multivariate analyses . Can. J. Plant Sci. , 81 : 789 – 803 .
- Bretag , T.W. , Price , T.V. and Keane , P.J. 1995 . Importance of seed-borne inoculum in the etiology of the ascochyta blight complex of field peas (Pisum sativum L.) grown in Victoria . Austr. J. Exp. Agric. , 35 : 525 – 530 .
- Chang, K.F., Howard, R.J., Briant, M.A., Turnbull, G.D., Hwang, S.F., Deneka, B.A., & Gorda, M.V. (1997). Efficacy of fungicidal seed treatments for the control of ascochyta seedling blight on chickpea. Pest. Manag. Res. Rep., 1997, Report #75. pp. 187–188. http://www.cps-scp. ca/pest_mangement-reports.shtm (http://www.cps-scp. ca/pest_mangement-reports.shtm)
- Duczmal , K.W. and Minicka , L. 1989 . Further studies on pea seed quality and seedling emergence in the field . Acta Hort. , 253 : 282 – 283 .
- Gossen , B.D. , Chongo , G. , Buchwaldt , L. , Hwang , S.F. , Kutcher , H.R. and Cho , C. 2001 . Final report, AFIF Project #96000300 . Integrated disease management for chickpea, lentil and field pea. SK Agric. & Food, Regina, SK. ,
- Gossen , B.D. , Hwang , S.F. , Conner , R. , Chang , K.F. and McDonald , M.R. 2008 . Final report, ADF Project #920030160 . Sources of resistance/tolerance to mycosphaerella blight of field pea. SK Agric. & Food, Regina, SK. ,
- Hwang , S.F. , Conner , R.L. , Chang , K.F. , Gossen , B.D. , Su , H. , Howard , R.J. and Turnbull , G.D. 2006a . Impact of seeding rate and depth on mycosphaerella blight and seed yield of field pea . Can. J. Plant Sci. , 86 : 845 – 853 .
- Hwang , S.F. , Gossen , B.D. , Chang , K.F. , Turnbull , G.D. and Howard , R.J. 2001 . Effect of seed damage and metalaxyl seed treatments on pythium seedling blight and yield of field pea in Alberta . Can. J. Plant Sci. , 81 : 509 – 517 .
- Hwang , S.F. , Gossen , B.D. , Conner , R.L. , Chang , K.F. , Turnbull , G.D. , Lopetinsky , K. and Howard , R.J. 2007 . Management strategies to reduce losses caused by rhizoctonia seedling blight of field pea . Can. J. Plant Sci. , 87 : 145 – 155 .
- Hwang , S.F. , Lopetinski , K. and Evans , I.R. 1991 . Effects of seed infection by Ascochyta spp., fungicide seed treatment, and cultivar on yield parameters of field pea under field conditions . Can. Plant Dis. Surv. , 71 : 169 – 172 .
- Hwang , S.F. , Zhang , R. , Chang , K.F. , Gossen , B.D. , Turnbull , G.D. and Bing , D.J. 2006b . Comparison of three methods for assessing disease reaction to Mycosphaerella pinodes in field pea (Pisum sativum) . J. Plant Dis. Prot. , 113 : 20 – 23 .
- Maude , R.B. 1966 . Pea seed infection by Mycosphaerella pinodes and Ascochyta pisi and its control by seed soaks in thiram and captan suspensions . Ann. Appl. Biol. , 57 : 193 – 200 .
- Morrall , R.A.A. 1997 . Evolution of lentil diseases over 25 years in western Canada . J. Plant Pathol. , 19 : 197 – 207 .
- Morrall , R.A.A. , Carriere , B. , Ernst , B. , Pearse , C. , Schmeling , D. and Thomson , L. 2006 . Seed-borne pathogens of pea in Saskatchewan in 2005 . Can. Plant Dis. Surv. , 86 : 109 – 111 .
- Morrall , R.A.A. , Carriere , B. , Ernst , B. , Pearse , C. , Schmeling , D. and Thomson , L. 2007 . Seed-borne pathogens of pea in Saskatchewan in 2006 . Can. Plant Dis. Surv. , 87 : 125 – 126 .
- Moussart , A. , Tivoli , B. , Lemarchand , E. , Deneufbourg , F. , Roi , S. and Sicard , G. 1998 . Role of seed infection by the ascochyta blight pathogen of dried pea (Mycosphaerella pinodes) in seedling emergence, early disease development and transmission of the disease to aerial plant parts . Eur. J. Plant Pathol. , 104 : 93 – 102 .
- Roger , C. and Tivoli , B. 1996 . Spatio-temporal development of pycnidia and perithecia and dissemination of spores of Mycosphaerella pinodes on pea (Pisum sativum) . Plant Pathol. , 45 : 518 – 528 .
- Sheridan , J.J. 1973 . The survival of Mycosphaerella pinodes on pea haulm buried in soil . Ann. Appl. Biol. , 75 : 195 – 203 .
- Skolko , A.J. , Groves , J.W. and Wallen , V.R. 1954 . Ascochyta diseases of peas in Canada – with special reference to seed transmission . Can. J. Agric. Sci. , 34 : 417 – 428 .
- Wallen , V.R. 1965 . Field evaluation and the importance of the ascochyta complex on peas . Can. J. Plant Sci. , 45 : 27 – 33 .
- Wang , T.F. , Gossen , B.D. and Slinkard , A.E. 2006 . Lodging increases severity and impact of mycosphaerella blight on field pea . Can. J. Plant Sci. , 86 : 855 – 863 .
- Xue , A.G. 2003 . “ Diseases of pea ” . In Diseases of field crops in Canada , Edited by: Bailey , K.L. , Gossen , B.D. , Gugel , R. and Morrall , R.A.A. 201 – 213 . Saskatoon , SK : Canadian Phytopathological Society .
- Xue , A.G. , Warkentin , T.D. , Greeniaus , M.T. and Zimmer , R.C. 1996 . Genotypic variability in seedborne infection of field pea by Mycosphaerella pinodes and its relation to foliar disease severity . Can. J. Plant Pathol. , 18 : 370 – 374 .
- Zhang , J.X. , Fernando , W.G.D. and Xue , A.G. 2005a . Effect of residue type and burial depth on survival of Mycosphaerella pinodes in Manitoba . Can. J. Plant Pathol. , 27 : 132 – 136 .
- Zhang , J.X. , Fernando , W.G.D. and Xue , A.G. 2005b . Daily and seasonal spore dispersal by Mycosphaerella pinodes and development of mycosphaerella blight of field pea . Can. J. Bot. , 83 : 302 – 310 .
- Zhang , R. and Gossen , B.D. 2007 . Heritability estimates and response to selection for resistance to mycosphaerella blight in pea . Crop Sci. , 47 : 2303 – 2307 .
- Zhang , R. , Hwang , S.F. , Chang , K.F. , Gossen , B.D. , Strelkov , S. , Turnbull , G.D. and Blade , S.F. 2006 . Genetic resistance to Mycosphaerella pinodes in 558 field pea accessions . Crop. Sci. , 46 : 2409 – 2414 .
- Zhang , R. , Hwang , S.F. , Gossen , B.D. , Chang , K.F. and Turnbull , G.D. 2007 . A quantitative analysis of resistance to Mycosphaerella pinodes in Pisum sativum . Crop Sci. , 47 : 161 – 167 .