Abstract
The temporal patterns of Sclerotinia sclerotiorum ascospore dispersal in canola fields were studied at two North Dakota locations between 2005 and 2007. Seven-day volumetric spore samplers were used to monitor airborne ascospore populations while electronic data loggers recorded hourly information on air temperature, relative humidity, soil moisture and soil temperature under the canola canopy. Ascospore dispersal occurred during single periods that lasted 4 to 6 hours. In 2005 and 2007, most ascospores were collected between 10 am and 1 pm; however, in 2006, a drier than normal year, most were collected between 2 am and 7 am. In 2005 and 2007, the first sharp increase in ascospore dispersal was preceded by a 10-unit drop in relative humidity from close to saturation, and an increase in air temperature of 5 °C. In 2006, however, no significant changes in relative humidity, which remained around 90%, or air temperature, which hovered around 15 °C, were recorded prior to the start of the discharges. These nighttime discharges lasted 2 hours longer than daytime discharges. Daily discharges, daytime and nighttime, started when relative humidity was ≥ 90% and air temperature was around 15–16 °C. Multiple peak-days, days with mean ≥ 20 ascospores m−3, were recorded in 2005 and 2007, but none were recorded in 2006. Peak-days were associated with preceding periods of seven consecutive days with mean relative humidity ≥ 85% in the canola canopy.
Résumé
Les modes de dispersion temporelle des ascospores de Sclerotinia sclerotiorum dans les champs de canola ont été étudiés, de 2005 à 2007, à deux endroits dans le Dakota du Nord. Des échantillonneurs volumétriques de spores, de type sept jours, ont été utilisés pour surveiller les populations aéroportées d'ascospores tandis que des enregistreurs électroniques de données compilaient, sur une base horaire, la température de l'air, l'humidité relative, l'humidité du sol et la température du sol sous le couvert de canola. La dispersion des ascospores se produisait durant des périodes uniques de 4 à 6 heures. En 2005 et 2007, la majorité des ascospores étaient collectées de 10 h à 13 h. Toutefois, en 2006, une année plus sèche que la normale, la collecte s'est effectuée de 2 h à 7 h. En 2005 et 2007, la première augmentation marquée de la dispersion des ascospores avait été précédée par une baisse de 10 unités de l'humidité relative à partir du point de saturation, ou presque, et d'une augmentation de la température de l'air de 5 °C. En 2006, toutefois, aucun changement notable de l'humidité relative, qui est restée à environ 90 %, ou de la température de l'air, qui avoisinait les 15 °C, n'a été enregistré avant le début des dispersions. Ces dispersions nocturnes duraient 2 heures de plus que les dispersions diurnes. Les dispersions quotidiennes, diurnes et nocturnes, débutaient quand l'humidité relative était ≥ 90 % et que la température de l'air avoisinait 15 ou 16 °C. Plusieurs jours de pointe, jours dont la moyenne était ≥ 20 ascospores/m3, ont été enregistrés en 2005 et 2007, mais pas en 2006. Les jours de pointe étaient associés à des périodes précédentes de sept jours durant lesquels l'humidité relative moyenne sous le couvert du canola était ≥ 85 %.
Introduction
Ascospores are considered the primary inoculum for diseases caused by the fungus Sclerotinia sclerotiorum (Lib.) de Bary in many crops, including Sclerotinia stem rot (SSR) of canola (Brassica napus L.) (Jamaux et al., Citation1995). Ascospores are produced on apothecia from overwintered sclerotia, and when mature, they are forcibly released from asci into the air (Hartill & Underhill, Citation1976). In canola, ascospores initially colonize petals, leaves and even pollen grains (McLean, Citation1958; Jamaux et al., Citation1995; Huang et al., Citation1998) but eventually fungal mycelia move into the main branches and stem where they destroy tissues and may kill the plants. A close association between ascospore concentrations in the air and SSR intensity has been demonstrated in canola (Qandah, Citation2008).
SSR is endemic in canola-growing regions of North Dakota, which is the largest producer of canola in the USA (USDA-NASS, Citation2009). Between 1991 and 2002, the annual state average SSR incidence was 13.6% (Bradley & Lamey, Citation2005) with an estimated direct economic impact of approximately US$94 million (Lamey, Citation2003). Commercial cultivars are susceptible to the disease and lose on average 0.5% of their yield potential for every percentage unit of SSR incidence (del Rio et al., Citation2007). SSR management in North Dakota is conducted mainly through fungicide applications.
Among the environmental factors that influence release of S. sclerotiorum ascospores into the atmosphere, temperature, relative humidity and light have received more attention by researchers. Newton and Sequeira (Citation1972) indicated that ascospores could be released at air temperatures ranging between 4 and 32 °C, although the optimum was around 22 °C. In their study, a limited number of ascospores was released when apothecia were incubated at 8 to 16 °C; but that amount was severely reduced at temperatures below 8 or above 28 °C. Observations of ascospore release during daytime in fields led Hartill (Citation1980) to suggest that light was involved in the process. Later, Raynal (Citation1990) working with S. trifoliorum in growth chamber experiments, speculated that ascospore discharge is most likely due to the increase in air temperature rather than the effect of light itself. Raynal's suggestion was substantiated by Clarkson et al. (Citation2003) who used a ‘spore clock apparatus’ in the laboratory to show that S. sclerotiorum spores could be released in darkness. While there is no apparent consensus on which factor is responsible for initiating ascospore discharge, researchers seem to agree that under field conditions discharge and dispersal of S. sclerotiorum ascospores only occurs during daylight (Newton & Sequeira, Citation1972; Ben-Yephet & Bitton, Citation1985; McCartney & Lacey, Citation1991; Gutierrez & Shew, Citation1998). The assumption that relative humidity close to saturation is required for ascospore release was rejected by Clarkson et al. (Citation2003) who showed that ‘light ascospores discharge’ could still occur at relative humidities as low as 65–75%. In their laboratory studies on relative humidity, Clarkson et al. (Citation2003) kept apothecia at constant 15 °C.
While the previous studies provide a general frame for the conditions when ascospores could be released, a more detailed characterization of the environment associated with ascospore discharge and dispersal in canola fields is necessary to better understand how SSR epidemics develop. Such information could then be used in the development of better disease management tools against this important disease. Thus, the objectives of this study were to characterize the daily and seasonal S. sclerotiorum ascospore dispersal patterns in canola fields and to determine if there was an association between the timing of ascospore dispersal and environmental factors like temperature, rainfall and relative humidity.
Materials and methods
Field locations
The patterns of daily and seasonal ascospore dispersal in a canola field and their association with weather parameters were studied during a period of three years, between 2005 and 2007, at Cando and Langdon in North Dakota. The experimental plots were established in commercial canola fields.
Ascospore sampling
Two seven-day volumetric spore samplers were positioned 96 m apart and 0.96 m above the ground in commercial canola fields at Cando and Langdon, North Dakota during the summers of 2005–2007. Samplers were operated in 2005 and 2006 during a five-week period that started on 24 June, approximately one week prior to the beginning of flowering, and lasted through it. In 2007, air sampling started in 4 June, three weeks prior to flowering, and lasted through it. The instruments, a Burkard (Burkard Manufacturing Co., Hertfordshire, UK) and a locally made volumetric sampler, were operated using 12 v car batteries that were replaced every week. Both instruments have an internal drum that completed one full rotation every seven days. Prior to use, the air flow of both types of instruments was estimated at 10 L per minute (Burkard samplers) and 5 L per minute (generic samplers). Spore counts were later adjusted to reflect the difference in air volumes sampled. Spores were collected by both sampler types on a cellulose tape that was wrapped around the outer edge of the drum and coated with a fine layer of gel. The gel, made by mixing 50 g petroleum gel, 6 g paraffin wax, 0.75 mL phenol and 71 mL toluene was mixed under heat and applied to the tape side exposed to the air flow using a fine brush. Every seven days, a new tape replaced the exposed one. The start and end of each sampling period was clearly marked on the tapes and the time of day when tapes were retrieved was recorded. This information was later used to properly match spore counts with time of day and to estimate daily totals. In the laboratory, the exposed tape was cut into sections representing each one-day exposure. Each section was stained with lactophenol cotton blue and observed using a compound light microscope at 400 × magnification. Ascospores were identified based on their morphology (single-celled, hyaline and ellipsoid spores 4–6 × 9–14 μm in size (Kohn, Citation1979). If there was uncertainty about the identification of spores, those spores were not counted. The total number of ascospores collected per day was estimated by scanning the entire section with traverse passes of the objective over the tape. To calculate the number of ascospores collected per hour, the number of sequential traverse passes needed to scan the whole section was divided by 24 and the spores observed in that many passes were assigned to specific hours depending on their position on the tape. Hourly dispersal was estimated on seven separate days per location and year. Ascospore concentrations were expressed as the number of ascospores m−3 of air per hour or day.
Weather data
Hourly air temperature (°C), relative humidity (%), precipitation (mm) and soil moisture (kPa) were recorded from 4 June through 4 August in each year in Langdon and Cando using a set of Watchdog weather sensors (Watchdog Models 450 and 200, Spectrum Technologies, Plainfield, IL). The temperature and humidity sensors were positioned within the canola canopy at 0.45 m above soil level in a protective shield. Precipitation gauges were located just above the canopy and the soil moisture sensors (Watermark soil moisture sensors) were buried within the first 6 cm of soil. A 30-year mean cumulative precipitation and daily mean air temperature for the period were obtained from the North Dakota Agricultural Weather Network (http://ndawn.ndsu.nodak.edu/index.html) which has weather stations located within 2 km of the fields. Each spore sampler had a set of sensors placed to its side.
Data analysis
Hourly and daily ascospore concentrations were averaged per location and year. To visualize daily patterns of ascospore dispersal, the amount of ascospores detected every hour was expressed as a percentage of the daily total and plotted against time expressed in hours of a day. To visualize seasonal patterns, daily means of ascospore concentrations were plotted against time expressed in day units. To associate ascospore dispersal events to weather variables and to develop a predictive model, a mean of 20 ascospores m− 3 day− 1 was considered the threshold to separate regular days from peak-days. Multiple regression analysis with the stepwise selection option from Statistical Analysis System package (version 9.1; SAS Institute, Inc., Cary, NC) was used to identify weather parameters associated with ascospore concentrations in the air at each location and year. The means of air temperature, relative humidity, precipitation and soil moisture for periods of three, five and seven consecutive days preceding peak- and several non peak-days, from all three years of data, were used as independent variables while ascospore concentrations were used as the dependent variable. The mean of the variables identified as significant in the multiple regression analyses were grouped by periods (three, five and seven consecutive days as described) and used to generate predictive models using logistic regression analysis. The statistical significance of the models was evaluated using the Likelihood ratio test. The fit of the model was evaluated using the Hosmer-Lemeshow goodness-of-fit test, and the model's predictive power was evaluated using Goodman–Kruskal's Gamma, the c statistic, and the percentages of concordant and discordant pairs. The ‘crossvalidate’ option in proc logistic was used to generate expected probabilities from which a 2 × 2 table was made. Information from the tables were used to calculate each model's sensitivity (true positive proportion) and specificity (true negative proportion), as well as their overall accuracy. Model accuracy, expressed as a percentage, was calculated as [sensitivity × (total observed positives /total observations) + specificity × (total observed negatives /total observations)].
Results
Daily ascospore discharge patterns
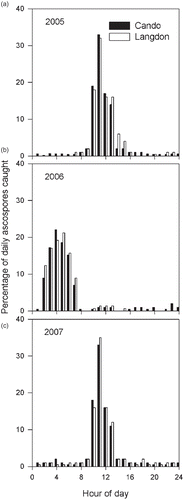
Very few differences were detected between locations in the percentage of ascospores trapped at specific hours of the day, although large differences were observed between years (). During 2005, > 85% of the ascospores trapped daily were collected between 10 am and 1 pm at both locations (). Ascospore concentrations in the air started to increase at about 9 am, with a five-fold increase at 10 am. By 2 pm, ascospore concentrations were back at the levels observed at 9 am. During 2006, the bulk of the discharge occurred between 2 am and 7 am (). Ascospore concentrations started to increase by midnight, with an almost 10-fold increase at 2 am. By 5:30 am, when the sun was still not visible in the horizon, almost 70% of the total ascospores trapped in a day had already been caught. During 2007, the daily discharge pattern was similar to that of 2005 ().
Fig. 1. Hourly Sclerotinia sclerotiorum ascospore dispersal pattern in Cando and Langdon, North Dakota in 2005 (a), 2006 (b) and 2007 (c). Each data point is the mean of 14 observations.
Daytime discharges during 2005 and 2007 were preceded by a 3–5 °C increase in temperature and a 10-unit drop in relative humidity that followed a prolonged period of high relative humidity. During both years, the hourly mean relative humidity remained > 95% between midnight and 8 am while the mean air temperature was 16 °C in Cando and 15 °C in Langdon ( and ). Between 8 and 10 am, humidity decreased an average of 10 units and the air temperature increased to 21 °C in Cando and to 19 °C in Langdon. During the next hour, relative humidity decreased by six units, air temperature increased 1 °C, and ascospore concentration reached its peak at both locations. During the third hour, ascospore concentrations decreased to the levels of the first hour while the relative humidity fell five units and the temperature increased one degree. During the fourth and final hour of heavy discharge, relative humidity decreased by another four units, while temperature climbed another degree. The driest and warmest period of the day occurred between 2 and 3 in the afternoon, when the mean temperature under the canopy reached 25 °C and the relative humidity was 67%. By then, the majority of ascospores had been discharged.
Fig. 2. Hourly mean relative humidity and air temperature in a 24-hour period in late June 2005 (a), 2006 (b) and 2007 (c) at Cando and Langdon, North Dakota. Each data point represents the mean of 14 observations.
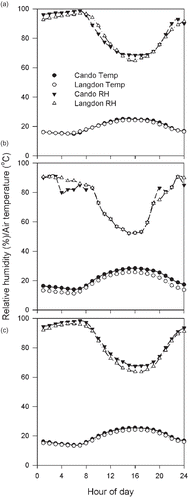
Nighttime discharges were preceded by a much shorter period of high relative humidity that was followed by less pronounced changes in relative humidity and air temperature (). The mean relative humidity between 11 pm and 2 am, the time preceding ascospore release, hovered around 90% and the mean temperature was 15 °C. During the second hour, relative humidity and temperature remained steady while the concentration of airborne ascospores increased by two-fold in Cando and by 30% in Langdon. During the third hour, ascospore concentrations increased by 23% and reached a peak in Cando while the relative humidity dropped by 10 units and the temperature remained steady; simultaneously, at Langdon, the relative humidity remained steady at around 90%, the temperature declined by one degree to 12 °C, and ascospore concentrations increased by 10%. During the fourth hour, ascospore concentrations returned to the levels of the second hour at Cando, and reached its peak at Langdon; during this time, air temperature reached its lowest point at both locations, but the relative humidity remained steady. In the last 2 hours of heavy discharge, relative humidity remained steady while the temperature increased by 1 °C as the sun rose over the horizon. By 10 am air temperature had climbed to 21 °C and the relative humidity decreased to 73%. The driest and warmest period of the day occurred between 3 and 5 pm when the air temperature was on average 27 °C and the relative humidity 53%.
Seasonal ascospore discharge patterns
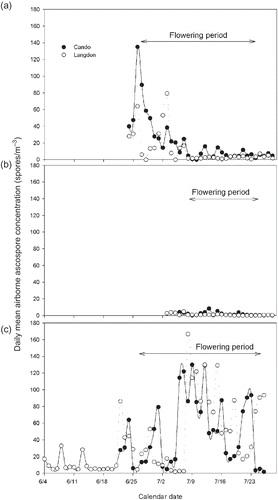
Ascospores were first detected on 24 June, 6 July and 4 June of 2005, 2006 and 2007, respectively, at both locations (–c). Several peaks of ascospore concentrations were detected through the flowering period during 2005 and 2007 at both locations, but no peaks were detected during 2006. Ten peak-days were recorded during the first 2 weeks of the flowering period in Cando in 2005 (), with the largest peak occurring on 26 June at 140 ascospores m−3 (). The average daily concentration of ascospores in Cando for the entire sampling period was 21.6 ascospores m−3. During the same year, at Langdon only four peak-days were recorded during the flowering season, but three occurred during the second week of flowering, with the highest concentration recorded on 3 July at 80 ascospores m−3 of air. The average daily concentration of ascospores for the entire sampling period at Langdon was 12.3 ascospores m−3 of air. During 2006, very low numbers of ascospores were recorded at both locations; none of them were considered peak-days (). The average daily concentrations of ascospores for the entire sampling period during 2006 were 2.1 and 1.1 ascospores m−3 of air at Cando and Langdon, respectively. During 2007, peak-days were more abundant and there were higher concentrations than in 2005 at both locations with an average daily ascospore concentration for the entire sampling period of 35.4 and 38.5 ascospores m−3 of air for Cando and Langdon, respectively. During the flowering period, which started on approximately 27 June at both locations, a total of 18 and 19 peak-days were recorded at Cando and Langdon, respectively (). Almost one third of these peak-days occurred during the second week of flowering, and the rest occurred during the second half of the flowering period at both locations. At Cando, three peak-days had concentrations >100 ascospores m−3 of air, whereas at Langdon five such peak-days were recorded.
Fig. 3. Sclerotinia sclerotiorum ascospore discharge patterns in Cando and Langdon, North Dakota for the period between 4 June and 28 July of 2005 (a), 2006 (b) and 2007 (c).
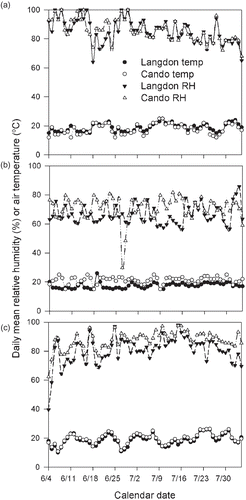
During 2005, the daily mean relative humidity during the first three weeks of the flowering period remained consistently above 80%, with many days at around 90%, but by the last week of flowering it fluctuated between 75 and 80% while the temperature fluctuated around 20 °C (). During 2006, Cando was a bit warmer and more humid than Langdon, but none of these locations had more than 2 days with daily mean relative humidity > 80% during the flowering season and the daily air temperature hovered around 20 °C (). Year 2007 was more humid although a bit cooler than 2005. The daily mean relative humidity in Cando remained > 90% during the flowering season, whereas in Langdon it hovered around 85% (). Air temperature was similar at both locations and ranged between 18 and 20 °C.
Association between weather and ascospore discharge
Of the three years of this study, 2005 had the most precipitation during the sampling period, with a total of 379 and 320 mm at Cando and Langdon, respectively (). That is 2.5 and 1.9 times higher than the 30-year mean for the period at each location, respectively. In 2005, during that same period, it rained almost every other day and almost one half of the period at both locations had days with daily mean relative humidity > 85%. The average daily temperature was around 18 °C at both locations, which is similar to the 30-year mean for the period. In contrast, 2006 was rather dry and warmer. There were only 8 and 12 rainy days during the period at Cando and Langdon, respectively, and the cumulative precipitation was one-tenth and one-third of that observed in 2005 at each location, respectively. The average temperature was 4 °C warmer than 2005 at both sites. In 2007, conditions at both locations were closer to what was observed in 2005 in terms of rain distribution, and daily mean air temperature and relative humidity (). However, some differences were detected in other environmental characteristics. Cando recorded 30% less rain, had fewer days with daily mean relative humidity > 85%, and had three additional days with air temperatures > 18 °C than in 2005; whereas Langdon had 30% fewer days with daily mean relative humidity > 85%, and had fewer rainy days.
Table 1. Environmental conditions under the canopy of a canola field at Cando and Langdon, ND during the period between 4 June and 4 August of 2005, 2006 and 2007
The multiple regression analyses revealed that a positive, statistically significant (α < 0.025, R2 > 0.85), association existed between ascospore concentrations and the mean relative humidity of the seven days preceding a peak-day at all locations in wet years. None of the other weather variables were significant. The mean relative humidity of groups of seven consecutive days that preceded peak-days was in general >85%.
All models, three- five- and seven-days, produced using logistic regressions were statistically significant and provided good fit to the data (); however, some differences were observed in their ability to predict peak-day occurrences. The latter two models were better than the three-day model since they had higher Gamma and c values, as well as a higher percentage of concordant and a smaller percentage of discordant pairs. The seven-day model had a greater true positive (higher sensitivity) and true negative (higher specificity) proportions than the three-day and five-day model and in general was more accurate than both as well (). The seven-day model was
Table 2. Statistical fitness of logistic regression models that associated Sclerotinia sclerotiorum ascospore peak-daysa to mean relative humidity under the canola canopy in North Dakota fields in 2005, 2006 and 2007
Discussion
The daily and seasonal patterns of ascospore dispersal by S. sclerotiorum were studied at two North Dakota locations between 2005 and 2007. The first and third years of the study were considered wet, based on the amount of precipitation that fell during the canola growing season. The second year was considered dry. In all three years, S. sclerotiorum ascospore dispersal occurred no more than once daily in a single event that lasted 4–6 hours. During dispersal, ascospore concentrations in the air followed a normal distribution curve with the highest peaks occurring around 11 am in wet years, and around 4 am in the dry year. During wet years, > 85% of ascospores were collected within a period of 4 hours (10 am to 1 pm) when air temperature was on average < 20 °C and relative humidity was > 80%. The ability of S. sclerotiorum to discharge ascospores in daylight has been reported by other researchers (Hartill, Citation1980; Raynal, Citation1990; McCartney & Lacey, Citation1991).
The ability of S. sclerotiorum to release ascospores in darkness under field conditions, as reported in this study, validates the laboratory observations made by Clarkson et al. (Citation2003); however, the epidemiological importance of this phenomenon, whether in the life cycle of this pathogen or in the development of SSR epidemics, is very likely small. Nighttime release of S. sclerotiorum ascospores was only observed during 2006, a dry year. During the other two years of this study, when precipitation was close to or above average, ascospores were released in daylight. Further, the ascospore concentrations detected during the dry year were very low, in general < 10% of what was observed during 2007, an ‘almost average year’ in terms of precipitation. The combination of low ascospore concentrations in the air, low relative humidity and scarce precipitation recorded during 2006 resulted in no visible symptom of infection on canola plants. However, since no efforts were made to observe plants other than canola in neighbouring areas, we cannot conclude that ascospores released in darkness during 2006 were ‘wasted away’ entirely, although it is very likely that that was their fate. In this sense, nighttime release of ascospores by S. sclerotiorum could be considered a simple reaction to adverse environmental conditions rather than an evolutionary strategy developed by this fungus; the fungus discharged ascospores at the only time when moisture conditions allowed build up of the pressure required for puffing (Hartill & Underhill, Citation1976).
To our knowledge, this is the first detailed description of the changes in air temperature and relative humidity that preceded and continued during release and dispersal of S. sclerotiorum ascospores in canola fields. The magnitude and timing of the changes observed support the conclusion that ascospore discharge is driven mainly by changes in relative humidity; although increases in air temperature could indirectly enhance the process by increasing the rate of relative humidity changes. While other researchers had recognized the importance of high relative humidity in the release process (Ramsdell et al., Citation1974; Laflamme & Archambault, Citation1990; Pearson et al., Citation1991; McCartney & Lacey, Citation1992; Hong & Michailides, Citation1998), this is the first time that detailed data supporting this observation is provided. The role of relative humidity in the discharge process was more evident during nighttime releases. Nighttime discharges were not triggered by changes in relative humidity or air temperature, although they took place only after a few hours of high relative humidity conditions had been accumulated. Once the release process had started, large drops in relative humidity coincided with significant increments in the number of airborne ascospores. These spikes in concentrations occurred when air temperatures remained more or less steady. During the last 2 hours of heavy discharge, however, relative humidity remained stable while the temperature increased by 1 °C. We cannot ascertain the exact mechanism that triggered nighttime ascospore discharge; however, wind gusts or even condensation of free water on the surface of mature apothecia could have been responsible for it (Laflamme & Archambault, Citation1990). While most ascospores were released between 10 am and 2 pm, during 2005 and 2007, few ascospores were detected at times when the relative humidity was at its lowest, around 65%. Similarly, small numbers of ascospores were detected during the dry year when humidity was around 57%. These observations validate results of laboratory studies that found S. sclerotiorum ascospores could be released under low humidity conditions (Clarkson et al., Citation2003).
Relative humidity had more influence in the production of ascospore peak-days during the canola flowering period than air temperature and precipitation. Since air temperature was always well within the normal range for S. sclerotiorum (Newton & Sequeira, Citation1972; Abawi & Grogan, Citation1979; Clarkson et al., Citation2003), it was not expected to be selected as a critical variable by the models. Lack of association between precipitation and ascospore dispersal on S. sclerotiorum has been reported by other researchers (Kora et al., Citation2005), although many agree that apothecia are usually produced after rain falls on closed crop canopies (Abawi & Grogan, Citation1979; Morral & Dueck, Citation1982; Kora et al., Citation2005). Since no two rain events are similar, they may differ in the total amount of precipitation, rate and temporal distribution (time of day, duration of rain), that variability may also contribute to the lack of statistical association. Yet, it is evident that rain could play an indirect effect on ascospore release by altering the duration of high relative humidity, influencing air temperature, as well as by forcing discharge of ascospores or washing them away.
Of the three models evaluated using logistic regression analysis, the seven-consecutive-day model provided the strongest predictive capability. The model indicates that the probability of a peak-day occurring increases dramatically when the seven-day mean relative humidity under the canola canopy increases from 85% to 90%. An apothecium could release ascospores during an average of nine days (Schwartz & Steadman, Citation1978) under laboratory conditions. If one assumes that apothecia may last one or two fewer days under field conditions, then the seven-day model would cover the entire period in which the apothecium is releasing ascospores. Further, S. sclerotiorum ascospores can infect plant tissues more readily when incubated at relative humidity ≥ 90% (Harikrishnan & del Rio, Citation2006). Thus, measuring relative humidity during seven consecutive days could provide an estimation not only of the risk of occurrence of peak-days but also of the risk of infection of plant tissues by ascospores. The characterization of the association between weather variables collected by standard weather stations and relative humidity under the canola canopy could then be used to develop disease-prediction systems that advise growers of risk of disease development.
Acknowledgements
This research was funded by award #58–5442–5–289 from the USDA-ARS Sclerotinia Initiative Program.
References
- Abawi , G.S. and Grogan , R.G. 1979 . Epidemiology of disease caused by Sclerotinia species . Phytopathology , 69 : 899 – 904 .
- Ben-Yephet , Y. and Bitton , S. 1985 . Use of a selective medium to study the dispersal of ascospores of Sclerotinia sclerotiorum . Phytoparasitica , 13 : 33 – 40 .
- Bradley , C.A. and Lamey , H.A. Canola disease situation in North Dakota, U.S.A., 1993–2004 . Proc. 14th Aust. Res. Assembly on Brassicas . Port Lincoln, Australia. pp. 33 – 34 .
- Clarkson , J.P. , Staveley , J. , Phelps , K. , Young , C.S. and Whipps , J.M. 2003 . Ascospore release and survival in Sclerotinia sclerotiorum . Mycol. Res. , 107 : 213 – 222 .
- del Rio , L.E. , Bradley , C.A. , Henson , R.A. , Endres , G.J. , Hanson , B.K. , Mckay , K. , Halvorson , M. , Porter , P.M. and Lamey , H.A. 2007 . Impact of Sclerotinia stem rot on yield of canola . Plant Dis. , 91 : 191 – 194 .
- Gutierrez , W.A. and Shew , H.D. 1998 . Identification and quantification of ascospores as the primary inoculum for collar rot of greenhouse-produced tobacco seedlings . Plant Dis. , 82 : 485 – 490 .
- Harikrishnan , R. and del Río , L.E. 2006 . Influence of temperature, relative humidity, ascospore concentration, and length of drying of colonized dry bean flowers on white mold development . Plant Dis. , 90 : 946 – 950 .
- Hartill , W.F.T. 1980 . Aerobiology of Sclerotinia sclerotiorum and Botrytis cinerea spores in New Zealand tobacco crops . New Zealand J. Agric. Res. , 23 : 259 – 262 .
- Hartill , W.F.T. and Underhill , A.P. 1976 . ‘Puffing’ in Sclerotinia sclerotiorum and S. minor . New Zealand J. Bot. , 14 : 355 – 358 .
- Hong , C. and Michailides , T.J. 1998 . Effect of temperature on the discharge and germination of ascospores by apothecia of Monilinia fructicola . Plant Dis. , 82 : 195 – 202 .
- Huang , H.C. , Kokko , E.G. , Erickson , R.S. and Hynes , R.K. 1998 . Infection of canola pollen by Sclerotinia sclerotiorum . Plant Pathol. Bull. , 7 : 71 – 77 .
- Kora , C. , Mcdonald , M.R. and Boland , G.J. 2005 . Epidemiology of Sclerotinia rot of carrot caused by Sclerotinia sclerotiorum . Can. J. Plant Pathol,. , 27 : 245 – 258 .
- Jamaux , I. , Gelie , B. and Lamarque , C. 1995 . Early stages of infection of rapeseed petals and leaves by Sclerotinia sclerotiorum revealed by scanning electron microscopy . Plant Pathol. , 44 : 22 – 30 .
- Kohn , L.M. 1979 . A monographic revision of the genus Sclerotinia . Mycotaxon , 9 : 365 – 444 .
- Laflamme , G. and Archambault , L. 1990 . Evaluation of microclimatic factors affecting ascospore release of Gremmeniella abietina var. balsamea . Can. J. Plant Pathol. , 12 : 190 – 194 .
- Lamey , H.A. The status of Sclerotinia sclerotiorum on canola in North America . Proc. 2003 Sclerotinia Initiative Annual Meeting . January 21–22 2003 , Bloomington, MN, USA.
- Mccartney , H.A. and Lacey , M.E. 1991 . The relationship between the release of ascospores of Sclerotinia sclerotiorum, infection and disease in sunflower plots in the United Kingdom . Grana , 30 : 486 – 492 .
- Mccartney , H.A. and Lacey , M.E. Release and dispersal of Sclerotinia ascospores in relation to infection . Proc. Brighton Crop Prot. Conf. – Pest and Diseases . Nov 23–26 1992 , Brighton, UK. Vol. 3A-2 , pp. 109 – 116 .
- Mclean , D.M. 1958 . The role of dead flower parts in infection of certain crucifers by Sclerotinia sclerotiorum (Lib.) de Bary . Plant Dis. Rep. , 42 ( 663 ) : 666
- Morral , R.A.A. and Dueck , J. 1982 . Epidemiology of Sclerotinia stem rot of rapeseed in Saskatchewan . Can. J. Plant Pathol. , 4 : 161 – 168 .
- Newton , H.C. and Sequeira , L. 1972 . Ascospores as the primary infective propagule of Sclerotinia sclerotiorum in Wisconsin . Plant Dis. , 56 : 798 – 802 .
- Pearson , R.C. , Siegfried , W. , Bodmer , M. and Schuepp , H. 1991 . Ascospore discharge and survival in Pseudopezicula tracheiphila, causal agent of Rotbrenner of grape . J. Phytopathol. , 132 : 177 – 185 .
- Qandah , I.S. 2008 . Epidemiological studies on Sclerotinia stem rot caused by Sclerotinia sclerotiorum (Lib.) de Bary on canola , Fargo, ND : Ph.D. Dissertation, North Dakota State University .
- Ramsdell , D.C. , Nelson , J.W. and Myers , R. 1974 . An epidemiological study of mummy berry disease of highbush blueberry . Phytopathology , 64 : 222 – 228 .
- Raynal , G. 1990 . Kinetics of the ascospore production of Sclerotinia trifoliorum (Eriks.) in growth chamber and under natural climatic conditions. Practical and epidemiological incidence . Agronomie , 10 : 561 – 572 .
- Schwartz , H.F. and Steadman , J.R. 1978 . Factors affecting sclerotium populations of, and apothecium production by, Sclerotinia sclerotiorum . Phytopathology , 68 : 383 – 388 .
- USDA–NASS . June 2009 2009 . North Dakota Agriculture. Ag Statistics No. 78 , June 2009 , North Dakota : National Agricultural Statistics Service .