Abstract
Barley genotypes with known field reactions to fusarium head blight (FHB) were evaluated for partial disease resistance (PDR) components using an in vitro detached leaf assay. The detached leaves were inoculated with Fusarium graminearum or F. culmorum and incubated at room (21 ± 2 °C) or low temperature (10 ± 1 °C). Both species were pathogenic and had a shorter incubation period and produced larger lesions at room temperature. Inoculation with F. culmorum produced less well-delineated necrotic lesions compared with those from inoculation using F. graminearum under both temperature regimes. On susceptible genotypes, inoculation with F. graminearum at room temperature resulted in significantly shorter latent and incubation periods, larger lesions and more macroconidial production compared with resistant genotypes. Inoculation with F. culmorum resulted in no significant differences in any PDR components measured. Several PDR components for F. graminearum, including latent period, lesion size and macroconidial production, were found to be significantly correlated. There was a negative correlation between incubation period and field ratings in one of three tests, latent period and field ratings in two of three tests, and a positive correlation between lesion size and field disease severity ratings for FHB only in one of three tests. Few PDR components for F. culmorum were found to be significantly correlated with each other. Overall, the best differentiation between resistant and susceptible barley genotypes resulted from inoculation with F. graminearum at room temperature, including a significant correlation between incubation and latent periods with field ratings. In general, given the variability observed, especially in relation to field ratings for FHB, measurement of PDR components cannot be routinely used to complement field-based ratings. However, the measurement of latent period did show promise as it was correlated to field ratings in two of three tests and as such measurement of latent period may be useful in identifying genotypes highly susceptible or resistant to FHB. Further research is required to evaluate the potential of using a detached leaf assay to complement field screening for FHB resistance.
Résumé
Des génotypes d'orges affichant des réactions connues à la fusariose de l'épi ont été évalués pour leurs composants responsables de la résistance partielle à la maladie (RPM) au moyen d'un test biologique in vitro effectuée sur des feuilles individuelles. Ces feuilles ont été inoculées avec Fusarium graminearum ou F. culmorum et ont été incubées à température ambiante (21 °C ±2°) ou à basse température (10 °C ±1°). Les deux espèces étaient pathogènes et, à température ambiante, leur période d'incubation était plus courte et les lésions qu'elles produisaient étaient plus volumineuses. L'inoculation avec F. culmorum a produit des lésions nécrotiques moins bien définies comparativement à celles produites par F. graminearum, et ce, peu importe le régime de température. Chez les génotypes vulnérables, l'inoculation avec F. graminearum à température ambiante induisait des périodes de latence et d'incubation significativement plus courtes, de plus volumineuses lésions et une production de macroconidies plus abondante que chez les génotypes résistants. Quant à l'inoculation avec F. culmorum, elle n'a affiché aucune différence significative relativement aux composants mesurés de la RPM. Plusieurs composants de la RPM relatifs à F. graminearum, y compris la période de latence, la taille des lésions et la production de macroconidies, se sont avérés significativement corrélés. Il y avait une corrélation négative entre la période d'incubation et les estimations en champ dans un test sur trois, la période de latence et les estimations en champ dans deux tests sur trois, et une corrélation positive entre la taille des lésions et les estimations de la gravité de la fusariose au champ dans un seul test sur trois. Par ailleurs, peu de composants de la RPM relatifs à F. culmorum se sont avérés significativement corrélés. En général, la meilleure différenciation entre les génotypes d'orge résistants et vulnérables a découlé de l'inoculation avec F. graminearum à température ambiante, y compris une corrélation significative des périodes d'incubation et de latence avec les estimations au champ. Dans l'ensemble, étant donné la variabilité observée, particulièrement en ce qui a trait aux estimations au champ pour la fusariose de l'épi, la mesure des composants de la RPM ne peut pas être couramment utilisée pour compléter les estimations basées sur les données de terrain. Toutefois, la mesure de la période de latence s'est avérée prometteuse, puisqu'elle était corrélée à l'estimation au champ dans deux tests sur trois, et qu'une telle mesure peut servir à identifier les génotypes qui sont hautement vulnérables ou résistants à la fusariose. Il faudra procéder à de plus amples recherches pour évaluer le potentiel des tests biologiques effectués sur des feuilles individuelles pour bonifier le dépistage de la résistance à la fusariose au champ.
Introduction
Fusarium head blight (FHB) occurs in all the major cereal-growing areas of the world (Wilcoxson et al., Citation1992), and is responsible for yield losses of up to 70% as well as mycotoxin accumulation in grain (Tusa et al., Citation1981). In the last 15 years, FHB has been the most significant disease of wheat (Triticum spp. L.) and barley (Hordeum vulgare L.) in parts of western Canada (Gilbert & Tekauz, Citation2000; Tekauz et al., Citation2000). Severe epidemics of FHB have been experienced in Manitoba in 1993, 1994 and 1996–1998 (Tekauz et al., Citation2000). The disease, caused by Fusarium spp. including F. graminearum Schwabe, is found annually in most barley fields in Manitoba, and its severity can be as high as that in wheat (Gilbert et al., Citation1999; McCallum et al., Citation1999). FHB has also been reported on barley in Saskatchewan and southern Alberta (Fernandez et al., Citation1999; Clear & Patrick, Citation2000). FHB was undetected in barley in Manitoba as recently as the mid 1990s (Tekauz et al., Citation1995), but barley appears to be as vulnerable as wheat to FHB, possibly due to changes in the pathogen population, the cultivars grown, and (or) environmental conditions conducive to the disease (Tekauz et al., Citation2000).
Considerable research has been devoted to the breeding of cereal cultivars resistant to FHB. However, breeding for resistance is expensive and time-consuming and requires the establishment and use of field disease nurseries. It is generally accepted that for accuracy, assessment of resistance should be undertaken over several years as a result of the considerable variability in interaction among genotype, environment and year (Bruehl, Citation1967; Legge et al., Citation2004). Consequently, it would be desirable to develop alternative approaches to screen for resistance that are as effective but less costly and time consuming.
Fusarium graminearum currently is not a common pathogen in Alberta, Canada, especially in central and northern cropping regions where barley is a major crop. Nevertheless, the cultivation of resistant cultivars in the province is warranted as one means to slow down disease spread from the epidemic areas of western Canada. Screening of existing cultivars and of advanced and experimental barley lines under contained conditions is preferred to avoid the need for field disease nurseries, which could exacerbate the occurrence and spread of F. graminearum in central Alberta. Such screening of germplasm in a controlled environment would facilitate the development of resistant barleys for Alberta, and would minimize potential disease intensification due to field screening.
A detached leaf assay was used by Diamond & Cooke (Citation1999), Browne & Cooke (Citation2004) and Browne et al. (Citation2005) to study the partial disease resistance (PDR) components in commercial cultivars and germplasm of winter and spring wheat having a range of FHB resistance. Several PDR components were found to be significantly correlated with whole plant reactions. These authors concluded that this assay can be used as a pre field-screening tool, as it offers the advantages that conditions that can be controlled, requires relatively little space, can be more readily repeated compared with whole plant tests, and individual measurement of a number of PDR components can be taken rather than just disease incidence or severity alone. Murakami & Ban (Citation2005) demonstrated that an oval lesion resulted when a spore suspension of F. graminearum was inoculated onto wounded portions of wheat leaves, and that lesion size increased significantly when leaf tissue was inoculated with both a spore suspension and purified deoxynivalenol (DON) toxin. Using wounded wheat leaves, their bioassay system was able to detect differences in disease reaction between resistant and susceptible cultivars.
The objective of the present study was to develop and test a detached leaf assay system for barley by determining the optimal conditions for leaf segment incubation using two pathogenic Fusarium species, and to assess which PDR components best represent an indirect assessment of FHB field resistance.
Materials and methods
Field evaluation
Six barley cultivars/genotypes were used for this study; two-rowed accessions I92130 and H94051001 and six-rowed ‘Chevron’, ‘Penco/Chevron’, ‘AC Lacombe’ and ‘Stander’. These were selected to represent a range of FHB reactions, as previously determined at the Agriculture and Agri-Food Canada, Brandon Research Centre, FHB nursery during 2003/2004 (B. Legge and J. Tucker, personal communication). The genotypes used included the moderately resistant two-rowed accessions I92130 and H94051001 and six-rowed ‘Chevron’ and ‘Penco/Chevron’, and the susceptible six-rowed ‘AC Lacombe’ and ‘Stander’.
Each of these lines was evaluated in 1.5 m plot replicated three times in the FHB disease nursery at Brandon, Manitoba in 2003 and 2004. Lines were inoculated using corn kernels infested with three isolates of F. graminearum spread onto the soil surface at 40 g m−2, three to five times at weekly intervals, beginning prior to heading of the earliest lines in the nursery. Misting/irrigation was applied at regular intervals to promote ascospore and (or) macroconidial development on infested corn kernels, and subsequent infection of spikes. Entries were rated visually on a 0–5 severity scale two to three weeks after heading, with ‘5’ representing the highest FHB severity (Legge et al., Citation2004).
Detached leaf assay
The six barley cultivars/genotypes listed above were used to evaluate PDR components using an in vitro detached leaf assay. Barley seedlings were grown in PROMIX ‘BX’, a soilless nutrient medium (Premier Horticulture Inc. Quakertown, PA, USA) in a greenhouse. A slow-release fertilizer (N-P-K, 14-14-14) was added at approximately 350 g per 30 kg medium. Each barley genotype was seeded as five seeds in a clump, four clumps per 15 cm diameter pot, and grown for 17–21 days, depending on each test, until leaves were harvested. Five-cm long segments of the first, second and third seedling leaves were excised and two to four such segments were placed on the surface of 0.5% water agar (Bacto, Difco, Detroit, MI) containing 10 mg L−1 kinetin as a senescence retarder in 100 mm diameter Petri dishes. Isolates PW027 #2 of F. graminearum and PWOT #6 of F. culmorum (W.G. Smith) Sacc., originally obtained from infected wheat kernels originating from Central Alberta, were cultured as single macroconidial colonies on Spezieller Nährstoffarmer agar (SNA, Nirenberg, Citation1981) for 7–10 days to harvest macroconidia. The centre of the adaxial surface of each leaf segment was inoculated with a 10 μL droplet of a suspension of 1 × 106 macroconidia mL−1 containing 0.08% of Tween 20. No wounding was employed. Control leaf segments were inoculated with sterile distilled water containing Tween 20. The leaf segments were incubated in a laboratory incubator at low temperature (10 ± 1 °C) under continuous white light or room temperature (21 ± 2 °C) under continuous white light, with three replicates (Petri dishes) per treatment.
The PDR components examined are defined as follows: Incubation period (IP) – the number of days from inoculation to the appearance of visible symptoms, monitored by daily observation under a compound microscope at 20×; Latent period (LP) – the number of days from inoculation to the first appearance of sporodochia, based on daily observation under a dissecting microscope; Macroconidial counts (MC) – the number of macroconidia present per Petri dish measured using a haemocytometer and compound microscope, following addition of 5 mL of sterile water per plate and agitation to suspend the macroconidia; Lesion size (LS) – the area of the necrotic and (or) water-soaked lesion in mm2 based on lesion length × lesion width measured using Varner's callipers (Digimatic, Mitutoyo Corporation, Japan) 12 or 13 days after inoculation for room temperature and 21 or 24 days for low temperature.
Three tests were performed. In Test 1, the PDR components listed above were evaluated following inoculation of the third seedling leaf with each of F. graminearum and F. culmorum at both low (10 ± 1 °C) and room (21 ± 2 °C) incubation temperatures. In Tests 2 and 3, only F. graminearum was used to inoculate the second and first seedling leaves, respectively. Two additional barley accessions, CI4196 and H93120, were added to Tests 2 and 3 that were not included in Test 1. Both of these accessions were rated as resistant to FHB in previous field trials. The PDR data were subjected to analysis of variance, and Fisher's least significant difference (LSD) was used for separation of means when the F-test was significant. The PDR data were standardized by converting values to a percentage of the control, and the relationship between components in each test was determined using Pearson's correlation coefficient based on the means of test replicates. Finally, the relationships of components among the three leaf positions were determined using Pearson's correlation coefficient based on the means of test replicates after data standardization.
Results
Symptoms on leaf segments at the point of inoculation initially appeared as small brown to dark brown flecks which rapidly enlarged to become water-soaked spots. In most cases, the water-soaked spots subsequently became oval-shaped necrotic lesions extending parallel to leaf veins in both directions (, ). In contrast, control leaf segments inoculated with distilled water displayed no visible lesions. Inoculation with F. graminearum generally produced larger lesions on susceptible cultivars than moderately resistant ones (). Fusarium graminearum and F. culmorum isolates both were pathogenic, and had shorter incubation periods when tested at room temperature compared with low temperature. However, inoculation with F. culmorum resulted in the development of less well-delineated necrotic lesions compared with those resulting from inoculation using F. graminearum ().
Fig. 1. Symptoms on barley seedling leaf segments on water agar inoculated with Fusarium graminearum and incubated for seven days at room temperature. Top left – I92130, moderately resistant; top right – ‘Stander’, susceptible; bottom left – H94051001, moderately resistant; and bottom right – ‘AC Lacombe’, susceptible.
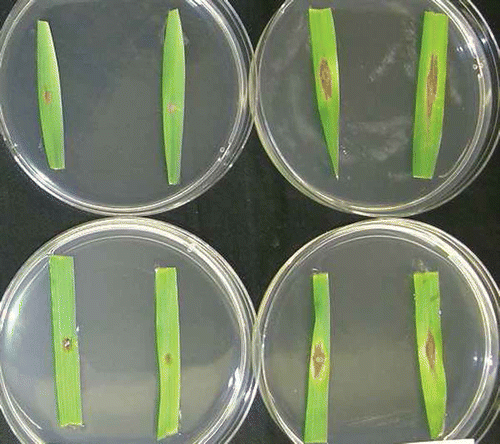
Fig. 2. Symptoms on barley ‘AC Lacombe’ seedling leaves inoculated with Fusarium culmorum (left) or F. graminearum (right) at room temperature. Photo was taken after seven days of incubation.

In Test 1, there were no significant differences in incubation period among the barley genotypes tested at either of the incubation temperatures ( and ). There were significant differences in latent period among genotypes inoculated with F. graminearum at the lower experimental temperature (). At room temperature, F. graminearum induced considerably larger lesions on the susceptible cultivars ‘AC Lacombe’ and ‘Stander’ compared with the resistant genotypes, H94051001, CI4196, I92130 and ‘Penco/Chevron’ (). There were no differences among genotypes in macroconidial counts at the low temperature (). When tested at room temperature F. graminearum macroconidial counts were considerably higher in susceptible ‘AC Lacombe’ and ‘Stander’ compared with the more resistant genotypes. The field disease rating data shown in based on symptom severity on spikes in 2003 and 2004 at the Brandon FHB nursery became available when the preliminary investigation of the present study was done in 2006. The field ratings reflect the resistant or moderately resistant ratings generally ascribed to ‘Chevron’ barley and the other more resistant accessions, as well as the susceptibility of ‘AC Lacombe’. Although ‘Stander’ barley is considered to be susceptible to FHB, field ratings at Brandon in 2003 and 2004 were of intermediate (MR-MS) values (). Detached leaf segment inoculation with F. culmorum resulted in non-significant differences among barley genotypes for all PDR components at both incubation temperatures ().
Table 1. Partial disease resistance components measured in detached leaves of barley inoculated with Fusarium graminearum under two temperature regimes (Test 1)
Table 2. Partial disease resistance components measured in detached leaves of barley inoculated with Fusarium culmorum under two temperature regimes (Test 1)
Results of correlation analyses among barley PDR components for F. graminearum in Test 1 are listed in . Latent period at both temperatures (Rows C and D) was significantly and negatively correlated with LS (Columns 5 and 6) and MC at low temperature (Column 7). Lesion size (LS) at low temperature (Row E) was positively correlated with LS at room temperature and MC at low temperature (Columns 6 and 7). Lastly, latent period (LP) at room temperature (Row D) was negatively correlated with field ratings for FHB severity at the Brandon FHB nursery (Column 9). The results of correlations among PDR components for F. culmorum are listed in . Incubation period at low temperature (Row A) was negatively correlated with LP (RT) (column 3) and positively correlated with LS and MC (Columns 5 and 6), while MC after 21 days at low temperature (Column 6) was negatively correlated with LP at room temperature (Row C) and positively correlated with LS at room temperature (Row E). The positive correlations between IP and LS and MC at low temperature for F. culmorum were unexpected. This may have resulted from the less well-delineated lesions produced by F. culmorum that led to difficulty in determining the initial appearance of a lesion in relation to quantifying macroconidia.
Table 3. Correlation coefficients for partial disease resistance components measured in detached leaves of barley inoculated with Fusarium graminearum and evaluated under two temperature regimes (Test 1)
Table 4. Correlation coefficients for partial disease resistance components measured in detached leaves of barley inoculated with Fusarium culmorum and evaluated under two temperature regimes (Test 1)
In Test 2, inoculation with F. graminearum resulted in a significantly shorter incubation period in susceptible ‘AC Lacombe’ compared with resistant genotypes H94051001, I92130 and H93120 at room temperature, but differences among genotypes were non-significant at low temperature (). Sporodochia appeared (LP) earlier at room temperature in susceptible ‘AC Lacombe’ and ‘Stander’ and this difference was significant compared with the moderately resistant accession I92130. Both ‘AC Lacombe’ and ‘Stander’ tended to develop larger lesions compared to all resistant genotypes except H94051001; however, the LS differences were non-significant. Macroconidial counts at both temperatures were generally higher for ‘AC Lacombe’ and ‘Stander’ than for the more resistant genotypes. There were no significant differences for IP at the low temperature. LP and LS at the low temperature were not measured for Test 2 because of the lack of clearly defined symptoms at LT.
Table 5. Partial disease resistance components measured in detached leaves of barley inoculated with Fusarium graminearum and evaluated at two temperatures (Test 2)
Correlations were determined for F. gramineaurm PDR components in Test 2. At room temperature, LP was found to be positively correlated with IP (r = 0.85**, data not shown), indicating that the early appearance of lesion promoted sporodochia production. No significant correlations were found for other PDR components evaluated, or with field ratings (data not shown).
In Test 3, inoculation of susceptible ‘AC Lacombe’ and ‘Stander’ with F. graminearum resulted in significantly shorter incubation periods (IP) compared with resistant genotypes ‘Chevron’, H94051001, I92130 and H93120 at low temperature (). Incubation period at room temperature tended to be shorter for the susceptible cultivars than the resistant ones, and the differences among genotypes were nearly significant at the 5% level (P = 0.06). The two susceptible cultivars had significantly shorter latent periods compared with all other genotypes at room temperature. Although lesions (LS) tended to be larger in the susceptible lines at low temperatures, these differences were not significant. However, these differences in lesion size were significant at room temperature. Macroconidial counts at both temperatures were significantly higher for ‘AC Lacombe’ and ‘Stander’ than for all the more resistant genotypes except CI4196 at low temperature.
Table 6. Partial disease resistance components measured in detached leaves of barley inoculated with Fusarium graminearum and evaluated at two temperatures (Test 3)
Results of Test 3 correlation analyses among PDR components for F. graminearum are listed in . Incubation period (IP) at room temperature (Column 2) and low temperature (Row A) was significantly correlated and latent period (LP) at room temperature (Column 3) was also correlated with incubation period (IP) (Row B). Lesion size (LS) at both temperatures (Columns 4 and 5) was negatively correlated with IP (Rows A and B), and LS (Column 5) was negatively correlated with LP (Row C) and positively with LS at low temperature (Row D). Macroconidial counts at low temperature (Column 6) were correlated with IP (Row A). Macroconidial counts at room temperature (Column 7) were negatively correlated with IP (Row B) and LP (Row C), and positively correlated with LS (Row E). Lastly, field ratings for FHB severity at the Brandon FHB nursery (Column 8) was negatively correlated with IP (Row B) and LP (Row C) and positively correlated with LS (Row E).
Table 7. Correlation coefficients for partial disease resistance components measured in detached leaves of barley inoculated with Fusarium graminearum and evaluated under two temperature regimes (Test 3)
Results of correlation analyses on PDR components for three tests using three different leaf positions are reported in . More significant correlations in PDR components were found between Leaves 2 and 3 and Leaves 1 and 3 than with Leaves 1 and 2. There were differences in correlations of PDR components among the three tests (), suggesting that seedling leaf position may play a role in causing unexplained variation in the PDR components parameters measured, or could be due to simple between-test variation. The correlations between Leaf 1 and 2, for instance, were found for MC at low temperature associated with IP, LP and LS that were tested at room temperature; the correlations between Leaf 1 and 3 were mostly located for LP and LS at room temperature associated with LS and MC, and those between Leaf 2 and 3 were found mostly for MC with LP and LS. The experiment in the present study was repeated using different leaf positions and the consistency in PDR components needs to be confirmed by more repeated testing.
Table 8. Correlation coefficients for partial disease resistance components evaluated using barley seedling leaves 1 (Test 3), 2 (Test 2) and 3 (Test 1) inoculated with Fusarium graminearum and evaluated under two temperature regimes
Discussion
Fusarium graminearum is the most frequently isolated causal agent of FHB in barley in Manitoba, but other Fusarium species are also involved (Tekauz et al., Citation2000, Citation2008). Fusarium culmorum has been isolated occasionally from FHB-affected barley in Alberta (Turkington et al., Citation2002). The two species were used in the present study to assess whether the detached leaf assay was a suitable protocol to screen for FHB resistance in barley. Our study showed that differentiation between susceptible and resistant genotypes based on PDR components such as latent period (LP), lesion size (LS) and macroconidial counts (MC) was more evident following inoculation with F. graminearum compared to F. culmorum. It was noted that inoculation with F. culmorum did not necessarily result in the development of discrete necrotic lesions even when mycelial growth was quite extensive over the leaf surface. This made unequivocal measurement of PDR components more difficult. This is in agreement with results reported for the interaction between the wheat detached leaf assay and F. culmorum (Browne and Cooke Citation2004). Measuring PDR components in barley for F. culmorum using the detached leaf assay may therefore be problematic. Consequently, F. graminearum was found to be the more suitable species to use for the detached leaf assay; additionally, this species is the most commonly isolated causal agent of FHB. Generally, head blight symptoms in barley are less well-defined than those in wheat (Steffenson, Citation2003), and this may increase variation in the assessment of field disease severity in barley. As a result, severity ratings are often supplemented by additional data such as mycotoxin content (DON and others) to assist in evaluating resistance or susceptibility in barley (Tekauz et al., Citation2000). If clearly defined foliar symptoms are produced as in the case for F. graminearum-mediated disease with the detached leaf assay, severity based on lesion size or other PDR components could be assessed with minimal ambiguity.
The room temperature experimental regime used in our tests resulted in more significant differences in the time to the initial appearance of sporodochia (LP), and the resulting number of macroconidia (MC) compared with the low temperature regime, for F. graminearum. Room temperature, a more readily available experimental condition, also resulted in more rapid symptom development (IP) compared with low temperature. While the barley genotypes evaluated consistently showed differentiation in F. graminearum macroconidial counts, considerably more macroconidia were observed at low temperature than at room temperature in Tests 1 and 2. Room temperature (21 ± 2 °C) and a prolonged 21-day incubation period were found to cause a rapid drying of both the leaf segment and subtending agar and this likely reduced sporulation. Consequently, macroconidia in Test 3 were counted 16 days following inoculation at room temperature. This resulted in relatively higher macroconidial counts compared with those in Tests 1 and 2. Among the significant correlations in PDR components demonstrated in the present study, latent period (LP) at room temperature was simple to measure, discriminated among resistant and susceptible cultivars, and was significantly associated with field ratings in two of three tests. Our results suggest that PDR components such as LP may have the potential to be evaluated in a detached leaf assay as a predictor to field reactions, especially in relation to identifying FHB-susceptible genotypes.
Genotypes previously identified or known to have a level of field resistance to FHB, exhibited resistance in the detached leaf assay based on measurement of latent period, lesion size or sporulation at room temperature, suggesting that measuring these PDR components to identify genotypes may have potential to complement assessments of FHB reaction in the field. Further research would be beneficial to evaluate a wider range of barley germplasm to identify which PDR components consistently provide the ability to identify susceptible genotypes such as ‘AC Lacombe’ and ‘Stander’ that demonstrated a shorter latent period and higher macroconidial counts compared with more resistant genotypes. If these differences are demonstrated to be consistent over a larger, more diverse number of susceptible and resistant barley genotypes, the detached leaf assay could be useful in separating broad genotype classes and providing a tool to reduce the number of lines tested in field nurseries. Additional research may also improve on the ability of the detached leaf assay and measurement of specific PDR components to identify sources of FHB resistance. For example, the initial development of discrete necrotic lesions following primary infection, followed by the subsequent arrest of pathogen (lesion) development as demonstrated in the detached leaf assay, could indicate a resistant barley response. Such a response may reflect resistance resulting from the combination of a longer incubation period, smaller lesion size and reduced sporulation.
The relationship among barley PDR components was inconsistent and several were poorly correlated based on the detached leaf assay. This was also reported by Browne and Cooke (Citation2004) for the association between wheat and F. graminearum. Latent period and lesion size were not always significantly correlated with incubation period in our study. This suggests that pathogen development as reflected by lesion expansion, and sporulation on detached leaf segments, may not simply be a function of incubation period, and that components of PDR may be under separate genetic control. Further investigation is needed to determine which individual components are best correlated to FHB resistance in whole plants. Moreover, there were sizeable differences in values for PDR components, such as incubation period at low temperature and lesion size at room temperature, among the tests. Such differences highlight the need for additional studies to determine the optimal conditions to generate consistent and meaningful data. Comparing the three leaf positions, Leaf 1 may be a better choice for measuring PDR components, given that more significant correlations between the components for Leaf 1 and field rating were identified () than with the two other leaf positions (). This was also found to be the case with the pathogen Microdochium majus (Wollenw.) Glynn & S.G. Edwards, a causal agent of the wheat FHB in Europe, for which the first and second leaves of wheat seedlings were found to correlate better than the third leaf with whole plant disease reactions (Browne et al., Citation2006).
The correlations between most PDR components in the detached leaf assay and FHB field severity ratings assessed at Brandon, MB were non-significant in our study. Brown and Cooke (Citation2005) similarly reported that some detached leaf assay-derived PDR components were not effective in identifying wheat lines with high levels of whole-plant FHB resistance. Some plant characteristics may confer disease escape, i.e. plant height (Steffenson, Citation2003), absence of awns (Mesterhazy, Citation1995) or flowering at the boot stage (Cooke, Citation1981). These are independent of the PDR variables measured using the detached leaf assay. This view is in agreement with the knowledge that a number of discrete types of resistances are responsible for overall FHB resistance and that these can be present in multiple combinations in different cereal genotypes (Parry et al., Citation1995). The lack of correlation between most individual PDR components and field disease ratings, may necessitate that several PDR components be examined to differentiate FHB reactions among barley genotypes. Nonetheless, this can be achieved during the same experimental trial. In a situation such as that in central Alberta, where producers require regionally adapted feed and malt barley cultivars with improved FHB resistance, and where the use of field disease nurseries is presently untenable, an informative detached leaf assay would be an asset to programmes breeding for FHB resistance.
Acknowledgements
This work was supported by research grant Project #2003A036R from the Alberta Crop Industry Development Fund. Technical assistance by Emily Andes and manuscript editing by Carol Dyson are greatly appreciated. The field data provided by B. Legge and J. Tucker of Agriculture and Agri-Food Canada, Brandon Research Centre, Brandon, MB are acknowledged.
References
- Browne , R.A. and Cooke , B.M. 2004 . Development and evaluation of an in vitro detached leaf assay for pre-screening resistance to fusarium head blight in wheat . Eur. J. Plant Pathol , 110 : 91 – 102 .
- Browne , R.A. and Cooke , B.M. 2005 . Resistance of wheat to Fusarium spp. in an in vitro seed germination assay and preliminary investigations into the relationship with fusarium head blight resistance . Euphytica , 141 : 23 – 32 .
- Browne , R.A. , Murphy , J.P. , Cooke , B.M. , Devaney , D. , Walsh , E.J. , Griffey , C.A. , Hancock , J.A. , Harrison , S.A. , Hart , P. , Kolb , F.L. , Mckendry , A.L. , Milus , E.A. , Sneller , C. and Van Sanford , D.A. 2005 . Evaluation of components of fusarium head blight resistance in soft red winter wheat germplasm using a detached leaf assay . Plant Dis. , 89 : 404 – 411 .
- Browne , R.A. , Mascher , F. , Golebiowska , G. and Hofgaard , I.S. 2006 . Components of partial disease resistance in wheat detected in a detached leaf assay inoculated with Microdochium majus using first, second and third expanding seedling leaves . J. Phytopathol , 154 : 204 – 208 .
- Bruehl , G.W. 1967 . “ Fusarium head blight or scab ” . In Wheat and wheat improvement , Edited by: Reitz , L.P. and Queensbury , K.S. 388 – 390 . Madison, WI : American Society of Agronomy .
- Clear , R.M. and Patrick , S.K. 2000 . Fusarium head blight pathogens isolated from fusarium-damaged kernels of wheat in western Canada, 1993–1998 . Can. J. Plant Pathol , 22 : 51 – 60 .
- Cooke , R.J. 1981 . “ Fusarium disease of wheat and other small grains in North America ” . In Fusarium: Diseases, biology and taxonomy , Edited by: Nelson , P.E. , Toussoun , T.A. and Cook , R.J. 39 – 52 . University Park, PA : The Pennsylvania State University Press .
- Diamond , H. and Cooke , B.M. 1999 . Towards the development of a novel in vitro strategy for early screening of Fusarium ear blight resistance in adult winter wheat plants . Eur. J. Plant Pathol. , 105 : 363 – 372 .
- Fernandez , M.R. , Holzgang , G. , Celetti , M.J. and Hughes , G. 1999 . The incidence of fusarium head blight of barley, common wheat and durum wheat grown in Saskatchewan during 1998 . Can. Plant Dis. Surv. , 79 : 80 – 83 .
- Gilbert , J. and Tekauz , A. 2000 . Review: recent developments in research on fusarium head blight of wheat in Canada . Can. J. Plant Pathol , 22 : 1 – 8 .
- Gilbert , J. , Tekauz , A. , Kaethler , R. , Mccallum , B. , Mueller , E. and Kromer , U. 1999 . 1998 Survey of fusarium head blight in spring wheat in Manitoba . Can. Plant Dis. Surv , 79 : 91
- Legge , W.G. , Therrien , M.C. , Tucker , J.R. , Banik , M. , Tekauz , A. , Somers , D. , Savard , M.E. , Rossnagel , B.G. , Lefol , E. , Voth , D. , Zatorski , T. , Harvey , B.L. and Scoles , G. 2004 . Progress in breeding for resistance to fusarium head blight in barley . Can. J. Plant Pathol , 26 : 436 – 442 .
- Mccallum , B. , Tekauz , A. , Gilbert , J. , Mueller , E. , Kaethler , R. , Stulzer , M. and Kromer , U. 1999 . Fusarium head blight of barley in Manitoba in 1998 . Can. Plant Dis. Surv , 79 : 84 – 85 .
- Mesterhazy , A. 1995 . Types and components of resistance against fusarium head blight of wheat . Plant Breed. , 114 : 377 – 386 .
- Murakami , J. and Ban , T. Development of novel bioassay system for FHB molecular interaction . Proceedings of the 4th Canadian Workshop on fusarium head blight . Nov 1–3 2005 . pp. 62 Ottawa, ON : Ottawa Congress Centre .
- Nirenberg , H.I. 1981 . A simplified method for identifying Fusarium spp. occurring on wheat . Can. J. Bot , 59 : 1599 – 1609 .
- Parry , D.W. , Jenkinson , P. and Mcloed , L. 1995 . Fusarium ear blight (scab) in small grain cereals – a review . Plant Pathol , 44 : 207 – 238 .
- Steffenson , B. 2003 . “ Fusarium head blight of barley: impact, epidemics, management, and strategies for identifying and utilizing genetic resistance ” . In Fusarium head blight of wheat and barley , Edited by: Bushnell , W.R. 241 – 295 . St. Paul, MN : American Phytopathological Society .
- Tekauz , A. , Gilbert , J. , Mueller , E. , Kaethler , R. and Kromer , U. 1995 . Foliar and head disease of barley in Manitoba in 1994 . Can. Plant Dis. Surv , 75 : 113 – 114 .
- Tekauz , A. , Mccallum , B. and Gilbert , J. 2000 . Review: fusarium head blight of barley in western Canada . Can. J. Plant Pathol , 22 : 9 – 16 .
- Tekauz , A. , Gilbert , J. , Mueller , E. , Stulzer , M. , Beyene , M. and Kaethler , R. 2008 . Survey for fusarium head blight of barley in 2007 in Manitoba . Can. Plant Dis. Surv , 88 : 45 – 46 .
- Turkington , T.K. , Clear , R.M. , Burnett , P.A. and Xi , K. 2002 . Fungal plant pathogens infecting barley and wheat seed from Alberta, 1995–1997 . Can. J. Plant Pathol. , 24 : 302 – 308 .
- Tusa , C. , Munteanu , I. , Capetti , E. , Pirvu , T. , Bunescu , S. , Sin , G.L. , Nicolae , H. , Ianu , A. , Caea , D. , Romascanu , O. and Stoika , V. 1981 . Aspects of the Fusarium attacks on wheat in Romania . Probleme de Protectia Plantelo , 9 : 15 – 31 .
- Wilcoxson , R.D. , Busch , R.H. and Ozmon , E.A. 1992 . Fusarium head blight resistance in spring wheat cultivars . Plant Dis , 76 : 658 – 661 .