Abstract
Field experiments were conducted during 2002–2004 at Saskatoon, Swift Current and Kyle, Saskatchewan to assess the effect of application frequency, timing and rotation of different fungicides on the suppression of ascochyta blight, and on yield and seed quality of chickpea cultivars ‘Myles’, ‘Sanford’ and ‘CDC Yuma’. Fungicides tested included chlorothalonil, azoxystrobin, pyraclostrobin, mancozeb and boscalid. Ascochyta blight severity ranged from 21 to 99% in untreated control plots and was significantly reduced through fungicide applications to levels below 25% in the most efficacious treatments. Increasing the number of fungicide applications was beneficial under high disease pressure, but was not always correlated with better ascochyta blight control. A pre-flower application had a positive effect by suppressing ascochyta blight severity, increasing seed yield or one-thousand seed weight in almost all experiments. Higher numbers of applications were not always correlated with higher seed yield and better seed quality. This may be due in part to poor yields in 2004 at Saskatoon and Kyle where above-average rainfall resulted in excessive vegetative growth. Different fungicide rotations had less of an impact on ascochyta blight management than the timing and the number of applications, but under moderate to high disease pressure, including strobilurin fungicides was beneficial. Five applications of mancozeb at two-week intervals were significantly less efficacious compared with rotations with other products.
Résumé
Des expériences ont été menées en champs, de 2002 à 2004, à Saskatoon, Swift Current et Kyle, en Saskatchewan, pour évaluer les effets de la fréquence, de la synchronisation et de la rotation des applications de divers fongicides sur l'inhibition de la brûlure ascochytique, sur le rendement ainsi que sur la qualité des semences chez les cultivars de pois chiches ‘Myles’, ‘Sanford’ et ‘CDC Yuma’. Les fongicides testés incluaient le chlorthalonil, l'azoxystrobine, la pyraclostrobine, le mancozèbe et le boscalide. La gravité de la brûlure ascochytique variait de 21 à 99 % sur les lots témoins non traités et était significativement réduite à moins de 25 % par l'application des fongicides les plus efficaces. L'augmentation du nombre d'applications de fongicide s'avérait bénéfique lorsque les plantes étaient soumises à de fortes pressions de maladie, mais n’était pas toujours corrélée à une gestion plus efficace de la brûlure ascochytique. Une application au stade préfloral avait un effet positif en ce sens qu'elle favorisait l'inhibition de la gravité de la brûlure ascochytique, accroissant ainsi le rendement en grains ou le poids de mille semences au cours de presque toutes les expériences. Des applications plus fréquentes n’étaient pas toujours corrélées à un rendement en grains plus élevé ni à une meilleure qualité des semences. Cela était peut-être dû en partie aux faibles rendements obtenus en 2004 à Saskatoon et à Kyle où des précipitations supérieures à la moyenne ont engendré une croissance végétative excessive. Différentes rotations de fongicides ont eu moins d'effets sur la gestion de la brûlure ascochytique que la synchronisation des applications et leur nombre, mais, sous des pressions de maladie de moyennes à élevées, l'ajout de fongicides à base de strobilurine a été bénéfique. Cinq applications de mancozèbe à intervalle de deux semaines ont été significativement moins efficaces que des rotations effectuées avec d'autres produits.
Introduction
Ascochyta blight of chickpea (Cicer arietinum L.) caused by Ascochyta rabiei (Pass.) Labrousse (tel. Didymella rabiei (Kovacheski) von Arx) has been a major biotic constraint to chickpea production in Saskatchewan where the vast majority of Canadian chickpea is grown. Under conditions conducive for disease development, yield losses of close to 100% have been encountered in the Canadian prairies (Chongo & Gossen, Citation2001), and the disease has contributed to a decline in chickpea area from more than 450 000 ha in 2001 to just over 30 000 ha in 2009 (Saskatchewan Ministry of Agriculture, 2010). Host plant resistance to the pathogen is partial and plants become increasingly more susceptible with the initiation of flowering (Chongo & Gossen, Citation2001). Major resistance break-down due to genetic changes in pathogen populations has been observed in Saskatchewan (Vail & Banniza, Citation2008) and the Palouse region of the northwestern USA (Peever et al., Citation2004), and has likely been promoted by the presence of the two mating types (Armstrong et al., Citation2000; Vail & Banniza, Citation2009).
The fungus survives on plant debris, and in and on infected seed, but air-borne ascospores also represent a major source of inoculum in Saskatchewan. Infection can occur as early as the seedling stage causing initially small whitish spots that enlarge to become tan coloured as cells in the developing lesion die. Lesions are surrounded by a distinct dark margin and eventually pycnidia form in circular patterns in the centre of lesions. Pycnospores are carried to other plant parts through rain splash. All plant parts can be infected, but in particular stem lesions are considered very damaging as they often cause stem breakage thereby destroying potentially healthy branches above the breakage point.
Wherever ascochyta blight occurs on chickpea, disease management relies on an integrated approach. This usually includes four-year crop rotations, the selection of cultivars with partial resistance, the use of disease-free seed and fungicidal seed treatments, best management practices in terms of seeding and weed control as well as foliar fungicide applications during the growing season (Reddy & Singh, Citation1990; Shtienberg et al., Citation2000; Chongo et al., Citation2003; Akem et al., Citation2004; Pande et al., Citation2005; Gan et al., Citation2006, Citation2009; Davidson & Kimber, Citation2007; Chandirasekaran et al., Citation2009). The timely and efficient use of fungicides has remained a major factor in the successful management of the disease and the economic viability of the crop. A wide range of fungicides have been tested for their efficacy in controlling ascochyta blight on chickpea (reviewed by Davidson & Kimber, Citation2007). The importance of fungicide applications for chickpea production in Saskatchewan is reflected in the increasing choice of fungicides that have been registered for ascochyta blight control on this crop in the past 10 years. Chlorothalonil (Bravo 500, Syngenta) became available for ascochyta blight control in chickpea in 1998, followed by azoxystrobin (Quadris, Syngenta), pyraclostrobin (Headline EC, BASF), and boscalid (Lance, BASF) in 2004, and prothioconazole (Proline 480 SC, Bayer CropScience) in 2008 (Saskatchewan Ministry of Agriculture, Citation1998, Citation2004, Citation2008). The use of pyraclostrobin against ascochyta blight was restricted to tank mixes with boscalid (also sold as Headline Duo, BASF) in 2008 over concerns of increasing strobilurin resistance in the population of A. rabiei in Saskatchewan (Chang et al., 2007). Mancozeb, a widely used fungicide against this disease in other countries such as Australia was never registered for this purpose in Canada, and is one of several other older chemicals currently under review by the Pest Management Regulatory Agency of Health Canada to determine whether they are safe based on current standards (Health Canada, Citation2011).
Earlier studies in Saskatchewan showed that fungicide applications at early, mid and/or late flower using chlorothalonil, azoxystrobin or mancozeb reduced ascochyta blight and increased yield under high disease pressure, but earlier and later applications at the pre-flower and late podding stages, respectively, were recognized as potentially beneficial to delay the onset of disease and to protect the forming seed in pods from infection (Chongo et al., 2003). The current study was initiated in 2002 among several others (Armstrong-Cho et al., Citation2008a , Citation2008b ) to further optimize the application of foliar fungicides and to develop recommendations for their efficient use. The objectives of this particular project were to investigate the effects of application frequency and timing from the pre-flower to the late podding stage, as well as the effect of different rotation of fungicides available at the time on ascochyta blight development, yield and seed quality in chickpea crops.
Materials and methods
Experiment 1: effect of application timing and frequency
Experiments were successfully established at Saskatoon (Kernen Research Farm, University of Saskatchewan) in 2003 and 2004 using chickpea cultivars ‘Myles’ and ‘CDC Yuma’, and in commercial chickpea fields at Kyle, Saskatchewan, with ‘Myles’ and ‘Sanford’ in 2004. Separate trials were established for each cultivar at these locations. Experiments were located in areas where chickpeas had been grown before, so none of the trials was artificially inoculated, but solely relied on natural inoculum. Cultivar ‘Myles’ is a desi chickpea with a fern leaf type and a fair rating (equivalent to a rating of 3–6 on a 0–9 scale as described by Chongo et al., Citation2004) for ascochyta blight resistance whereas ‘CDC Yuma’ and ‘Sanford’ are large-seeded kabuli chickpea cultivars with fern leaf type and unifoliate leaves, respectively, and a poor (equivalent to a rating of 7–8 on a 0–9 scale as described by Chongo et al., 2004) and very poor rating (equivalent to a rating of 9 on a 0–9 scale as described by Chongo et al., 2004) for ascochyta blight resistance (Saskatchewan Agriculture, Citation2007). At Saskatoon, plots of 2.25 × 6 m were established on 22 May 2003 and 25 May 2004, and at Kyle plots of 4 × 5 m were seeded on 15 May 2004.
Fungicides included in this experiment were chlorothalonil (Bravo 500, Syngenta, 1000 g active ingredient [a.i.] ha−1), pyraclostrobin (Headline, BASF, 100 g a.i. ha−1) and boscalid (Lance, BASF, and 300 g a.i. ha−1) applied as single, double or triple applications in various combinations (). The objective was to restrict the number of compounds in order to place the emphasis on timing while simultaneously testing different modes of actions. The experimental design was a randomized complete block design (RCBD) with four replicates.
Table 1. Fungicide treatment schedule in field experiments with desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ conducted at Saskatoon in 2003 and 2004, and at Kyle in 2004 to assess the effect of application timing and frequency on ascochyta blight development, yield and seed quality
Fungicides were applied in 200 L ha−1 carrier volume at 275 kPa using standard flat fan nozzles. Disease severity was assessed using a 0–11 scale (Horsfall & Barratt, Citation1945) at five arbitrary spots in each plot prior to each fungicide application and 10–14 days after the last fungicide treatment. Fungicides were applied as outlined in . Plant density was determined about four weeks after seeding. Plots were harvested on 19 September (‘Myles’) and 25 September (‘CDC Yuma’) 2003 and on 22 September 2004 at Saskatoon, and on 24 August 2004 at Kyle. Plots of ‘Myles’ established in a commercial field at Kyle in 2004 were not harvested because of operational errors. Seed yield, thousand-seed weight (TSW) and per cent seed infection were also determined.
Testing for infection of chickpea seeds was limited to 40 seeds per plot due to the high number of treatments and limitations in the availability of seed in some trials. Testing was conducted by surface sterilizing the seeds, plating them onto potato dextrose agar in Petri dishes and incubating the plates for one to two weeks. Ascochyta blight incidence was evaluated by counting the number of seeds with infection by A. rabiei.
Experiment 2: effect of fungicide rotations and application frequency
Experiments were conducted at Saskatoon (Kernen Research Farm, University of Saskatchewan) and at Swift Current (Semiarid Prairie Agricultural Research Centre Swift Current, Agriculture and Agri-Food Canada) from 2002 to 2004 using cultivars ‘CDC Yuma’ and ‘Myles’. Plots of 2.25 × 6 m were established on 13 May 2002 and 10 May 2004 at Swift Current, and on 22 May 2003 and 25 May 2004 at Saskatoon. A split-plot design with cultivar as main-plots and fungicides as sub-plot factors was used at Swift Current. Separate RCBD experiments with four replicates seeded for each cultivar at Saskatoon.
Fungicides included mancozeb (Dithane, Dow-Agro Sciences) applied at a low (1680 g a.i. ha−1, mancozeb-L) and a high (2440 g a.i. ha−1, mancozeb-H) rate, and chlorothalonil, azoxystrobin (Quadris, Syngenta, 125 g a.i. ha−1), pyraclostrobin and boscalid at single rates. These were the compounds available or becoming available during the period of the study. Mancozeb was included as it was a frequently used compound in Australian chickpea production at the time. The treatments included various sequences of fungicides as well as a tank-mix treatment of chlorothalonil and azoxystrobin (). Fungicide application, disease rating and yield data collection were conducted as described above. TSW was not recorded for Swift Current in 2002, and seed infection rates were not tested for this location in 2004.
Table 2. Fungicide treatment schedule in field experiments with desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivar ‘CDC Yuma’ conducted at Saskatoon in 2003 and 2004, and at Swift Current in 2002, 2003 and 2004 to assess the effect of different fungicide rotations and application frequency on ascochyta blight development, yield and seed quality
Weather data
Daily weather data were collected at the experimental locations at Saskatoon (Kernen Research Farm) and at Swift Current (Agriculture and Agri-Food Canada, Semiarid Prairie Agricultural Research Centre). No weather station was available at Kyle, so summary data collated by the Saskatchewan Ministry of Agriculture from the nearest location with weather records at Lacadena 24 km west of Kyle are presented to give an overview of local conditions in 2003 and 2004 (Anonymous, Citation2003, Citation2004). Comparisons of rainfall patterns with 30-year averages were based on data from Environment Canada for the respective locations (Environment Canada, Citation2011).
Statistical analysis
All statistical analyses were conducted using the Statistical Analysis System (SAS) version 9.2 (SAS Institute Inc., Cary, NC, USA). Grade values of the Horsfall–Barratt scale were converted to percentage values as described by Horsfall & Barratt (Citation1945), and mean percentage disease severity was calculated for each replicate of each treatment and is used for descriptive purposes in the text for ease of understanding. For descriptive purposes also, experiments with disease severity values up to 25% in control plots were considered to have low levels of disease, those with values between 25 to 50% moderate, and experiments with more than 50% ascochyta blight severity in control plots were referred to as having high disease levels. The percentages of disease severity assessed for each treatment during the course of the experiments were summarized by calculating the area under the disease progress curve (AUDPC) by trapezoidal integration (Shaner & Finney, Citation1977). All data were tested for normality and homogeneity of variance. Heterogeneous variances were modelled using the mixed procedure in SAS (Littell et al., Citation2006). Data were analyzed separately for each experiment using a mixed model with block or cultivar × block as random factors for experiments with a RCBD or a split-plot design, respectively, whereas all other factors were considered fixed. In the split-plot experiments, disease severity between cultivars varied significantly, so treatments were compared for each cultivar separately. Linear contrasts were used to determine differences between sprayed and unsprayed plots and to compare treatments with different application frequencies. Means were separated using Fisher's least significant difference (LSD).
Results
Weather conditions
A wide range of disease severity levels were observed over the three seasons of field studies triggered by variable precipitation patterns at the three test locations (Anonymous, 2003, 2004). Based on 30-year averages, Swift Current experienced a wet year in 2002, with June to September precipitation of 144, 73, 102 and 59 mm, which was well above the normal. Overall wet conditions also prevailed in 2004 at this location, with above normal rainfall in May, July, August and October (84, 61, 72 and 22 mm) whereas April, June and September had normal amounts of rain. At Saskatoon in 2003, rainfall was above normal in April (35 mm) and well above normal in July with 70 mm. The season of 2004 at Saskatoon started dry with no precipitation in April, but above normal rainfall in June, July and August (77, 66 and 74 mm). The area around Kyle experienced above normal rain in May (61 mm) and August (98 mm) in 2004.
Experiment 1: effect of fungicide timing and frequency
At the first assessment date at Saskatoon in 2003 (4 July) and 2004 (13 July), ascochyta blight severity was at or below 3% in control plots of ‘Myles’ and had reached 21% at the last assessment date on 26 August 2003, and 41% on 22 September 2004 (). In 2004 at Kyle, higher values of up to 7% were observed at the first assessment date (29 June) that increased to 28% ascochyta blight severity at the last assessment date on 24 August. Significant effects of fungicides on reducing disease severity were observed in all three trials, whereas yield effects were only observed at Saskatoon in 2003 (). However, fungicides improved TSW and lowered seed infection.
Fig. 1. Daily precipitation between day 160 (8 June) and day 270 (26 September 2002 and 2003; 27 September 2004) at Saskatoon and Swift Current, and progress curves of ascochyta blight in unsprayed control plots of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivar ‘CDC Yuma’ in experiments to evaluate the effect of application timing and frequency (Exp. 1), and to assess the effect of fungicide rotations and application frequency (Exp. 2) on ascochyta blight severity.
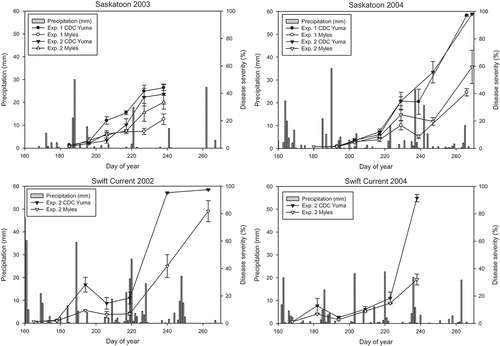
Table 3. Effects of fungicide application timing and frequency on ascochyta blight severity measured as Area Under the Disease Progress Curve (AUDPC), yield (kg ha− 1), thousand-seed weight (TSW) and seed infection rates (%) with Ascochyta rabiei of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004
Although ascochyta blight severity in kabuli plots was similar to those in plots of ‘Myles’ at the beginning of the season, higher ascochyta blight levels were recorded at the last assessment dates, with 44 and 97% in control plots of ‘CDC Yuma’ at Saskatoon in 2003 and 2004, and 99% in ‘Sanford’ at Kyle in 2004 (assessment dates as for ‘Myles’) (). Significant fungicide effects were observed at all three site years on disease severity, but on yield, TSW and seed infection rates only at Saskatoon and Kyle in 2004 (). Usually, ascochyta blight severity was reduced significantly through the best fungicide treatments, to levels below 10% under light to moderate disease pressure, and to 10 to 25% under high disease pressure.
Seed yields varied significantly among experiments, with highest yields obtained at Saskatoon in 2003 with 2082 kg ha−1 and 1970 kg ha−1 in control plots of ‘Myles’ and ‘CDC Yuma’, respectively, and lowest seed yields of 22 kg ha−1 in heavily diseased control plots of ‘Sanford’ at Kyle in 2004. Although higher than at Kyle, seed yields were also depressed in plots of ‘Myles’ and ‘CDC Yuma’ at Saskatoon in 2004.
Treatment effects on disease
In plots of ‘Myles’ at Saskatoon in 2003, fungicide applications reduced ascochyta blight severity in all treated plots compared with the control with the exception of Treatment 5 consisting of early and mid-flowering applications and one at late podding with chlorothalonil (). A two-application schedule with pyraclostrobin at early and mid-flowering, and boscalid at late podding (Treatment 11) also provided less control compared with all other treatments with three applications except for Treatment 5. Lack of a pre-flower application in both treatments likely allowed for early infection during major rain following this very early application ().
Table 4. Area Under the Disease Progress Curve (AUDPC) of ascochyta blight on desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide application timing and frequency
Effects of the remaining treatments were very similar, and linear contrasts indicated that one fungicide application (Treatment 2) could be as efficacious as two or three (Treatments 3–7) in controlling ascochyta blight (P > 0.05) under these conditions, although triple application treatments increased TSW compared with a single application treatment (P < 0.05). This suggests that protecting plants before flowering from infection that occurred shortly after the pre-flower application was the most critical application for preventing further significant disease increases (). This was also confirmed when comparing similar treatments with and without a pre-flower application (Treatments 5 vs. 7 and Treatments 11 vs. 13), which revealed a very highly significant impact of the pre-flower application on ascochyta blight control (P < 0.0001), that was also beneficial in terms of seed yield (P = 0.0012).
Only pre- and early flower applications with pyraclostrobin, followed by a late flower application with chlorothalonil (Treatment 8) resulted in better control compared with treatments with one or two applications at pre-, mid or late flowering with chlorothalonil (Treatments 2–4) ().
Results from the kabuli cultivar ‘CDC Yuma’ at Saskatoon in 2003, where moderate levels of ascochyta blight developed, also highlighted the importance of a pre-flower application. Treatments 5 and 11 that lacked the pre-flower fungicide applications provided significantly less control than the comparable Treatments 7 and 13 with pre-flower applications, respectively (P = 0.0002) and were also inferior to all other fungicide treatments (). Among those remaining treatments, efficacy in controlling ascochyta blight was similar.
Treatment effects were different at moderate ascochyta blight severity in ‘Myles’ at Saskatoon and at Kyle in 2004. A single application of chlorothalonil (Treatment 2) was insufficient to control ascochyta blight, resulting in disease severity levels similar to those in control plots at both locations (). Linear contrast analysis indicated that double and triple applications were superior to single application treatments at both locations in terms of ascochyta blight severity (P < 0.005), but only at Saskatoon were triple applications marginally outperforming double applications (P = 0.0440). Regular rain events, in particular in the second half of the growing season, were observed at this location (), resulting in further benefit from a third application. In 2004, pre-flower applications at Kyle and Saskatoon did not affect disease severity (P > 0.05), likely because other applications were equally important due to frequent rain events. However, both treatments without this earliest application (Treatments 5 and 11) performed poorly, although similarly to treatments with a pre- and mid-flower application of chlorothalonil (Treatment 3), and a pre- and mid-flower, and a late podding application of chlorothalonil (Treatment 7) (). Under slightly higher disease pressure at Saskatoon, the remaining two treatments using only chlorothalonil (Treatments 4 and 6) were equally poor in performance compared with Treatment 5. The remaining treatments that included pyraclostrobin were equally efficient (). Results suggested that chlorothalonil alone in a spray regime was insufficient for control during the 2004 season characterized by frequent rain events.
Under high disease pressure in ‘CDC Yuma’ plots at Saskatoon in 2004, double and triple applications of fungicides were superior to a single application (P < 0.0001). Indeed, a single application of chlorothalonil at the pre-flower stage had no effect compared with the control at this location, whereas it slightly reduced disease severity in ‘Sanford’ plots at Kyle in 2004 (). At Saskatoon, two applications with chlorothalonil, regardless of the timing (Treatments 3 and 4) as well as three applications of this fungicide lacking the pre-flower spray (Treatment 5) resulted in inferior control compared with the majority of the remaining treatments. Weak performances for Treatments 4 and 5 were also evident at Kyle (). Lack of a pre-flower application in Treatment 11 had less impact on ascochyta blight control in both trials than evident under moderate and very low disease pressure. However, linear contrast analysis indicated that a pre-flower application was still beneficial under high disease pressure (P = 0.0137 at Saskatoon, 2004, P = 0.0002 at Kyle 2004). At Kyle and Saskatoon plots of Treatment 11 had significantly less disease than those of Treatment 5 with the same timing of applications but a different fungicide (). Treatments with three applications that included two pyraclostrobin applications, applied at pre-flowering, followed by early, mid or late flowering or late podding (Treatments 8, 12, 13 and 14) were among the most efficacious treatments (). High AUDPC values indicated that three applications were insufficient during the 2004 season where frequent rain events promoted vegetative growth and infection on plant tissue not protected by a fungicide.
Treatment effects on seed yield and quality
Poor performance in ascochyta blight control of Treatment 5 (early and mid-flowering applications and one at late podding with chlorothalonil) in plots of ‘Myles’ at Saskatoon in 2003 was also reflected in seed yield of this treatment that was significantly lower than that of almost all other plots except for control plots and those of Treatment 11 lacking a pre-flower application as well (). Comparing similar treatments with and without a pre-flower application (Treatments 5 vs. 7 and Treatments 11 vs. 13) also revealed the beneficial impact of the pre-flower application on seed yield (P = 0.0012). TSW, however, was not greatly affected. Seed yield of Treatment 12 with a pre- and early flower application of pyraclostrobin followed by a late flower application with boscalid was significantly higher than that of most other treatments except for Treatments 2 and 3, although TSW of seed from the latter was significantly lower than that of Treatment 12 (). Seed infection rates were very low and A. rabiei was not detected in seeds of many plot samples, hence data were not further analyzed (data not presented). Seed infection was observed in seed from Treatments 1, 2, 5, 10 and 13.
Table 5. Yield (kg ha−1) of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide application timing and frequency to control ascochyta blight
In 2004 at Saskatoon where plots of ‘Myles’ had been harvested, yield was severely affected by early frost, resulting in a high percentage of underdeveloped green seed. Unsprayed control plots yielded as much seed as any of the treated plots (data not presented). Lack of ascochyta blight control of some treatments also reduced TSW and increased seed infection rates at this location, and double and triple application treatments outperformed the single application treatment (P < 0.05). This was particularly obvious for TSWs in control plots and those with Treatments 2, 4 and 6 (). In contrast, seeds of plots with Treatment 7 with three applications of chlorothalonil at pre- and mid-flower and at podding were among the largest seeds in the experiment (). Very few differences were observed among the remaining treatments in yield response and TSW, but treatments with applications at late flowering or podding tended to have larger seeds. Seed infection rates were very high in control plots (85%) and in plots of Treatments 2, 3 and 5 compared with Treatments 9, 10, 12 and 13 which had average infection rates of 10% and lower ().
Table 6. Thousand-seed weight (TSW) (g) of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide application timing and frequency to control ascochyta blight
Table 7. Ascochyta blight severity (%) in seeds of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide application timing and frequency to control ascochyta blight
Under moderate disease pressure in ‘CDC Yuma’ plots at Saskatoon in 2003, fungicide treatments had no effect on seed yield and TSW (data not presented). Seed infection rates were low and in the majority of plots no A. rabiei was recovered from the seed, so data were not further analyzed (data not presented). In contrast, under high disease pressure in 2004 treatments with three applications that included two pyraclostrobin applications, applied at pre-flowering, followed by early, mid- or later flowering or late podding (Treatments 8, 12, 13 and 14) were among the most efficacious treatments in ascochyta blight control with overall highest seed yields and the largest seeds ( and ). Lower disease and higher yields were, however, not always correlated with the lowest seed infection rates, nor were applications at late podding always those with lowest infection rates. Few differences were evident in yields, TSW and seed infection of Sanford at Kyle in 2004 (, , ).
Experiment 2: effect of fungicide rotations and application frequency
A wide range of ascochyta blight severity was also observed in experiments comparing different fungicide rotations. Trace levels of ascochyta blight were observed at the first assessment dates in all plots at both locations (Swift Current: 13 May 2002; 10 May 2004; for Saskatoon see above). At the end of the season, moderate ascochyta blight levels were observed in control plots of ‘Myles’ with 32% at Swift Current in 2004, and 33% at Saskatoon in 2003 (). Moderate disease levels of 39% were also encountered in control plots of ‘CDC Yuma’ at Saskatoon in 2003. More severe epidemics developed in control plots of ‘CDC Yuma’ at Saskatoon in 2004 with 56% disease severity and in plots of ‘Myles’ in 2004 with 59%. At Swift Current, ascochyta blight severity in plots of ‘CDC Yuma’ reached 91% in 2004 and 95% in 2002, and 82% in ‘Myles’ in 2002 (). Significant treatment effects on disease severity were observed in all trials, though yield effects were only evident in plots of ‘Myles’ at Saskatoon in 2003, and at Swift Current in 2002 ( and ). TSW was affected in Myles’ plots and ‘CDC Yuma’ plots at Saskatoon in 2003, and in ‘CDC Yuma plots’ in 2004. This cultivar was also highly diseased at Saskatoon in 2004 where disease severity levels reached 98%. Similar to trials described above, the most efficacious fungicide treatments reduced ascochyta blight severity to levels at or below 10%, even under high disease pressure.
Table 8. Effects of fungicide rotations and application frequency on ascochyta blight severity measured as Area Under the Disease Progress Curve (AUDPC), yield (kg ha− 1), thousand-seed weight (TSW) and seed infection rates (%) with Ascochyta rabiei of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ at Saskatoon in 2003 and 2004
Table 9. Effects of fungicide rotations and application frequency on ascochyta blight severity measured as Area Under the Disease Progress Curve (AUDPC), yield (kg ha−1), thousand-seed weight (TSW) and seed infection rates (%) with Ascochyta rabiei of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ at Swift Current in 2003 and 2004
Treatment effect on disease
Under low disease pressure in ‘Myles’ plots at Saskatoon in 2003 and at Swift Current in 2004, all fungicide applications reduced ascochyta blight severity in comparison to the unsprayed control plots (). For the most part, two applications were as effective as three or more applications irrespective of product choice. In both trials, Treatments 5 and 6 with a mancozeb-L and chlorothalonil application at pre- and early flower, followed by an application at late podding with azoxystrobin or pyraclostrobin showed inferior disease suppression. There were no differences among treatments with three or four applications, but at Saskatoon, Treatment 11 with five applications including two pyraclostrobin sprays was superior to Treatment 9 with five mancozeb-L applications, but was similar to five mancozeb-H applications (Treatment 10) ().
Table 10. Area Under the Disease Progress Curve (AUDPC) of ascochyta blight on desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ Saskatoon in 2003 and 2004, and at Swift Current in 2002 and 2004 in response to different fungicide rotations and application frequency
In plots of ‘Myles’ at Saskatoon in 2004 with 59% ascochyta blight in control plots at the end of the season (), all fungicide applications reduced ascochyta blight severity in comparison to the unsprayed control plots except for Treatment 5 with a pre-flower application of mancozeb-L, followed by an early flower application with chlorothalonil, and a late podding application with azoxystrobin (). Among two application regimes, Treatment 2 (chlorothalonil and azoxystrobin at pre- and mid- to late flowering, respectively) controlled ascochyta blight as well as Treatment 3 (chlorothalonil and pyraclostrobin at the same growth stages), but a chlorothalonil/azoxystrobin tank mix applied at those growth stages resulted in significantly lower disease than in plots of Treatment 2. Two applications were often as efficacious as treatments with four or five applications. There were no differences among treatments with three or four applications at both locations (). When comparing fungicide rotation regimes with five applications, Treatment 11 with two mancozeb-L, one mancozeb-H and 2 pyraclostrobin applications resulted in lower disease compared with Treatments 9 and 10 with 5 mancozeb-L or mancozeb-H applications, respectively.
Under moderate disease pressure in plots of ‘CDC Yuma’ at Saskatoon in 2003 and under high disease pressure in ‘Myles’ at Swift Current in 2002 (data not presented), all fungicide rotation treatments equally reduced ascochyta blight severity compared with the control, except for disease levels in ‘CDC Yuma’ plots of Treatments 5 and 6 that did not differ from those of control plots ().
Under higher disease pressure in ‘CDC Yuma’ plots at Saskatoon in 2004, all fungicide treatments decreased ascochyta blight compared with the control (). Apart from Treatments 2 and 3, all treatments had similar effects on disease development. A similar response was observed in ‘CDC Yuma’ plots at Swift Current in 2004 where ascochyta blight severity reached 91% in control plots (). At this location, however, Treatments 5 and 6 with an initial mancozeb-L application had disease levels as high as in control plots. Under slightly higher disease pressure at this location in 2002, Treatment 2 with a chlorothalonil and azoxystrobin application was inferior to the other two double applications (Treatments 3 and 4) in controlling ascochyta blight in ‘CDC Yuma’. There was no difference among treatments with four fungicide applications, but Treatment 11 with two mancozeb-L and one mancozeb-H applications and two pyraclostrobin applications outperformed Treatments 9 and 10 with mancozeb-L or mancozeb-H applications, respectively.
Effects on seed yield and quality
Under low disease pressure, poor ascochyta blight control of Treatments 5 and 6 in plots of ‘Myles’ was illustrated by low yields comparable to that of control plots at Saskatoon in 2003, and significantly lower yields than almost all other treatments at Swift Current in 2004 (). TSW was also negatively affected by Treatments 5 and 6 at Saskatoon, but no fungicide effects on TSW were apparent at Swift Current. Yield differences among the remaining treatments were minimal. At Saskatoon in 2003, Treatments 8 and 11 outperformed several of the other treatments in terms of yield, and the latter also resulted in larger TSW. Seed infection was not evaluated in ‘Myles’ seed at Swift Current in 2004, and was too low at Saskatoon in 2003 to warrant further analysis (data not presented).
Table 11. Seed yield (kg ha− 1) of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or ‘Sanford’ at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide rotations and application frequency to control ascochyta blight
Under moderate disease pressure in plots ‘CDC Yuma’ at Saskatoon in 2003, no yield effect (data not presented) and only minor effects on TSW were observed (). At Swift Current, where high disease levels prevailed in control plots of ‘Myles’, no differences in seed yield were observed among fungicide rotations with equal numbers of applications, except for Treatment 9 with mancozeb-L that resulted in lower yield than the other two treatments with five fungicide applications (Treatments 10 and 11) (). Lower yields were encountered in treatments that included mancozeb-L (Treatments 5, 6 and 9) and in two of the treatments restricted to two applications (Treatments 2 and 3), but not in the third (Treatment 4) with a chlorothalonil/azoxystrobin tank mix. Seed infection rates were too low at Saskatoon to warrant analysis, and revealed no differences among fungicide treatments at Swift Current (data not presented).
Table 12. Thousand-seed weight (TSW) (g) of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ at Saskatoon in 2003 and 2004, and at Swift Current in 2004 in response to different fungicide rotations and application frequency to control ascochyta blight
No effects of fungicide on yield and TSW were observed under high disease pressure in plots of ‘Myles’ at Saskatoon in 2004 (data not presented). Testing of seeds revealed significant differences in seed infection among treatments (). All fungicide treatments reduced seed infection compared with the control where 71% of seeds were infected. Treatments varied widely in their efficacy to protect seeds from infection. Among double applications, a tank mix of chlorothalonil and azoxystrobin was superior to the other two double applications, reducing seed infection by over 20% in comparison. Among triple application treatments, including pyraclostrobin (Treatment 6) rather than azoxystrobin (Treatment 5) more than halved infection rates. Among treatments with four fungicide applications, Treatment 15 was superior to all other treatments in protecting seeds, whereas Treatment 11 was the best among five-application treatments ().
Table 13. Ascochyta blight severity (%) in seeds of desi chickpea cultivar ‘Myles’ and kabuli chickpea cultivars ‘CDC Yuma’ or Sanford at Saskatoon in 2003 and 2004, and at Kyle in 2004 in response to different fungicide application timing and frequency to control ascochyta blight
No yield effect was observed in ‘CDC Yuma’ plots at Saskatoon in 2004 (data not presented), whereas at Swift Current in 2004 all treatments except for Treatment 6 equally improved yield of CDC Yuma compared with the control (). At Swift Current in 2002, clear yield increases were recorded in plots with four fungicide applications and Treatment 11 compared with other treatments, but no differences among fungicide rotations were observed other than a higher efficacy in Treatment 11 vs. 9 and 10 that included only mancozeb at two concentrations. TSW at Saskatoon in 2004 was affected by fungicide treatment, and was reduced in treatments with mancozeb (). Double application of fungicides, irrespective of product, and Treatments 9, 10 and 12 that were dominated by mancozeb, were inferior in protecting seeds from infection compared with the other treatments at this location (). In 2004, no effect on TSW was apparent in ‘CDC Yuma’ from Swift Current, and in 2002, seeds were not assessed for TSW. Seeds from 2002, but not from 2004, were tested for ascochyta blight infection. Treatments with mancozeb (Treatments 5, 6, 9, 10 and 12) and Treatment 2 (pre-flower chlorothalonil and mid-late flower azoxystrobin applications) failed to control seed infections.
Discussion
Variable rainfall patterns and use of susceptible and partially resistant chickpea cultivars allowed for fungicide application regimes to be tested under a range of disease severities ranging from 21 to 99% in control plots. Experiments revealed that when low to moderate levels of disease developed, such as in the ‘Myles’ plots of this project, fewer applications were usually sufficient to significantly decrease ascochyta blight severity; yet, a single application was only comparable to two or three applications in terms of disease suppression at Saskatoon in 2003. However, higher numbers of applications were not beneficial in terms of yield, and only increased TSW at Saskatoon in 2004. Previous studies had shown that fungicide applications did not suppress disease severity compared with unsprayed control plots under low and moderate disease pressure (Chongo et al., 2003). Increasing the number of applications was advantageous under high disease pressure in ‘CDC Yuma’ plots at Saskatoon, Kyle and Swift Current in 2004. Three applications in the first study on timing appeared to be insufficient to fully control the disease. Four applications in the second study at Saskatoon and Swift Current in 2004 looking at different fungicide rotations suppressed disease levels further, in particular with regard to seed infection rates. In contrast, Chang et al. (Citation2007) using up to five azoxystrobin or pyraclostrobin applications, found that more than three applications did not result in significantly further ascochyta blight suppression, but seed infection was not considered.
Adding additional fungicide applications obviously increases production costs and is only economical as long as the increase in the value of the crop through higher seed yield and/or quality is at least equivalent to this additional cost. Positive linear relationships have been described for disease severity and yield loss (Chongo et al., 2003; Shtienberg et al., Citation2005), indicating that as long as disease is suppressed through additional fungicide applications, yield increases can be expected.
If fungicide costs are based on suggested retail prices from 2009 (Saskatchewan Ministry of Agriculture, Citation2010), and the value for grade one desi and kabuli chickpeas on September 2010 prices of $0.56 kg−1 and $0.68 kg−1 (http://www.agriculture.gov.sk.ca/MarketTrends/), 64 kg ha−1 of grade 1 kabuli and 77 kg ha−1 of grade 1 desi seed would be required to cover the cost for an additional application of pyraclostrobin, and 57 kg ha−1 of kabuli and 70 kg ha−1 for each additional chlorothalonil spray. For each additional boscalid application an additional 105 kg ha−1 of kabuli and 127 kg ha−1 of desi would have to be produced, whereas for an additional application of azoxystrobin 67 kg ha−1 of kabuli and 82 kg ha−1 of desi seed would be required. These calculations do not, however, include application costs. Considering the more normal yield data from ‘CDC Yuma’ plots of the fungicide rotation study at Swift Current in 2004, it appears that adding two more applications (Treatments 2 vs. 7 and 4 vs. 8) would be economical, assuming the increases in seed yield are of grade 1 quality. However, TSW data from this trial indicate that TSW was on average smaller than the 9 mm (TSW ∼ 415 g) required for grade 1 for this cultivar. In addition, yield differences between those treatments were in fact not significant, indicating a high level of variability. Harvested seeds were not graded, which would be required to truly assess the economic viability of extra fungicide applications.
A second factor to be considered when assessing application efficacy and its economics based on seed yield data is the fact that high precipitation during the growing season, the driver for major epidemics, can also induce excessive vegetative growth in chickpea, resulting in generally poor seed set and seed development irrespective of disease. This confounding effect was most obvious in the second study when comparing disease, yield and seed infection data of ‘CDC Yuma’ plots at Saskatoon and Swift Current in 2004. Disease control was comparable in both trials, but seed yields at Saskatoon were a fraction of what was harvested at Swift Current, and well below the average of approximately 1200 kg ha−1 expected for a commercially grown large-sized kabuli chickpea.
No one single application regime with regard to application timing appeared to exceed others at all locations and years, emphasizing the importance of field scouting and spraying in response to local weather conditions. This was previously shown in Israel (Shtienberg et al., 2000) and more recently in Australia, where a per calendar spray schedule was compared with a more targeted three-application regime with mixed results (Shtienberg et al., 2006). Detailed analyses of data in Australia revealed that inconsistent ascochyta blight control in some of the trials was primarily due to lack of optimal application timing of the protectant fungicides prior to rain events. A pre-flower application in the current project had a positive effect on ascochyta blight control, seed yield or TSW in all fungicide timing trials except in ‘Myles’ plots at Kyle in 2004. This was clearly because of significant rain early in the season in those years, but it also highlighted how important preventing or delaying early disease onset is for the ultimate outcome of an epidemic. Therefore, a pre-flower application has been incorporated as a recommendation into an integrated ascochyta blight management guide developed by the Saskatchewan Ministry of Agriculture. Shtienberg et al. (Citation2006) suggested that initial spraying should be based on symptoms in the field, but this has proven less successful in Saskatchewan as initial symptoms were too easily overlooked by chickpea producers. Fungicide applications at late podding did not reduce seed infection rates consistently. As a plant with indeterminate growth, chickpea pods on any one plant develop over a long period of time, so consistent suppression of the disease throughout the growing season is required to prevent seed infection. A fungicide application toward the end of the growing season may only have an impact if weather conditions are conducive for infection at that point.
Overall, the fungicide rotation study showed that the choice of active ingredient appeared to be less critical for ascochyta blight management compared with the timing and frequency of application in response to local conditions. However, the use of only mancozeb at low and high rates when disease pressure was moderate or high was usually inferior to a fungicide rotation that included pyraclostrobin, and even compared with many of the treatments with fewer applications. Regular application of mancozeb, a fungicide cheaper than most others used on chickpea, throughout the chickpea growing season has been one management strategy in Australia (Shtienberg et al., 2006). In both studies of the current project, it was obvious that the inclusion of two strobilurin fungicides improved ascochyta blight control particularly in highly diseased ‘CDC Yuma’ plots in 2004, although this did not consistently increase yield and TSW, nor did it always reduce seed infection rates. Application timing was based on plant growth stage rather than rain events, so it is likely that at least some of the higher efficacy of these strobilurin fungicides was based on their semi-systemic characteristics in situations where the application occurred after a rain event. In Israel, field trials showed that tebuconazole, which is not registered for chickpea in Canada, had a post-infection activity of up to three days (Shtienberg et al., 2000). Increasing fungicide insensitivity in A. rabiei to strobilurin fungicides has become a major concern in Saskatchewan (Chang et al., 2007). Hence, the use of different compounds throughout the growing season when azoxystrobin and pyraclostrobin are used is very important for the management of fungicide insensitivity, especially in years with high disease pressure and frequent applications.
Based on this study, and in support of research done elsewhere, it is obvious that the optimal timing of fungicide application is the most critical aspect of ascochyta blight management in chickpea in terms of efficacy and number of applications necessary. Application timing is critical both in reference to growth stage of the crop, as well as to rain events. Contact fungicides need to be applied prior to a rain event and semi-systemic or systemic fungicides within a short window after a rain event. Ideally, disease forecasting models should be employed to predict critical periods of infection ahead of time based on weather forecasts. In Israel, where airborne ascospores represent the primary source of initial inoculum, an empirical model based on pseudothecial maturation in response to temperature and moisture conditions has been developed and successfully used commercially since 2001 (Shtienberg et al., 2005). Based on trap plants placed into fields with chickpea stubble in Saskatchewan, it is assumed that ascospores are already released prior to emergence of chickpea seedlings (Y. Gan, unpublished data). Furthermore, as Shtienberg et al. (Citation2006) pointed out, the successful use of a forecasting model is only feasible if reliable weather forecasts are available three to four days ahead of rain. An assessment of precipitation forecasts up to five days ahead of time at Saskatoon in 2000 revealed that a ‘wet’ forecast had an accuracy of 55% on the day it was issued which declined to 53% three days ahead, and further to 50% for the forecast four to five days ahead (Ripley & Archibold, Citation2002). It can be speculated that seasonal accuracy in rain predication varies and is even lower during the growing season in Saskatchewan as a lot of the precipitation during that period occurs as thunderstorms that are notoriously very difficult to forecast. This likely represents the biggest obstacle in implementing disease-forecasting systems for ascochyta blight control in chickpea in Saskatchewan.
In conclusion, experiments showed that control of ascochyta blight through fungicide applications was highly dependent on location and year. A pre-flower application was beneficial in several trials, indicating that delaying the onset of disease is very important for the management of the disease. In general, applying a fungicide at the right time was more important than the choice of active ingredient although using two strobilurin fungicides under high disease pressure was often beneficial in reducing disease severity and seed infection.
tcjp_a_561875_sup_18220130.pdf
Download PDF (88.9 KB)Acknowledgements
We are grateful for financial support from the Agricultural Development Fund of the Saskatchewan Ministry of Agriculture. Technical support was provided by Teri Ife, Gerry Stuber, Cal MacDonald, Mark Thompson and Megan England.
References
- Akem , C. , Kabbabeh , S. and Ahmed , S. 2004 . Integrating cultivar resistance with a single fungicide spray to manage ascochyta blight for increased chickpea yields . Plant Pathol. J. , 3 : 105 – 110 .
- Anonymous . 2003 . Crop Report. Report Number 28, October 22, 2003 , Regina, SK : Saskatchewan Ministry of Agriculture .
- Anonymous . 2004 . Crop Report. Report Number 34, December 3, 2004 , Regina, SK : Saskatchewan Ministry of Agriculture .
- Armstrong-Cho , C. , Chongo , G. , Gossen , B.D. and Duczek , L.J. 2000 . Mating type distribution and incidence of the teleomorph of Ascochyta rabiei (Didymella rabiei) in Canada . Can. J. Plant Pathol. , 23 : 110 – 113 .
- Armstrong-Cho , C. , Chongo , G. , Wolf , T. and Banniza , S. 2008a . Effect of carrier volume on ascochyta blight control in chickpea . Crop Prot. , 27 : 1020 – 1030 .
- Armstrong-Cho , C. , Chongo , G. , Wolf , T. , Hogg , T. , Johnson , E. and Banniza , S. 2008b . The effect of spray quality on ascochyta blight control in chickpea . Crop Prot. , 27 : 700 – 709 .
- Chandirasekaran , R. , Warkentin , T. , Gan , Y. , Shirtliffe , S.J. , Gossen , B.D. , Tar'an , B. and Banniza , S. 2009 . Improved sources of resistance to ascochyta blight in chickpea . Can. J. Plant Sci. , 89 : 107 – 118 .
- Chang , K.F. , Ahmed , H.U. , Hwang , S.F. , Gossen , B.D. , Strelkov , S.E. , Blade , S.F. and Turnbull , G.D. 2007 . Sensitivity of field populations of Ascochyta rabiei to chlorothalonil, mancozeb and pyraclostrobin fungicides and effect of strobilurin fungicides on the progress of ascochyta blight of chickpea . Can. J. Plant Sci. , 87 : 937 – 944 .
- Chongo , G. , Buchwaldt , L. , Gossen , B.D. , Lafond , G.P. , May , W.E. , Johnson , E.N. and Hogg , T. 2003 . Foliar fungicides to manage ascochyta blight [Ascochyta rabiei] of chickpea in Canada . Can. J. Plant Pathol. , 25 : 135 – 142 .
- Chongo , G. and Gossen , B.D. 2001 . Effect of plant age on resistance to Ascochyta rabiei in chickpea . Can. J. Plant Pathol. , 23 : 358 – 363 .
- Chongo , G. , Gossen , B.D. , Buchwaldt , L. , Adhikari , T. and Rimmer , S.R. 2004 . Genetic diversity of Ascochyta rabiei in Canada . Plant Dis , 88 : 4 – 10 .
- Davidson , J.A. and Kimber , R.B.E. 2007 . Integrated disease management of ascochyta blight in pulse crops . Eur. J. Plant Pathol. , 119 : 99 – 110 .
- Environment Canada (2011). National Climate Data and Information Archive http://www.climate.weatheroffice.gc.ca/Welcome_e.html (http://www.climate.weatheroffice.gc.ca/Welcome_e.html) (Accessed: 26 January 2011 ).
- Gan , Y.T. , Siddique , K.H.M. , Macleod , W.J. and Jayakumar , J. 2006 . Management options for minimizing damage by ascochyta blight in chickpea . Field Crops Res,. , 97 : 121 – 134 .
- Gan , Y.T. , Warkentin , T. , Chandirasekaran , R. , Gossen , D.B. , Wolf , T. and Banniza , S. 2009 . Effects of planting pattern and fungicide application systems on ascochyta blight control and seed yield in chickpea . Agron. J. , 101 : 1548 – 1555 .
- Health Canada (2011). Re-evaluation Note REV2009-07, PMRA Re-evaluation Workplan (April 2009 to March 2010) http://www.hc-sc.gc.ca/cps-spc/pubs/pest/_decisions/rev2009-07/index-eng.php (http://www.hc-sc.gc.ca/cps-spc/pubs/pest/_decisions/rev2009-07/index-eng.php) (Accessed: 20 January 2011 ).
- Horsfall , J.G. and Barratt , R.W. 1945 . An improved grading system for measuring plant diseases . Phytopathology , 35 : 65
- Littell , R.C. , Milliken , G.A. , Stroup , W.W. , Wolfinger , R.D. and Schabenberger , O. 2006 . SAS for mixed models , 2nd , Cary, NC : SAS Publishing .
- Pande , S. , Siddique , K.H.M. , Kishore , G.K. , Bayaa , B. , Gaur , P.M. , Gowda , C.L.L. , Bretag , T.W and Crouch , J.H . 2005 . Ascochyta blight of chickpea (Cicer arientinum L.): a review of biology, pathogenicity and disease management . Aust. J. Agr. Res. , 56 : 317 – 332 .
- Peever , T.L. , Salimath , S.S. , Su , G. , Kaiser , W.J. and Muehlbauer , F.J. 2004 . Historical and contemporary multilocus population structure of Ascochyta rabiei (teleomorph: Didymella rabiei) in the Pacific Northwest of the United States . Mol. Ecol. , 13 : 291 – 309 .
- Reddy , M.V. and Singh , K.B. 1990 . Management of ascochyta blight of chickpea through integration of host plant tolerance and foliar spraying of chlorothalonil . Indian J. Plant Prot. , 18 : 65 – 69 .
- Ripley , E.A. and Archibold , O.W. 2002 . Accuracy of Canadian short-and medium-range weather forecasts . Weather , 57 : 448 – 457 .
- Saskatchewan Ministry of Agriculture . 1998 . Guide to crop protection , Regina, SK : Saskatchewan Agriculture and Food .
- Saskatchewan Ministry of Agriculture . 2004 . Guide to crop protection , Regina, SK : Saskatchewan Agriculture and Food .
- Saskatchewan Ministry of Agriculture . 2007 . Varieties of grain crops 2007 , Saskatoon, SK : Western Producer .
- Saskatchewan Ministry of Agriculture . 2008 . Guide to crop protection , Regina, SK : Saskatchewan Ministry of Agriculture .
- Saskatchewan Ministry of Agriculture (2010). 2009 speciality crop report. Statistics. Saskatchewan Ministry of Agriculture http://www.agriculture.gov.sk.ca/Specialty_Crop_Report (http://www.agriculture.gov.sk.ca/Specialty_Crop_Report)
- Shaner , G. and Finney , R.E. 1977 . The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat . Phytopathology , 67 : 1051 – 1056 .
- Shtienberg , D. , Gamliel-Atinsky , E. , Retig , B. and Brener , S. 2005 . Significance of preventing primary infection by Didymella rabiei and development of a model to estimate the maturity of pseudothecia . Plant Dis. , 89 : 1027 – 1034 .
- Shtienberg , D. , Kimber , R.B.E. , Mcmurray , L. and Davidson , J.A. 2006 . Optimisation of the chemical control of ascochyta blight in chickpea . Australas. Plant Pathol. , 35 : 715 – 724 .
- Shtienberg , D. , Vintal , H. , Brener , S. and Retig , B. 2000 . Rational management of Didymella rabiei in chickpea by integration of genotype resistance and postinfection application of fungicides . Phytopathology , 90 : 834 – 842 .
- Vail , S. and Banniza , S. 2008 . Structure and pathogenic variability in Ascochyta rabiei populations on chickpea in the Canadian prairies . Plant Pathol. , 57 : 665 – 673 .
- Vail , S. and Banniza , S. 2009 . Molecular variability and mating-type frequency of Ascochyta rabiei of chickpea from Saskatchewan, Canada . Australas. Plant Pathol. , 38 : 392 – 398 .