Abstract
Fusarium avenaceum isolates causing fusarium head blight (FHB) in wheat were collected from the Canadian Prairies and Mixed Wood Plains Ecozones. A total of 91 isolates were grouped into 15 phenotypes and 10 VCGs which formed seven ITS rDNA phylogenetic clades and three EF-1α PCR-DGGE groups. Variation in virulence within F. avenaceum isolates ranged from high to avirulent on wheat. In general, virulent isolates demonstrated greater host range and geographic distribution, while avirulent isolates had a limited spread. Virulent isolates demonstrated greater heat tolerance, colour plasticity and growth rate compared with avirulent isolates. Overall, data indicate that pathogen dynamics and outbreaks might be influenced by the abundance ratio of virulent/avirulent isolates related to climate characteristics of each agro-region. The characterization of F. avenaceum ‘sensu lato’ isolates, obtained from western and eastern ecozones, provided baseline information on the variation that exists for this FHB-causing species. Combined with environmental or ecological information, this knowledge could establish a consistent, long-term management of the pathogen.
Résumé
Des isolats de Fusarium avenaceum, causant la fusariose de l'épi (FE) chez le blé, ont été collectés dans les Prairies canadiennes et dans les écozones des plaines à forêts mixtes. En tout, 91 isolats ont été regroupés en 15 phénotypes et en 10 groupes de compatibilité végétative, qui ont formé sept variantes phylogénétiques d'ADNr de l'ITS ainsi que trois groupes de l'EF-1α par PCR-DGGE. Chez le blé, la virulence des isolats de F. avenaceum variait d'élevée à nulle. De façon générale, les isolats virulents affichaient une plus grande variété d'hôtes ainsi qu'une plus vaste distribution géographique, alors que la distribution des isolats non virulents était limitée. Comparativement aux isolats non virulents, les isolats virulents ont fait preuve d'une plus grande tolérance à la chaleur, ont été moins sujets au changement de couleur et ont présenté un meilleur taux de croissance. Globalement, les données indiquent que la dynamique de l'agent pathogène et les épidémies peuvent être influencées par le rapport d'abondance des isolats virulents/non virulents, relatif aux caractéristiques climatiques de chaque région agricole. La caractérisation des isolats, au sens large, de F. avenaceum, provenant des écozones de l'ouest et de l'est, fournit l'information de base quant à la variation spécifique de cette espèce qui cause la FE. Combinées aux données environnementales ou écologiques, ces connaissances pourraient contribuer à gérer de façon cohérente et à long terme l'agent pathogène en cause.
Introduction
Gibberella Sacc. (teleomorph)/Fusarium Link (anamorph) species cause fusarium crown/root rot (FRR), fusarium head blight (FHB), fusarium damaged kernels (FDK), scab, damping-off and wilt disease symptoms. Several Fusarium species have been associated with the most significant cereal diseases of small kernel crops in Canada and North America (Symons et al., Citation2002; Kurt & Bushnell, Citation2003). Fusarium avenaceum is one of the most commonly encountered pathogenic species of Fusarium on cereals in the Canadian prairies (Fernandez et al., Citation2005; Pearse et al., Citation2006), northern Europe and Russia (Kristensen et al., Citation2005; Kulik et al., Citation2007; Vogelgsang et al., Citation2008). Prevalence of this wide host range fungus in Canada is associated with cold regions (Vujanovic et al., Citation2006), diversified and continuous cropping sequences, and adoption of conservation tillage practices (Fernandez, Citation2007).
To achieve detection of F. avenaceum in crop plants, Yli-Matilla et al. (Citation1996) developed isozyme and RAPD-PCR analyses, while Yergeau et al. (Citation2005) developed PCR–DGGE (Polymerase Chain Reaction–Denaturing Gradient Gel Electrophoresis) fingerprinting tools based on the elongation factor 1 alpha (EF-1 alpha) gene. Vujanovic et al. (Citation2009) characterized the whole cell protein profile for F. avenaceum intraspecific detection in crops. However, no efficient agronomic practices or cultivars have been developed to suppress initiation of FRR, FHB and FDK toxin contamination in grains. Meanwhile, cereal grain contamination by F. avenaceum mycotoxins like moniliformin, butenolide, aurofusarin, beauvericin and enniatins (A-A1 and B-B3) threatens food and feed safety by rendering the grain unsuitable for human and animal consumption (Morrison et al., Citation2002; Desjardins, Citation2006; Sorensen et al., Citation2009). Unavailable control measures are due to the broad host range of the pathogen, its adaptability to overcome crop resistance (Leslie & Summerell, Citation2006) and the difficulty of fungicides to prevent FRR/FHB damage and FDK toxin contamination (Champeil et al., Citation2004).
The aim of our study was to define the differences between F. avenaceum species complex isolates originating from different host species/crops grown in the western Canadian Prairie Ecozone of Saskatchewan (CPES) in comparison with the eastern Canadian Mixed Wood Plains Ecozone (CMPE). Information obtained by DGGE profiling of Canadian F. avenaceum isolates combined with virulence and heat tolerance data provide valuable insights on the risk of pathogen dispersion with respect to the increasing effects of climate change. It may also prove useful in developing disease management strategies in CPES, representing 40% of the total Canadian agricultural land. On a larger scale, it could allow a better understanding of F. avenaceum adaptation and distribution throughout the northern hemisphere.
Materials and methods
Phenotypic characters and VCG grouping of F. avenaceum
Eighty-five CPES isolates were obtained from Agriculture and Agri-Food Canada (Swift Current Research Station, SK) and maintained in the Saskatchewan Microbial Collection and Database (SMCD). The isolates originated from various agro-regions and soil types (brown, dark brown, black and grey), as well as various crops including wheat, barley, oats, flax, pea, chickpea, canola and durum (). Based on the level of July precipitation (30-year average), Saskatchewan's prairies are divided into three distinct areas: Area I: the northern Saskatchewan 5A-5B, 8A-8B and 9A-9B districts with ≥ 70 mm of precipitation; Area II: southeastern Saskatchewan 1A-1B and 2A-2B districts with 60–69 mm; and Area III: southwestern Saskatchewan 3A-3B, 4A-4B, 6A-6B and 7A-7B with ≤ 59 mm. (CGC, 2006).
Table 1. Diversity of Fusarium avenaceum isolates in relation to origin and phenotype
Six isolates were recovered from wheat heads collected throughout the CMPE of Ontario and Quebec and the prairie ecozone of Alberta and used for comparison. Four isolates originated from eastern Quebec (CEROM, Quebec City, QC, Canada) and central Ontario (University of Guelph, Ridgetown, ON, Canada) with 60–120 mm precipitation in July and two from Alberta (AAFC, Lethbridge), a western Canadian prairie (Saskatchewan neighbour) province with ≤ 45 precipitation in July (CGC, 2006).
Collected isolates were grown on potato dextrose agar (PDA; Becton Dickinson, Sparks, MD) in sealed Petri dishes at 21 °C in darkness. Colonies were characterized using phenotypic and colour features or stimulated spore formation after transfer onto carnation leaf agar (CLA) medium (Summerell et al., Citation2003). Following taxonomic identification, 91 Canadian isolates were retained (Vujanovic et al., Citation2009). From those, 15 F. avenaceum phenotypic groups were identified and assigned letters A–J (). Nit mutants were also generated and classified based on ability to form heterokaryons. Vegetative compatibility was assessed by pairing nit mutants according to Vujanovic et al. (Citation2009). Isolates were assigned to the following 10 vegetative compatibility groups: VC-A, VC-B, VC-C, VC-D, VC-E, VC-F, FC-G, VC-H, VC-I, VC-J ( and ), each showing considerable variability in their colour and growth characteristics.
Table 2. Fusarium avenaceum VCGs, cultural/colour and growth characteristics
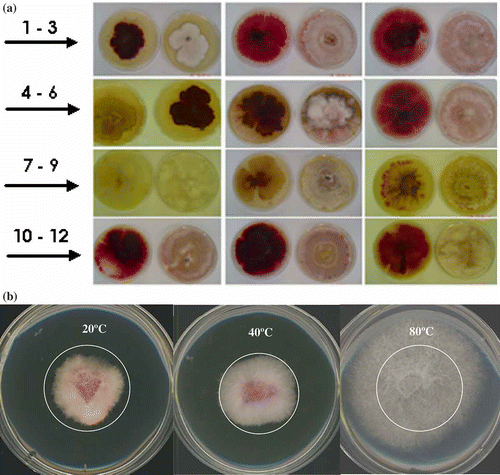
Fig. 1. a, Fusarium avenaceum ‘sensu lato’ phenotypes and colour identification numbers (CIN) based on abaxial colony surface on PDA: 1 – Co #560C13, 2 – Da #7A1A24, 3 – Ea #67151E, 4 – Gss #53050E, 5 – Is #5E1616, 6 – Ds #71232B, 7 – Js #E68A11, 8 – Bs #A86608, 9 – Gs #68400B; 10 – Hs #9B1523; 11 – Es #4E040B, 12 – Fs #78101B. CINs were generated with Hex Color Code Chart (http://www.2createawebsite.com/build/hex-colors.html#colorgenerator). b, Three-day-old Ds (highly virulent) strain on PDA at 40 °C in darkness showing increased growth and changing colour (from red to white) after treatment at 40 °C and 80 °C for 4 h.
Virulence and heat tolerance assays
The virulence assay was performed on spring wheat ‘TEAL’ – a susceptible cultivar to fusarium seedling blight (SMA, Citation2008). Seeds were obtained from the Department of Plant Science, University of Saskatchewan. Virulence test is an important indication of VCGs genetic affinities in F. avenaceum population (Satyaprasad et al., Citation2000) and could be correlated to fungal origin (Khalil et al., Citation2003). Effect of dry heat treatment on F. avenaceum physiological properties was also tested (Clear et al., Citation2002) by the induced shift in its growth and colour () on PDA (Summerell et al., Citation2003). The ability of seed to germinate when infected with standard inoculum (grown at 21 °C) or with treated strains (grown at 40 °C or 80 °C) was tested in vitro following the procedure of Vujanovic et al. (Citation2009). Uninfected seeds were used as control. Six replicate plates, containing 10 seeds each, were used per each treatment.
Representative isolates of each VCG were cultured onto PDA and incubated at room temperature for five days under darkness. A 3 mm diameter mycelial plug of each isolate was transferred onto PDA plates and incubated at room temperature for three days in the dark. Colonies and spring wheat ‘TEAL’ seeds inoculated with each isolate were separately incubated at 40 °C and 80 °C for 4 h and 24 h. After heat treatment, colonies of each isolate or seeds were transferred onto fresh PDA and incubated at room temperature for five days in the dark. For each treatment, five replicate plates were used.
Fungal isolates were also grown at standard 20 °C (control) or at 40 °C and 80 °C for 4 h, as well as repeatedly treated twice at 40 °C for 4 h and 24 h, and transferred onto fresh PDA and incubated at room temperature for three days under dark conditions. After three days of incubation, colony growth rate and colour changes were assessed by colony diameter measure and colour identification numbers (CIN) based on abaxial colony surface. CIN were generated with Hex Color Code Chart (http://www.2createawebsite.com/build/hex-colors.html#colorgenerator).
Each experiment combination was repeated three times. Statistical analyses were carried out using ANOVA via the SAS program (SAS Institute, Cary, NC, USA). Differences in virulence between F. avenaceum pathogenic groups of isolates were determined by Tukey's studentized range tests and were considered significant at P < 0.05.
Molecular analyses
Total DNA from two-week-old cultures representative of each phenotype/VCG was extracted using an Ultra Clean microbial DNA Isolation Kit (MO BIO Laboratories Inc, Canada) following manufacturer's instructions. The purified DNA was resuspended in 50 μL of elution buffer and stored at −20 °C until further analyses. PCR reactions were performed according to Qiagen kit instructions. The internal transcribed spacer (ITS) of the rDNA gene was amplified using fungi specific primers, ITS1F (Gardes & Bruns, Citation1993) and ITS4 (White et al., Citation1990). PCR amplicons were purified using QIAquick PCR purification kit (Qiagen) and sequenced (Plant Biotechnology Institute, Saskatoon, SK). Sequence homology searches were performed in the GenBank database using the NCBI-BLAST search algorithm. Sequences were aligned using Clustal X, and distance trees were produced with the PAUP* program (Swofford, Citation2000) using a neighbour-joining approach. Fusarium avenaceum ITS rDNA sequences have been deposited in the GenBank (http://www.ncbi.nlm.nih.gov/) under accession numbers EU255791 to EU255805.
PCR-Denaturing Gradient Gel Electrophoresis (DGGE)
DNA from isolates in each phenotype/VCG was extracted using DNeasy Plant Mini Kit (Qiagen). PCR amplification was carried out using Fusarium specific primer set EF1/EF2. Amplicons were then 100× diluted and reused as templates for a second PCR amplification with primers alfie1-CG/Alfie2 (Yergeau et al., Citation2005). DGGE analyses were performed using DGGE 2001 (C.B.S. Scientific Company, Inc, Canada). PCR amplicons were electrophoresed for 18 h at 60 V on a 7.5% acrylamide/bis-acrylamide (37.5:1) gel with a 40–60% denaturant gradient (100% denaturant corresponding to 7M urea and 40% (v/v) formamide). Electrophoresed gels were stained with ethidium bromide (10 μg mL−1) and digitized using a Bio Doc-IT Imaging System (UVP Inc. CA) apparatus. A molecular marker composed of known species (F. avenaceum, F. sambucinum and F. proliferatum) was loaded.
Statistical analyses
The frequency of a single fungal VCG in overall F. avenaceum collection of randomly chosen isolates was calculated (Ni/Nt × 100; where Ni is the total number of specific phenotype/VCG strains and Nt the total number of F. avenaceum strains). Data were then submitted to SYSTAT Correspondence Plot analyses in order to test the similarity of phenotype/VCGs assemblages between different hosts, organ types and Saskatchewan's geographic or agro-regions.
Cluster analyses were carried out by NTSyspc, Numerical Taxonomy System (version 2.2) and APS Image Analysis software 2002 for F. avenaceum species complex groups ordination based on detected physiological (growth and colour) stability of strains after heat shock treatments. Unweighted Pair-Group Arithmetic Average (UPGMA) clustering method was used to show the variability of strain's temperature tolerance based on growth rate and morphological-colour changes before and after heat treatments.
Results
Biogeography, host and tissue specificity
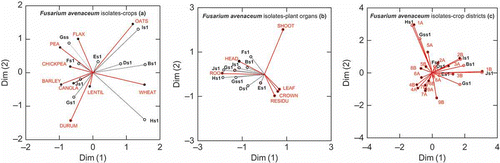
In Prairie Ecozone of Saskatchewan, predominant isolates were Ds, Fs and Es representing 32.5%, 25.5% and 25.0% of isolates, respectively. Remaining isolates represented less than 17.0%. Fifteen phenotypic groups comprised of 10 identified VCGs, including UC-unclassified VCGs as presented in , were also characterized by virulence assay. Disease severity of these groups was determined by in vitro testing on spring wheat ‘TEAL’ and barley seedlings. These groups were subsequently divided (P < 0.05) into four virulence groups according to Vujanovic et al. (Citation2009). They are highly virulent (HV), moderately virulent (MV), weakly virulent (WV) and non-virulent (NV), respectively (). Ds isolate showed high virulence whereas isolate Bs was non-virulent. The most virulent F. avenaceum HV-Ds strain originated from wheat and oat root tissues and residues in southern 1B and central 5A Saskatchewan crop districts ( a, b and c). Non-virulent NV-Bs strain was also associated with wheat and oat and occurred on roots and heads of 1B, 2B and 3A crop districts ( and ). Among weakly virulent groups, WV-Fs and WV-Es strains predominated in similar crop districts as HV-Ds. In contrast to HV-Ds, WV-Fs frequently originated from barley and oat root and head, whereas WV-Es preferred wheat, oats and canola roots, head and residue. Moderately virulent MV-Gs was rarely recovered from barley and durum, while MV-Js was exclusively associated to canola crop.
Fig. 2. Fusarium avenaceum virulence on germinating seed of ‘TEAL’ spring wheat at standard temperature (20 °C) in vitro. Pathogenic group scale: highly virulent (HV), moderately virulent (MV), weakly virulent (WV) and non-virulent (NV).
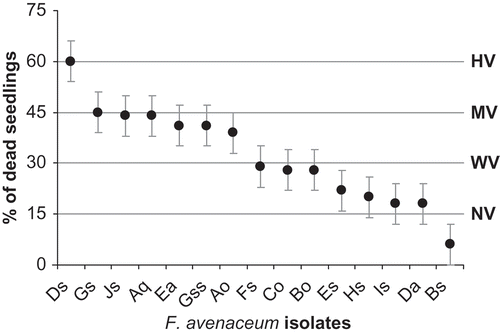
Fig. 3. Correspondence plot analysis showing the association of Fusarium avenaceum with a, various crops/plant, b, host plant organs and c, crop districts/locations. Agriculture districts and locations in Saskatchewan: 1A – Estevan, 1B – Moosomin, 2A – Scott, 2B – Indian Head, 3A – Coteau, 3B – Swift Current, 4A – Maple Creel, 4B – Leader, 5A – Langenberg, 5B – Kelvington, 6A – Watrous, 6B – Saskatoon, 7A – Rosetown, 7B – Unity, 8A – Tisdale, 8B – Humbolt, 9A – Prince Albert, 9B – Meadow Lake.
Heat treatment, colour plasticity and virulence
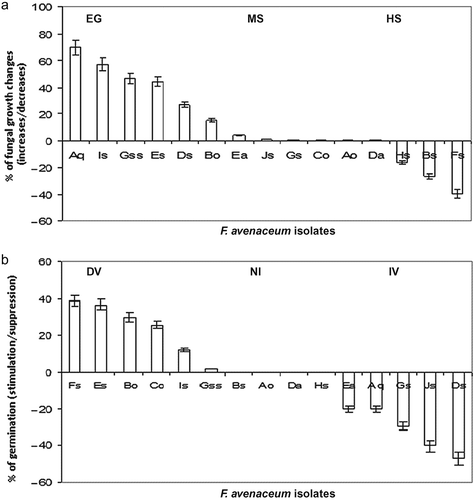
Both F. avenaceum phenotypic groups and VCGs showed distinctive initial colour and morphological features (before heat treatment), ranging from red to white colonies (). The 15 phenotypic groups exposed to elevated temperatures at 40 °C and 80 °C were transferred onto PDA and incubated at 20 °C for three days in the dark. Noticeable heat-induced changes were observed in terms of colour () and growth rate () compared with control. Twelve isolate groups were able to resist 40 °C for 4 h; the remaining three died (NV-Bs, WV-Hs, WV-Fs). HV-Ds and MV-Bo isolates survived even after extended re-incubation for 24 h at 40 °C. When the 15 isolates were exposed to 80 °C for 4 h, nine isolate groups were able to tolerate it, whereas only HV-Ds and MV-Bo survived after prolonged incubation for 24 h at 80 °C. No fungal mutation was observed in colonies transferred on fresh PDA medium.
Fig. 4. a, F. avenaceum growth alteration after heat treatment at 40 °C for 4 h. Heat tolerance scale: highly susceptible (HS)/decreasing growth, moderately or non-susceptible/stable growth (MS) and tolerant/enhanced growth (EG); b, F. avenaceum virulence alteration after heat treatment at 40 °C for 4 h. Virulence stability scale: increasing virulence (IV), non-increasing virulence (NI) and decreasing virulence (DV).
Marked colour changes were observed in the red group of isolates, which adopted a white colour when exposed to 40 °C for 4 h. The same change was observed in cultures incubated at 40 °C for 24 h and at 80 °C for 4 and 24 h (). It is to be noted that higher temperatures correlated with a distinct shift towards a paler shade of colour (). Growth rates based on colony growth area and measured by APS Image analysis software (2002) also varied when isolates were exposed to the above conditions (). Clearly, some isolate groups grew faster while others, marked by pale colours, were slower when compared to control cultures grown at 20 °C (, ).
A wide range of plasticity in growth alterations due to heat shock has been observed in F. avenaceum isolates, going from highly susceptible (HS)/decreasing growth to moderately and non-susceptible (MS) to tolerant/enhanced growth (EG) (). Highly susceptible strains such as NV-Bs, WV-Hs and WV-Fs died at 40 °C (cardinal max temp.) after 4 h of exposure time.
Highly susceptible, highly tolerant as well as moderately susceptible isolates demonstrated growth plasticity under heat shock; they also demonstrated a capacity for increased virulence level after heat treatment. Examples are HV-Ds, MV-Js, MV-Gs, MV-Aq and WV-Es. Considering the change in virulence level, three differential groups were recognized (): IV – increasing virulence, as mentioned above, NI – non-increasing virulence (NV-Bs, WV-Da, WV-Hs and MV-Ao) and DV – decreasing virulence, all remaining strains.
Cluster analyses or multiple-parameter combination of heat tolerance, colour and growth changes at 40 °C and 80 °C () indicated an isolate-specific predisposition of each of the F. avenaceum isolates for changing virulence under heat treatments (). More specifically, it seems that virulence changes occurring under high temperature are colour related. In this study, the group ‘A’ fungal colonies having ‘white colour mycelium’ (although of different geographic origin) seemed to be predisposed to low heat-shock tolerance. This was also observed for group ‘C’. Groups ‘A’ and ‘C’ also presented low levels of virulence after heat treatment (NI-Ao, NI-Bs, NI-Hs, DV-Co and DV-Fs) compared with group ‘B’ strains with ‘red colour mycelium’, which include highly virulent IV-Ds, IV-Gs, IV-Aq and IV-Ea (). Contrary to strains from ‘A’ and ‘C’ clusters, the majority of strains from cluster ‘B’ survived at 80 °C for 4 h.
Molecular analyses
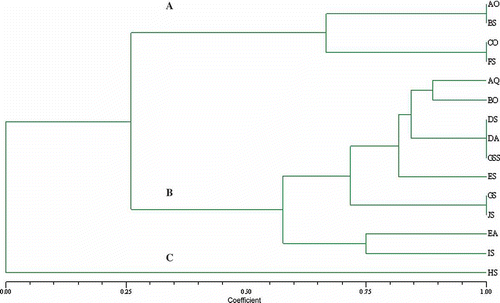
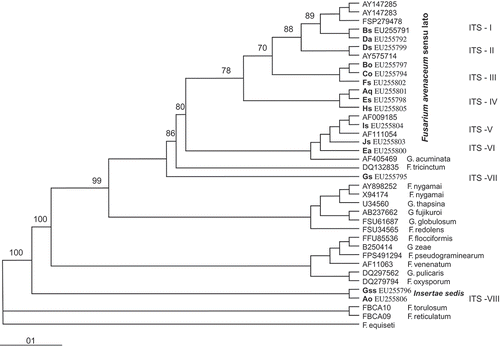
The isolates from the 15 phenotypic groups, comprising 10 identified VCGs, were analysed based on ITS rDNA sequences and showed considerable homology (98–100%) with F. avenaceum sequences retrieved from GenBank database. However, a constructed ITS phylogenetic tree indicated high variation among these isolates, in contrast to simple clustering. They were distributed within seven separate groups, going from ITS-I to ITS-VI, and two additional ITS-VII and ITS-VIII groups (). Fusarium avenaceum isolates from groups ITS-I to ITS-VI formed a single F. avenaceum population. In contrast and based on phylogeny, the strains Ao, Gs and Gss belonging to ITS-VII and ITS-VIII (although with 98% homology) were not included in the relatively broad F. avenaceum clade ().
Fig. 6. Rooted neighbour-joining tree showing similarities between ITS gene sequences of each F. avenaceum VCG. Bootstrapping values greater than 80% calculated from 1000 replicates are given above the branches. Scale bar indicates the number of substitutions per site. Identification number of DNA samples retrieved from GenBank are given before species names.
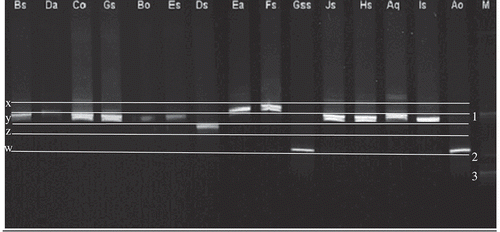
Furthermore, EF-1alpha gene sequences were amplified and a PCR-DGGE molecular profile was constructed. PCR-DGGE bands varied slightly between phenotypic/VCGs strains showing the multiple-banding pattern of strains within F. avenaceum species. However, these differences were considerable in the case of highly virulent Ds showing z-migration position. Moderately virulent Ea and Fs strains showed an x-migration position (). Moreover, Ao and Gss strains had the most significant difference in DGGE profile (EF-1 alpha) and showed a w-migration position. Consequently, the EF-1 alpha combined with ITS data confirmed the exclusion of Ao and Gss strains from F. avenaceum sensu lato. Further, the remaining isolates showed a y-migration position, which is typical for the majority of F. avenaceum isolates, with minor variations in their banding pattern ().
Discussion
Fusarium avenaceum isolates originating from several cereal and non-cereal hosts in the western CPES ecozone were analysed based on heat tolerance and virulence. Based on VCG analyses, no CPES isolate was matched with an eastern MWPE isolate compatibility group, originating from wheat. A majority of F. avenaceum isolates colonized wheat, barley and oat and showed multiple virulence activities. Interestingly, highly virulent Ds and non-virulent Bs isolates occurring in wheat showed the common ITS-I ancestry. Several other dominating Fs and Es isolates, from the ITS-III group, frequently were isolated from wheat, barley and oat hosts. Infrequently, Ds was isolated from flax/wheat, Fs from durum/flax/pea/chickpea and Es from canola/lentil/flax. Only small numbers of the very rare isolates were specifically associated with one single host, i.e. Js with canola and Hs with wheat. A range of F. avenaceum isolates often colonized root and head tissue as well as residue, as the most important sites for inocula production (Bailey et al., Citation2003). This genetic and ecologic plasticity seems to allow F. avenaceum populations to occupy various ecological niches in the CPES ecozone, mainly comprising wheat and barley crops.
Growth on PDA did not induce any phenotypic change in F. avenaceum during frequent isolate transfers and was thus retained as a suitable medium (Summerell et al., Citation2003). Considerable variability in heat tolerance between isolates was found. It was measured by assessing colour change (red vs. white) and colony growth alteration (increased vs. decreased). Both characteristics (colour and colony growth) seemed to be directly associated with pathogenic behaviour in tested isolates. This observation was also reported for F. graminearum strains (Ward et al., Citation2008). In this study, disease severity was directly related with greater colony growth and red colour stability of F. avenaceum colonies after prolonged heat (40 °C) treatment. Cluster analyses () showed that most white-coloured isolates belonged to the low heat tolerance ‘cluster A’, which cannot tolerate prolonged treatment at 40 °C, whereas the red-coloured ‘cluster B’ isolates may tolerate a very high temperature (80 °C) over 4 h. A prolonged heat treatment of wheat seed (70 °C) to control F. avenaceum, as recently proposed for F. graminearum control (Gilbert et al., Citation2005), would be inappropriate as this could negatively influence the selection of tolerant and highly aggressive isolates. Moreover, we found that various heat-treated F. avenaceum strains become even more aggressive after such exposure.
It has been proposed that the colour or pigment in fungi is related with melanin conidial pigments and aurofusarin red colony pigment found in F. avenaceum (Uhlig et al., Citation2007) as well as in several other ‘red Fusaria’ species (Frandsen et al., Citation2006). These fungal polyketide pigments are recognized virulence factors involved in fungal plant diseases, and indicators of fungal propagule survival capacity or longevity (Gomez & Nosanchuk, Citation2003). Our study showed that F. avenaceum pigments may be temperature-dependent and variable between different genetic (VCGs) groups. It was also found that the whitest F. avenaceum isolates, having the lowest virulence, were isolated from Canada's Mixed Wood Plains Ecozone. However, further work is required since this sample size was small (four isolates). In contrast, the most pigmented F. avenaceum isolates were isolated from western Canadian provinces, including Saskatchewan. In addition, heat tolerance increased with darker pigmentation, which could indicate a greater capacity to maintain temperature stability within a changing climate. In our study, the loss and gain of F. avenaceum pigment were related to changes in virulence and temperature tolerance in distinct genetic groups of F. avenaceum isolates retrieved from Saskatchewan fields. These findings could be useful for future field studies in order to predict F. avenaceum disease outbreaks throughout Saskatchewan agricultural regions.
ITS rDNA grouping did not fully concur with phenotypic analysis (O'Donnell & Cigelnik, 1997; Yli-Mattila et al., Citation2002) but did so with whole protein and VCG profiles (Vujanovic et al., Citation2009). Furthermore, ITS-based phylogeny () corresponded well with virulence profiles (). The most abundant and aggressive strains in terms of virulence and adaptability belong to ITS-II, ITS-V, ITS-VI and ITS-VII clades. Low virulence has been found in ITS-I, ITS-III and ITS-IV. DGGE efficiently discriminates highly virulent Ds from less virulent strains. Moreover, DGGE generated correct EF-1alpha profile for groups Ao and Gss and confirmed the ITS (ITS-I to ITS-VI) rDNA phylogenetic exclusion of ITS-VIII group from F. avenaceum ‘sensu lato’ populations. This clearly indicates that, when combined with the phylogenetic method, the DGGE approach could also be helpful to discriminate potentially cryptic species or isolates within F. avenaceum population morphotypes. However, until taxonomical resolution for F. avenaceum becomes available for Canadian/North American isolates, the term ‘sensu lato’ seems appropriate or in agreement with accepted approaches in population studies of Fusarium species (Britz et al., Citation2002; Lepoint et al., Citation2005).
We suggest that pronounced variability within F. avenaceum isolates, based on degree of pigment stability and maintaining of virulence under temperature stress, could play a crucial role under a climate change scenario (Williams et al., 1998; Bradshaw et al., Citation2004) and could influence the species' geographic distribution. Kim et al. (Citation2005) reported that red pigment (aurofusarin and rubrofusarin) biosynthesis in F. graminearum and F. culmorum was related with fungal virulence on cereal crops. However, further field studies are needed to confirm this with F. avenaceum isolates from Canada and other northern countries.
Acknowledgements
This research was financially supported by NSERC and Saskachewan-ADF to VV and by Agriculture Agri-Food Canada. The authors thank P. Daida and S.M. Ranogajec-Vujanovic for technical assistance, B. Rossnagel and P. Hucl for providing wheat seeds, and K. Turkington, L. Tamburic-Ilincic and S. Rioux for providing fungal cultures.
References
- Bailey , K.L , Gossen , B.D. , Gugel , R.K. and Morrall , R.A.A. 2003 . Diseases of field crop crops in Canada , 3rd , Saskatoon : The Canadian Phytopathological Society/Discovery Seeds Lab Ltd .
- Bradshaw , B. , Dolan , H. and Smit , B. 2004 . Farm-level adaptation to climatic variability and change: crop diversification in the Canadian prairies . Climatic Change , 67 : 119 – 141 .
- Britz , H. , Streetcamp , E.T. , Coutinho , T.A. , Wingfield , B.D. , Marasas , W.F.O. and Wingfield , M.J. 2002 . Two new species of Fusarium section Liseola associated with mango malformation . Mycologia , 94 : 722 – 730 .
- CGC (Canadian Grain Commission). (2007). Fusarium head blight in western Canada http://www.grainscanada.gc.ca/Information/fhb-e.htm (http://www.grainscanada.gc.ca/Information/fhb-e.htm)
- Champeil , A. , Dore , T. and Fourbet , J.F. 2004 . Fusarium head blight: epidemiological origin of the effects of cultural practices on head blight attacks and the production of mycotoxins by Fusarium in wheat grains . Plant Sci. , 166 : 1389 – 1415 .
- Clear , R.M. , Patrick , S.K. , Turkington , T.K. and Wallis , R. 2002 . Effect of dry heat treatment on seed-borne Fusarium graminearum and other cereal pathogens . Can. J. Plant Pathol. , 24 : 489 – 498 .
- Desjardins , A.E. 2006 . Fusarium mycotoxins , St. Paul , MN : Chemistry, genetics, and biology, American Phytopathological Society Press .
- Fernandez , M.R. 2007 . Fusarium populations in roots of oilseed and pulse crops grown in eastern Saskatchewan . Can. J. Plant Sci. , 87 : 945 – 952 .
- Fernandez , M.R. , Selles , F. , Gehl , D. , DePauw , R.M. and Zentner , R.P. 2005 . Crop production factors associated with fusarium head blight in spring wheat in eastern Saskatchewan . Crop Sci. , 45 : 1908 – 1916 .
- Frandsen , R.J.N. , Nielsen , N.J. , Maolanon , N. , Sørensen , J.C. , Olsson , S. , Nielsen , J. and Giese , H. 2006 . The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between the naphthoquinones and naphthopyrones . Mol. Microbiol. , 61 : 1069 – 1080 .
- Gardes , M. and Bruns , T.D. 1993 . ITS primers with enhanced specificity of basidiomycetes: application to the identification of mycorrhizae and rusts . Mol. Ecol. , 2 : 113 – 118 .
- Gilbert , J. , Woods , S.M. , Turkington , T.K. and Tekauz , A. 2005 . Effect of heat treatment to control Fusarium graminearum in wheat seed . Can. J. Plant Pathol. , 27 : 448 – 452 .
- Gomez , B.L. and Nosanchuk , D.J. 2003 . Melanin and fungi . Curr. Opin. Infectious Dis. , 16 : 91 – 96 .
- Khalil , M.S. , AbdelSattar , M.A. , Aly , I.N. , AbdElsalam , K.A. and Verreet , J.A. 2003 . Genetic affinities of Fusarium spp. and their correlation with origin and pathogenicity . Afri. J Biotech. , 2 : 109 – 113 .
- Kim , J.E. , Han , K.H. , Jin , J. , Kim , H. , Kim , J.C. , Yan , S.H. and Lee , Y.W. 2005 . Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae . Appl. Environ. Microbiol. , 71 : 1701 – 1708 .
- Kristensen , R. , Torp , M. , Kosiak , B. and Holst-Jensen , A. 2005 . Phylogeny and toxigenic potential is correlated in Fusarium species as revealed by partial translation elongation factor 1 alpha gene sequences . Mycol. Res. , 109 : 173 – 186 .
- Kulik , T. , Pszczólkowska , A. , Fordonski , G. and Olszewski , J. 2007 . PCR approach based on the esyn1 gene for the detection of potential enniatin-producing Fusarium species . Food Microbiol. , 116 : 319 – 324 .
- Kurt , J.L. and Bushnell , W.R. , eds. 2003 . Fusarium head blight of wheat and barley , St. Paul Minn : APS Press .
- Leslie , J.F. and Summerell , B.A. 2006 . The Fusarium laboratory manual , Oxford : Blackwell .
- Morrison , E. , Kosiak , B. , Ritieni , A. , Aastveit , A.H. , Uglig , S. and Bernhoft , A. 2002 . Mycotoxin production by Fusarium avenaceum strains isolated from Norwegian grain and the citotoxicity of rice culture extracts of porcine kidney epithelial cells . J. Agric Food Chem. , 50 : 3070 – 3075 .
- O'Donnell , K. , Kistler , H.C. , Cigelnik , E. and Ploetz , R. 1998 . Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene geanologies . Proc. Nat. Acad. Sci. USA , 95 : 2044 – 2049 .
- Lepoint , P.C.E. , Munaut , F.T.J. and Maraite , H.M.M. 2005 . Gibberella xylarioides sensu lato from Coffea canephora: a new mating population in the Gibberella fujikuroi species complex . Appl. Environ. Microbiol. , 71 : 8466 – 8471 .
- Pearse , P.G. , Holzgang , G. , Harris , C.L. and Fernandez , M.R. 2006 . Fusarium head blight in common and durum wheat in Saskatchewan in 2005 . Can. Plant Dis. Surv. , 86 : 75 – 76 .
- Satyaprasad , K. , Batemen , G.L. and Ward , E. 2000 . Comparisons of isolates of Fusarium avenaceum from white lupin and other crops by pathogenicity tests, DNA analyses and vegetative compatibility tests . J. Phytopathol. , 4 : 211 – 219 .
- SMA (Saskatchewan Ministry of Agriculture) . 2008 . SaskSeed guide. Wheat – main characteristics of varieties , Regina , , SK : Saskatchewan Ministry of Agriculture .
- Sørensen , L. , Phipps , R.K. , Nielsen , K.F. , Schroers , H.-J. , Frank , J. and Thrane , U. 2009 . Analysis of Fusarium avenaceum metabolites produced during wet apple core rot . J. Agric. Food Chem. , 57 : 1632 – 1639 .
- Summerell , B.A. , Salleh , B. and Leslie , J.F. 2003 . A utilitarian approach to Fusarium identification . Plant Dis. , 87 : 117 – 128 .
- Swofford , D.L. 2000 . PAUP. Phylogenetic Analysis Using Parsimony, Version 4.0b10 , Sunderland , MA : Sinauer Associates .
- Symons , S.J. , Clear , R.M. , Bell , K. and Butler , C. 2002 . “ Identifying wheat and barley seed affected by fusarium head blight ” . In Grain Biology Bulletin, 2 , 3rd , 1 – 5 . Winnipeg , MB : Canadian Grain Commission .
- Uhlig , S. , Jestoi , M. and Parikka , P. 2007 . Fusarium avenaceum – the North European situation . Food Microbiol. , 119 : 17 – 24 .
- Vogelgsang , S. , Sulyok , M. , Hecker , A. , Jenny , E. , Bänziger , I. , Schuhmacher , R. , Bucheli , T.D. and Forrer , H.R. 2008 . Toxigenicity and phytogenicity of Fusarium poae and Fusarium avenaceum on wheat . Eur. J. Plant Pathol. , 122 : 265 – 276 .
- Vujanovic , V. , Hamel , C. , Yergeau , E. and St-Arnaud , M. 2006 . Biodiversity and biogeography of Fusarium species from northeastern North American asparagus fields based on microbiological and molecular approaches . Microb. Ecol. , 51 : 242 – 255 .
- Vujanovic , V. , Vidovic , S. , Fernandez , M.R. , Daida , P. and Korber , D. 2009 . Whole-cell protein and ITS rDNA profiles as diagnostic tools to discriminate Fusarium avenaceum intraspecific variability and associated virulence . Can. J. Microbiol. , 55 : 117 – 125 .
- Ward , T.J. , Clear , R.M. , Rooney , A.P. , O'Donnell , K. , Gaba , D. , Patrick , S. , Starkey , D.E. , Gilbert , J. , Geiser , D.M. and Nowicki , T.W. 2008 . An adaptive evolutionary shift in Fusarium head blight pathogen populations is driving the rapid spread of more toxigenic Fusarium graminearum in North America . Fungal Genet. Biol. , 45 : 473 – 484 .
- White , T.J. , Bruns , T. , Lee , S. and Taylor , J.W. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: Guide to Methods and Applications , Edited by: Innis , M.A. , Gelfand , D.H. , Sninsky , J.J. and White , T.J. 315 – 322 . New York : Academic Press .
- Williams , G.D.V. , Faultey , R.A. , Jones , K.H. , Stewart , R.B. and Wheaton , E.E. 1988 . “ Estimating the effects of climatic change on agriculture in Saskatchewan ” . In The impacts of climatic variations on agriculture. Vol. 1: Assessments in cool, temperate and cold regions , Edited by: Parry , M.L. , Carter , T.R. and Konjin , N.T. 219 – 379 . Dordrecht : Kluwer .
- Yergeau , E. , Filion , M. , Vujanovic , V. and St-Arnaud , M. 2005 . A PCR-denaturing gradient gel electrophoresis (DGGE) approach to assess Fusarium diversity in asparagus . J. Microbiol. Meth. , 60 : 143 – 154 .
- Yli-Mattila , T. , Paavanen-Huhtala , S. , Bulat , S.A. , Alekhina , I.A. and Nirenberg , H.I. 2002 . Molecular, morphological and phylogenetic analysis of the Fusarium avenaceum/F. arthrosporioides/F. tricinctum species complex – a polyphasic approach . Mycolog. Res. , 106 : 655 – 669 .
- Yli-Mattila , T. , Paavanen-Huhtala , S. , Hannukkala , A. , Parikka , P. , Tahvonen , R. and Karjalainen , R. 1996 . Isozyme and RAPD-PCR analyses of Fusarium avenaceum strains from Finland . Plant Pathol. , 45 : 126 – 134 .