Abstract
Phoma stem canker, caused by Leptosphaeria maculans, is the most destructive disease of canola (Brassica napus) in the world. A study was conducted to compare mean disease severity and incidence at three different growth stages on naturally infected canola plants under field conditions. To determine the main source of inoculum over two consecutive years in Manitoba, three different approaches were employed: inspection of infested stubble pieces for the presence of pseudothecia and/or pycnidia, detection of ascospores using Burkard and rotorod spore samplers, and lastly, utilizing trap plants to study subsequent disease development. The results showed that the mean disease severity was significantly higher (P < 0.001) on plants infected at 1, 0 (cotyledon) than at 1, 3 (3-leaf) but no significant difference in disease incidence was observed between these two stages. However, these plants showed significantly (P < 0.01) higher disease severity and incidence than plants infected at 1, 6 (6-leaf) stage. A high density of pycnidia and low density of pseudothecia on stubble pieces, absence of ascospores in most weeks and high levels of disease incidence and disease severity on infected plants suggested that pycnidiospores can be considered as a main source of inoculum during these two years in Western Canada. These findings can be used to better understand the relationship between pycnidiospore inoculum and subsequent disease development on canola under field conditions.
Résumé
La jambe noire, causée par Leptosphaeria maculans, est la maladie la plus destructrice du canola (Brassica napus) au monde. Une étude a été menée pour comparer la gravité moyenne de la maladie et sa fréquence à trois différents stades de croissance sur des plants de canola infectés en conditions naturelles. Afin de déterminer la principale source d'inoculum au cours de deux années consécutives au Manitoba, trois approches différentes ont été utilisées : l'inspection de morceaux de chaume infectés pour y déceler des pseudothèces ou des pycnides, la détection d'ascospores au moyen des échantillonneurs de spores Burkard et Rotorod et, finalement, l'utilisation de plantes-pièges pour étudier le développement subséquent de la maladie. Les résultats ont montré que la gravité moyenne de la maladie était significativement plus haute (P < 0,001) chez les plants au stade 1, 0 (cotylédon) que chez ceux au stade 1, 3 (trois feuilles). Par ailleurs, aucune différence significative quant à la fréquence de la maladie n'a été observée à ces deux stades. Toutefois, ces plants ont affiché une fréquence et une gravité de la maladie significativement plus élevées (P < 0,01) que celles infectées au stade 1, 6 (six feuilles). Une forte densité de pycnides et une faible densité de pseudothèces sur les morceaux de chaume, l'absence d'ascospores durant la plupart des semaines et une fréquence ainsi qu'une gravité de la maladie élevées chez les plants infectés ont suggéré que les pycnidiospores peuvent être considérés comme une source primaire d'inoculum durant ces deux années dans l'Ouest canadien. Ces conclusions peuvent servir à mieux comprendre la relation entre les pycnidiospores en tant qu'inoculum et le développement subséquent de la maladie chez le canola en conditions naturelles.
Introduction
Phoma stem canker (blackleg), caused by Leptosphaeria maculans (Desm) Ces and de Not (anamorph: Phoma lingam (Tode ex Fr.) Desm), is a devastating disease of Brassica species in many areas of the world (Hall, Citation1992; Williams, Citation1992; West et al., Citation2001). In severely infected plants, cankers appear on the stem base and can completely girdle the stem during pod filling which is the main reason for yield reductions (Bailey et al., Citation2003). The disease is monocyclic (Hall, Citation1992; Petrie, Citation1995) in which the ascospores are considered to be the primary source of inoculum (Mac Gee & Petrie, Citation1979; Fitt et al., Citation1989; Koch et al., Citation1991; Petrie, Citation1995; Mac Gee, Citation1997). The role of ascospores in disease dispersal, subsequent canker development and yield loss is well documented (Hall, Citation1992; West et al., Citation1999; Zhou et al., Citation1999; West et al., Citation2001; Fitt et al., Citation2006). However, the epidemiology of the disease based on pycnidiospores as a source of inoculum has not been adequately assessed in natural conditions due to the possibility of ascospore contamination. Pycnidiospores are single-celled, hyaline, cylindrical (3–5 × 1.5–2 μm) and are embedded in light red or pinkish mucilage. They are formed inside pycnidia which are usually black, immersed, globose and ostiolate, measuring 200–600 μm in diameter. Pycnidia are produced in the lesions on recently killed stem tissue (Williams, Citation1992) and the pycnidiospores are dispersed by rain-splash or direct contact (Fitt et al., Citation1989; Hall, Citation1992; Howlett et al., 2001). In an experiment with a rain simulator under controlled conditions, Travadon et al. (Citation2007) showed that most pycnidiospores can be detected within 40 cm of the source of inoculum. However, there are some reports showing that spore dispersal can be as far as 105 cm from inoculated rows (Barbetti, Citation1976) and 216 cm from plants grown from infected seeds (Hall, Citation1996).
Some studies defining the importance of pycnidiospores on disease development have been conducted using artificial inoculation techniques in either controlled and/or field conditions and focused on the role of pycnidiospores following ascospore infection. For example, Barbetti (Citation1976) studied the role of pycnidiospores in phoma stem canker spread and subsequent development of the disease on different growth stages of canola plants under field conditions after artificial inoculation of plants. He showed that inoculation of the plants at three weeks resulted in the greatest disease spread compared to older plants. In another study, under controlled conditions, Li et al. (Citation2006b ) showed that co-inoculation of ascospores and pycnidiospores could increase the ability of pycnidiospores to cause more disease severity than when plants were inoculated with pycnidiospores alone. They also concluded that phoma stem canker may be considered to be a polycyclic disease due to pathogen's ability to cause several infection cycles by pycnidiospores during the growing season. Barbetti (Citation1975) studied the role of pycnidiospores on late phoma stem canker infections under field conditions following initial infection of the plants with ascospores. In Europe, it is believed that disease development is due to ascospore showers (West et al., Citation2001; Fitt et al., Citation2006) while in Australia, it is thought that the disease occurs in combination with pycnidiospores (Barbetti, Citation1975, 1976). However, in Western Canada, phoma stem canker incidence has been observed in years where no ascospores were detected (Guo & Fernando, Citation2005). Petri (1995) also showed that ascospore showers could not be expected each year after 16 years of surveys in Saskatchewan. This phenomenon can be possibly attributed to infection and subsequent disease development occurring from pycnidiospore inoculum.
Response of plant growth stage to disease was also studied under field conditions following exposure to ascospores or in controlled conditions (Poisson & Peres, Citation1999; Marcroft et al., Citation2005; Li et al., Citation2006a ). This response was also examined under field conditions following artificial inoculation with pycnidiospores (Barbetti, Citation1975). To better understand blackleg epidemiology in Western Canada, the relationship between disease severity and incidence at infection time with weather conditions were modeled (Ghanbarnia et al., Citation2009). To our knowledge, there are no previous studies comparing the susceptibility of naturally infected canola plants at different growth stages when they are exposed to P. lingam pycnidiospores at infection time or to indicate the role of pycnidiospores as a main source of inoculum under field conditions. Therefore, the objectives of this study were to: (1) compare susceptibility of different growth stages of B. napus to pycnidiospore exposure at infection time under field conditions; and (2) study the main source of inoculum and subsequent disease development.
Materials and methods
Field experiment
A two year study was conducted under field conditions to compare the susceptibility of naturally infected canola plants at different growth stages when they are exposed to P. lingam pycnidiospores at infection time (each set for one week) and subsequent development of phoma stem canker disease in the greenhouse on cultivar ‘Westar’ (susceptible to phoma stem canker disease). The experiment was carried out in a 50 × 60 m plot from June 1st to July 7th in 2004 for five weeks and from May 27th to August 10th in 2005 for 10 weeks at the Ian Morrison Research Station, Carman, Manitoba. Seeds of B. napus ‘Westar’ were sown in plastic flats fitted with 48-celled packs containing MetroMix (W. R. Grace and Co. Ltd, Canada). Seedlings were fertilized with a solution of N-P-K (20-20-20, Plant Products Ltd, Canada) and maintained in a growth room at 22 and 16 °C, day and night (12/12 hr), with continuous light. After one week, each seedling was transplanted into a 14.5 cm (diam.) pot. Ten plants at growth stage (GS) 1, 0 (both cotyledons unfolded and green), 1, 3 (third true leaf exposed) and 1, 6 (sixth true leaf exposed) (Howlett et al., 2001) were each placed in a phoma stem canker-infested canola field for one week (infection time). The field experiment plots contained remnant stubble pieces from phoma stem canker infected canola plants from previous years. Ten control plants were left in the greenhouse without exposure to inoculum. After one week outdoors, the plants were returned to the greenhouse with temperature controlled at 20–24 °C and 15–18 °C, day and night, and left to grow until the pod-filling stage. Plants were watered every other day.
Inspection of infested stubble
Thirty-five phoma stem canker-infested stubble pieces were selected randomly from the field site each week during the experiment in the years 2004 and 2005 to examine for the presence of pycnidia or pseudothecia using a stereomicroscope (WILD M3Z, Heerbrugg, Switzerland, ×65). The pycnidia and pseudothecia were characterized based on the shape and size of the fruiting body (Shoemaker & Burn, Citation2001). The maturity of pseudothecia in each week was grouped in one of the five maturity classes (A, B, C, D and E) using a simplified scale described by Tuscano-Underwood et al. (2003). To confirm the identification, 10 fruiting bodies from each stubble piece were crushed and microscopic slides were prepared for observation under a microscope (Hund Wetzlar, Germany) at ×400. Ascospores and/or pycnidiospores were characterized based on the size and shape of the spores (Shoemaker & Burn, Citation2001). To calculate the density of fruiting bodies (fruiting bodies per cm2), the total number of fruiting bodies counted on 10 cm of each stubble piece was divided by the surface area of the stubble piece. The surface area of each piece was calculated as Area = L × C, in which L is the length of each piece (10 cm of each piece was selected for counting the fruiting bodies) and C is circumference of each piece which was measured using flexible seamstress tape.
Spore sampling
Two types of spore traps were used to increase the chance of capturing the ascospores in the field. Rotorod spore samplers were used to capture ascospores from local areas and Burkard spore sampler was used to trap ascospores from long distance or other plots in the local area. Five rotorod spore samplers (Aerobiology Company, Nepean, ON) were set up in a diagonal orientation within the experimental plot to measure ascospore concentrations based on daily dispersal. Four rotorod samplers were set up 15 m from each corner and the last was set up in the middle of the field. They were placed 30 cm above ground. A CR10 datalogger (Campbell Scientific, Logan, UT) was programmed to operate the rotorods for 5 min/h. A thin coat of petroleum jelly was brushed on each rotorod. The rotorods were replaced every 24 h. Spore numbers counted from rotorod spore samplers were used for the analysis of spore release in different weeks. The dimensions of the rod (length and width) used for collecting ascospores was 20 × 0.1 mm. The mean daily concentration of ascospores was calculated as the total number of spores on one rod divided by the rod rotations per minute (2400), the rotation constant (0.097), the sampling period in minutes per day (120 min), and 24 h. All areas on the rods were inspected under a microscope at ×400. In addition, a seven-day Burkard spore trap (Burkard Scientific Ltd., Uxbridge, and Middlesex, UK) was set up in the center of the plot. The seven-day-tape in the Burkard spore trap was coated with a thin layer of petroleum jelly and replaced weekly. All areas of the tape were inspected under a microscope at ×400. Each Burkard tape was divided into seven equal sections, each representing one day. Ascospore concentration per day in the air was calculated as the number of spores per section, divided by the throughput at the orifice of the Burkard trap, which was 0.6 m3/h and 24 h.
Disease assessment
Recording of leaf lesions on the canola plants was started after two weeks in the greenhouse. Stem disease severity and disease incidence were evaluated at the pod-filling stage. Stem disease severity was evaluated using a 0-5 scale where 0 = uninfected, 1 = lesion area less than 25% of the cross-section area of the crown, 2 = 25 to 50%, 3 = >50% girdled, stem firm, 4 => 50% girdled, stem weak, and 5 = plant dead (West et al., Citation2002). Disease incidence was evaluated based on the percentage of symptomatic plants each week. To confirm the presence of the pathogen in the stem lesions or cankers, three diseased plants from each growth stage were selected. Four pieces, of 0.5 cm in size, were cut from each infected stem and sterilized in 2% sodium hypochlorite for 2 min, and then washed in sterilized water and plated on V-8 agar plates. Plates were incubated at room temperature under continuous fluorescent light. Spores were collected by scraping off the submerged pycnidia 10 days after plating. The identification of the pathogen was confirmed based on size and shape of pycnidia and pycnidiospores (Shoemaker & Burn, Citation2001). Pathogenicity tests on canola ‘Westar’, ‘Glacier’ and ‘Quinta’, stage 1, 0 s, were performed to determine the pathogenicity group (PG) (Koch et al., Citation1991) by inoculation of 1 ×107 pycnidiospores on 12-day-old seedlings as previously described (Chen & Fernando, Citation2006).
Data analyses
A generalized randomized complete block design with growth stages (three different stages) of plant as the treatment and week as the block was performed to assess phoma stem canker severity. Ten plants were observed for each growth stage treatment in each week. There were five replicate weeks (blocks) in year one and 10 replicate weeks in year two. Analysis of variance was performed using PC SAS (SAS Institute, Cary, NC) with PROC MIXED. Treatment, year and their interaction were considered as fixed effects. Week within year, and interaction of treatment with week within year were considered as random effects. The error term for testing treatment effects is the interaction of treatment and week within year. If a significant F test (P<0.05) was obtained among treatments, significance of difference among means was determined using Fisher's least significant difference test. For disease incidence, the percentage of affected plants per 10 plants in each batch was analyzed as in a model similar to that used for phoma stem canker severity but there was only one observation per batch. Also a randomized complete block design with fruiting bodies (pycnidia and pseudothecia) as treatment and week as the block was performed to compare the presence of main fruiting bodies in the local area. Thirty-five stubble pieces were observed each week. Arcsine square root transformations were performed for the data of disease incidence and square root transformation were applied to mean disease severity and mean fruiting bodies density per week due to slight departure of the residuals from normality.
Results
Development of phoma stem canker
The highest mean disease severity was observed on plants infected at the 1, 0 stage (4.7) and the lowest was observed in plants infected at 1, 6 stage (0.45) for both years (). In both years, treatment had a significant effect on the mean disease severity (2004 and 2005, P < 0.001, ). Mean disease severity for all three treatments were significantly different from each other. The mean disease severity was significantly higher for the plants infected at 1, 0 than at 1, 3 (P < 0.001) and 1, 6 (P < 0.0001) stages (). ANOVA also showed that the plants infected at the 1, 0 and 1, 3 stages had significantly higher disease incidence than the plants infected at 1, 6 stage (P <0.01) but no significant difference in phoma stem canker incidence was observed between the plants at 1, 0 and 1, 3 stages (). Also, mean disease severity for the plants infected at 1, 3 stage was significantly higher than the plants infected at 1, 6 stage (P < 0.01). Analysis of variance (ANOVA) indicated that treatment (P = 0.001) and week (P < 0.0001) significantly affected disease incidence but year did not (). It must be mentioned that no leaf lesion was observed on diseased plants during two years of experiments.
Fig. 1. Mean disease severity (a) and disease incidence (b) for the plants infected at 1, 0 (cotyledon), 1, 3 (3-leaf) and 1, 6 (6-leaf) stages of cultivar ‘Westar’. The experiment was carried out in a 50 × 60 m plot from June 1st to July 7th in 2004 for five weeks and from May 27th to August 10th in 2005 for 10 weeks at the Carman Research Station, Carman, Manitoba. Ten plants at 1, 0; 1, 3; and 1, 6 stages were each placed in a phoma stem canker-infested canola field for one week. After one week of field exposure, the plants were returned to greenhouse until the pod-filling stage. Stem disease severity was evaluated using a 0-5 scale where 0 = uninfected, 1 = lesion area less than 25% of the cross-section area of the crown, 2 = 25 to 50%, 3 = >50% girdled, stem firm, 4 => 50% girdled, stem weak, and 5 = plant dead (West et al., Citation2002). The square root transformation was applied to mean disease severity.
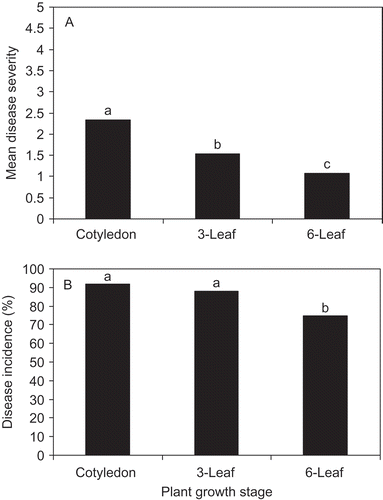
Table 1. Mean fruiting bodies* density on stubble pieces, mean weakly concentration of ascospores†, phoma stem canker severity and incidence‡ of canola plants infected at three different growth stages in each week at the Carman Research Centre in Manitoba for 2004 and 2005
Table 2. Analysis of variance for the effects of treatment, year and their interaction on the mean disease severity and disease incidence of blackleg on the canola plants infected at three different growth stages of cultivar ‘Westar’ in 2004 and 2005*
Inspection of infested stubble
The results of analysis of variance indicated that the density of pycnidia on stubble pieces was significantly higher than the density of pseudothecia (P<0.0001) in both years (). The highest density of pycnidia was observed in week one for both years (4.1 and 4.6 pycnidia/cm2 in 2004 and 2005, respectively) and the lowest was in week five in the first year (3.8 pycnidia/cm2) and week four in the second year (3.1 pycnidia/cm2). No immature and/or mature pseudothecia was observed in most weeks in 2004 and 2005. However, low density of pseudothecia was observed in week two (0.002 pseuodothecia/cm2) and three (0.003 pseuodothecia/cm2) in the first year and week two (0.02 pseuodothecia/cm2) and seven (0.004 pseuodothecia/cm2) in the second year. All these pseudothecia belonged to group A (pseudothecia and asci immature, ascospores absent). These results confirmed that pycnidia were the main fruiting bodies present in the local area in 2004 and 2005.
Table 3. Mean comparison of pycnidia and pseudothecia of Leptosphaeria maculans on blackleg infected canola stubble pieces in each week at the Carman Research Centre in Manitoba for pooled data*
Spore sampling and disease estimation on trap plants
The results showed that no ascospores were trapped in 2004 and only a low concentration of ascospores were observed (by Burkard spore trap) in weeks two (0.028 ascospores/cm3/week), five and seven (0.008 ascopsore/cm3/week) in 2005 (). These results using both Burkard and rotorod spore samplers indicated that very low concentrations of ascospores were trapped from local and/or long distance areas in most weeks in both years. The level of mean disease severity and disease incidence was studied using canola plants at three different growth stages as living traps in the field. This experiment showed that disease incidence in all weeks was very high in both years (). The results indicated that the total number of weeks with phoma stem canker incidence greater than 50 percent for both years (15 weeks) was 15, 13 and 12 in the plants infected at 1, 0, 1, 3 and 1, 6 stage, respectively (). Plating of the infected segments of the tissues on V8 agar showed that all isolates were L. maculans based on the colony characteristics and morphology of pycnidia and pycnidiospores. Pathogenicity tests on ‘Westar’, ‘Glacier’ and ‘Quinta’ indicated that all isolates belong to pathogenicity group 2 (PG2) except one isolate from week five that was PG3 collected in 2005. No leaf lesion was recorded during the maturation of plants in greenhouse.
Discussion
Previous studies (Petrie, Citation1994, 1995; Guo et al., 2005; and personal communications with Dr. Roger Rimmer) reported that there are some years with no ascospore showers but high disease severity and incidence on canola plants in Western Canada (Manitoba and Saskatchewan specifically). Therefore, we considered this unique condition as an ideal opportunity to compare blackleg severity and incidence on naturally infected canola plants by pycnidiospores as a main source of inoculum under field conditions. Our results showed that the mean disease severity and incidence on plants infected at 1, 0 stage were significantly higher than the other two stages. These results also indicated that mean disease severity of plants infected at 1, 3 stage was significantly higher than those of plants infected at 1, 6 stage but there was no significant difference in the disease incidence in the plants infected at these two growth stages. These results confirm those from other studies that showed most yield losses in the phoma stem canker epidemics is due to infection of the plants at early seedling stage, especially 1, 0 stage (Barbetti, Citation1975; Barbetti & Khangura, Citation1999). Cargeeg and Thurling (Citation1979) reported that there was strong correlation between development of crown canker at plant maturity and inoculation of seedlings 10 days after sowing. Marcroft et al. (Citation2005) indicated that the incidence of external stem lesions was significantly greater on plants inoculated in controlled conditions with pycnidiospores at the cotyledon, 3-leaf and 5-leaf stage compared to plants inoculated at inflorescence or flowering. They also found that cankers developed on most plants inoculated at 1, 0, 3-leaf and 5-leaf stage compared to the plants at inflorescence or flowering. They also reported that there were no significant differences among the first three growth stages in terms of developing external lesions or cankers. McGee and Petrie (Citation1979) indicated that rapeseed was most susceptible to infection by P. lingam at the 1, 6 stage of growth compared to older growth stages. Li et al. (Citation2006a ) studied the relationship of time of inoculation of four cultivars of B. napus including ‘Westar’ with a pycnidiospore suspension of L. maculans. They used two temperature regimes 18°C/24°C (night/day) and 11°C/18°C (night/day) in growth cabinets. They showed that crown canker severity of the plants inoculated at the 1, 0 stage (1, 0) was significantly higher than plants inoculated at 1, 3 (1, 03) and 5-leaf (1, 05) stages at 11/18 °C temperature regime. The results from this study showed that there was no significant difference in disease severity on plants inoculated at 1, 3 (1, 03) and 5-leaf (1, 05) on ‘Westar’. The crown canker severity of plants inoculated at 1, 0 stage was higher than plants inoculated at the 1, 3 stage but was the same as plants inoculated at 5-leaf stage at 18/24 °C temperature regime. This discrepancy in results may be due to different virulent isolates of L. maculans used in the two studies, point inoculation of plants versus natural exposure and infection of plants in the current field study and continuous favourable temperature regime in the controlled conditions compared to fluctuating temperature regime under field conditions. The lower level of disease in older plants may due to the size of the plants, as the pathogen takes longer to grow from the leaf to the crown in larger (mature) plants than in smaller (young) plants (Petrie, Citation1995).
The results from our study also provide some evidence for the role of pycnidiospores as main source of inoculum (in addition to ascospores) in Western Canada. The high number of pycnidia compared to pseudothecia on local stubble pieces, lack of ascospores in local and/or from long distance area and high disease incidence on the trap plants implied that pycnidiospores can be considered as main or at least an alternative source of inoculum at the beginning of growing season for these two years of study in Manitoba. Since the growing season in Western Canada begins at the end of April to early June, it is reasonable to assume that the high level of phoma stem canker incidence on the trap plants at the beginning of growing season could be attributed to the existence of pycnidiospores in these two years. Ascospores have been shown to be the main source of primary inoculum compared to pycnidiospores in most canola growing regions of the world (Petrie, Citation1995; Mahuku et al., Citation1997; West et al., Citation2001). The importance of ascospores compared to pycnidiospores in epidemiology of phoma stem canker is due to faster germination, penetration and disease development (Li et al., Citation2004). Woods and Barbetti (Citation1977) showed that fewer ascospores are needed for infection than pycnidiospores. Nevertheless, Hall (Citation1992) and Williams (Citation1992) mentioned the possibility of a role for pycnidiospores as a source of primary inoculum. To our knowledge, this is the first report showing pycnidiospores of P. lingam as a main source of inoculum or at least alternative source of inoculum in Western Canada. It is likely that pycnidia can survive the cold winters in Manitoba and release pycnidiospores at the beginning of the growing season and subsequently cause phoma stem canker in years in which ascospores do not exist or are in very low concentration due to environmental conditions not favouring their production and release. However, more studies are needed to prove the overwintering of the pathogen as an asexual stage in Manitoba. Pycnidial overwintering is not uncommon as it has been reported in other fungi (Petrie, Citation1995; Baird et al., Citation1999) and also in different species of Phoma such as P. pinodella, P. melilotia and P. sclerotioides (Arsenuik & Goral, Citation1998; Agostini et al., Citation2003). Low ascospore levels could be due to a delay in pseudothecial maturation due to extreme weather conditions as shown in other studies (Petrie, Citation1994; West et al., Citation2001; Toscano-Underwood, Citation2003). Petrie (Citation1994) showed that ascocarp development could be interrupted by three-week periods of freezing without catastrophic reductions in ascospore numbers but longer periods of freezing suppressed sporulation. He also mentioned that reductions in ascospore numbers, often observed following overwintering of two year-old and older stubble residue in Saskatchewan, can be due to the harmful effects of long periods of freezing or repeated freezing and thawing. Petrie (Citation1994) showed that hot spring weather may cause sporulation on stubble to be delayed until 12 months after crop harvest. Also, a 16-year study on pseudothecia maturation and ascospore release in Saskatchewan revealed that higher than average temperatures and lower than average rainfall in summer contributed substantially to poor, delayed or interrupted ascospore release in eight of the 16 years (Petrie, Citation1995). It may be that severe temperatures in Manitoba winters of 2003 and 2004 and higher than normal summer temperatures in 2003 and 2004 (above 30°C in July and August) may have contributed to a delay in maturation of pseudothecia and ascospore release in 2004 and 2005. These findings also confirmed that ascospore showers could not be expected in Western Canada every year during the growing season. This study may help explain the incidence of the disease in the absence of sexual spores during the growing season. It also confirmed previous findings that the level of infection on plants at 1, 0 stage strongly correlates to severity and incidence of subsequent stem cankers.
Acknowledgements
The authors thank Paula Parks and Alvin Iverson for assisting in the field. The authors also thank NSERC Discovery Grant and ARDI Manitoba for financial contributions to carry out this work. The authors would like to thank Dr. Rob Gulden and Besrat Demoz for critically reviewing the manuscript, especially the statistical analysis of the work.
References
- Agostini , J.P. , Bushang , P.M. , Bhatia , A. and Timmer , L.W. 2003 . Influence of environmental factors on severity of citrus scab and melanose . Plant Dis. , 87 : 1102 – 1106 .
- Arseniuk , E. and Goral , T. 1998 . Seasonal patterns of spore dispersal of Phaeosphaeria spp. and Stagonospora spp . Plant Dis. , 82 : 187 – 194 .
- Bailey , K.L. , Gossen , B.D. , Gugel , R.K. and Morall , R.A.A. , eds. 2003 . Diseases of field crops in Canada , Saskatoon : University Extension Press, University of Saskatchewan .
- Baird , R.E. , Phillips , D.V. , Mullinix , B.G. and Alt , P.J. 1999 . Relative longevity of Leptosphaeria maculans and associated mycobiota on canola debris . Phytoprotection , 80 : 1 – 11 .
- Barbetti , M.J. 1975 . Late blackleg infections in rape are important . Aust. Plant Pathol. Soc. Newslett. , 4 : 3 – 4 .
- Barbetti , M.J. 1976 . The role of pycnidiospores of Leptosphaeria maculans in the spread of blackleg disease in rape . Aust. J. Exp. Agric. Anim. Husb. , 16 : 911 – 914 .
- Barbetti , M.J. and Khangura , R.K. Management of blackleg in disease-prone environments of Western Australia . Proceedings of the 10th International Rapeseed Congress . Sept 26–29 1999 , Canberra, Australia. pp. 100 The Regional Institute Ltd .
- Cargeeg , L.A. and Thurling , N. 1979 . Seedling and adult plant resistance to blackleg (Leptosphaeria maculans (Desm.) Ces. Et de Not. ) in spring rape (Brassica napus L.) . Aust. J. Agric. Res. , 30 : 37 – 46 .
- Chen , Y. and Fernando , W.G.D. 2006 . Induced resistance to blackleg (Leptosphaeria maculans) disease of canola (Brassica napus) caused by a weakly virulent isolate of Leptosphaeria biglobosa . Plant Dis. , 90 : 1059 – 1064 .
- Fitt , B.D.L. , McCartney , H.A. and Walklate , P.J. 1989 . The role of rain in dispersal of pathogen inoculum . Annu. Rev. Phytopathol. , 27 : 241 – 270 .
- Fitt , B.D.L. , Brun , H. , Barbetti , M.J. and Rimmer , S.R. 2006 . World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus) . Eur. J. Plant Pathol. , 114 : 3 – 15 .
- Ghanbarnia , K. , Fernando , W.G.D. and Crow , G. 2009 . Developing rainfall-and temperature-based models to describe infection of canola under field conditions caused by pycnidiospores of Leptosphaeria maculans . Phytopathology , 99 : 879 – 886 .
- Guo , X.W. and Fernando , W.G.D. 2005 . Seasonal and diurnal patterns of spore dispersal by Leptosphaeria maculans from canola stubble in relation to environmental conditions . Plant Dis. , 89 : 97 – 104 .
- Hall , R. 1992 . Epidemiology of blackleg of oilseed rape . Can. J. Plant Pathol. , 14 : 46 – 55 .
- Hall , R. , Chigogora , J.L. and Phillips , L.G. 1996 . Role of seedborne inoculum of Leptosphaeria maculans in development of blackleg on oilseed rape . Can. J. Plant Pathol. , 108 : 73 – 80 .
- Holett , B.J. , Idnurum , A. and Pedras , M.S.C. 2001 . Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas . Fungal Genet. Biol. , 33 : 1 – 14 .
- Huang , Y.J. , Li , Z.Q. , Evans , N. , Rouxel , T. , Fitt , B.D. and Balesdent , M.H. 2006 . 2006. Fitness cost associated with loss of the AvrLm4 avirulence function in Leptosphaeria maculans (phoma stem canker of oilseed rape) . Eur. J. Plant Pathol. , 114 : 77 – 89 .
- Koch , E. , Song , K. , Osborne , T.C. and Williams , P.H. 1991 . Relationship between pathogenicity and phylogeny based on restriction fragment length polymorphism in Leptosphaeria maculans . Mol. Plant-Microbe Interact. , 4 : 341 – 349 .
- Li , H. , Sivasithamparam , K. , Barbetti , M.J. and Kuo , J. 2004 . Germination and invasion by ascospores and pycnidiospores of Leptosphaeria maculans on spring-type Brassica napus canola varieties with varying susceptibility to blackleg . J. Gen. Plant Pathol. , 70 : 261 – 269 .
- Li , H. , Smyth , F. , Barbetti , M.J. and Sivasithamparam , K. 2006a . Relationship between Brassica napus seedling and adult plant responses to Leptosphaeria maculans is determined by plant growth stage at inoculation and temperature regime . Field Crops Res. , 96 : 428 – 437 .
- Li , H. , Tapper , N.D.N. , Barbetti , M. and Sivasithamparam , K. 2006b . Enhanced pathogenicity of Leptosphaeria maculans pycnidiospores from paired co-inoculation of Brassica napus cotyledons with ascospores . Ann. Bot. , 97 : 1151 – 1156 .
- MacGee , D.C. 1997 . Blackleg (Leptosphaeria maculans (Desm.) Ces. Et de Not.) of rapeseed in Victoria: sources of infection and relationships between inoculum, environmental factors and disease severity . Aust. J. Agric. Res. , 28 : 53 – 62 .
- MacGee , D.C. and Emmet , R.W. 1977 . Blackleg (Leptosphaeria maculans) of rapeseed in Victoria: crop losses and factors which affects disease severity . Aust. J. Agric. Res. , 28 : 47 – 51 .
- MacGee , D.C. and Petrie , G.A. 1979 . Seasonal patterns of ascospore discharge by Leptosphaeria maculans in relation to blackleg of oilseed rape . Phytopathology , 69 : 586 – 589 .
- Mahuku , G.S. , Goodwin , P.H. , Hall , R. and Hasiang , T. 1997 . Variability in the highly virulent type of Leptosphaeria maculans within and between oilseed rape fields . Can. J. Bot. , 75 : 1485 – 1492 .
- Marcroft , S.J. , Sosnowski , M.R. , Scott , E.S. , Ramsey , M.D. , Salisbury , P.A. and Howlett , B.J. 2005 . Brassica napus plants infected by Leptosphaeria maculans after the third to fifth leaf growth stage in south-eastern Australia do not develop blackleg stem canker . Eur. J. Plant Pathol. , 112 : 289 – 292 .
- Petrie , G.A. 1986 . Consequences of survival of Leptosphaeria maculans (blackleg) in canola stubble residue through an entire crop rotation sequence . Can. J. Plant Pathol. , 8 : 353
- Petrie , G.A. 1994 . Effects of temperature and moisture on the number, size and septation of ascospores produced by Leptosphaeria maculans (blackleg) on rapeseed stubble . Can. Plant Dis. Surv. , 74 : 141 – 151 .
- Petrie , G.A. 1995 . Long-term survival and sporulation of Leptosphaeria maculans (blackleg) on naturally-infected rapeseed/canola stubble in Saskatchewan . Can. Plant Dis. Surv. , 75 : 23 – 34 .
- Poisson , B. and Peres , A. Study of rapeseed susceptibility to primary contamination of Leptosphaeria maculans in relation to plant vegetative stage . Proceedings of the 10th International Rapeseed Congress . Sept 26–29 1999 , Canberra, Australia. pp. 100 The Regional Institute Ltd .
- Rempel , C.B. and Hall , R. 1993 . Dynamics of production of ascospores of Leptosphaeria maculans in autumn on stubble of the current year's crop of spring rapeseed . Can. J. Plant Pathol. , 15 : 182 – 184 .
- Shoemaker , R.A. and Burn , H. 2001 . The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans . Can. J. Bot. , 79 : 412 – 419 .
- Toscano-Underwood , C. , Huang , Y.J. , Fitt , B.D.L. and Hall , A.M. 2003 . Effects of temperature on maturation of pseudothecia of Leptosphaeria maculans and L. biglobosa on oilseed rape stem debris . Plant Pathol. , 52 : 726 – 736 .
- Travadon , R. , Bousset , L. , Saint-Jean , S. and Sache , I. 2007 . Splash dispersal of Leptosphaeria maculans pycnidiospores and the spread of blackleg on oilseed rape . Plant Pathol. , 56 : 595 – 603 .
- West , J.S. , Biddulph , J.E. , Fitt , B.D.L. and Gladders , P. 1999 . Epidemiology of Leptosphaeria maculans in relation to forecasting stem canker severity on winter oilseed rape in the UK . Ann. Appl. Biol. , 135 : 535 – 546 .
- West , J.S. , Fitt , B.D.L. , Leech , P.K. , Biddulph , J.E. , Huang , Y.-J. and Balesdent , M. H. 2002 . Effects of timing of Leptosphaeria maculans ascospore release and fungicide regime on phoma leaf spot and phoma stem canker development on winter oilseed rape (Brassica napus) in southern England . Plant Pathol. , 51 : 454 – 463 .
- West , J.S. , Kharbanda , P.D. , Barbetti , M. J. and Fitt , B.D.L. 2001 . Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe . Plant Pathol. , 50 : 10 – 27 .
- Williams , P.H. 1992 . Biology of Leptosphaeria maculans . Can. J. Plant Pathol. , 14 : 30 – 35 .
- Woods , P.M. and Barbetti , M.J. 1977 . A study on the inoculation of rape seedlings with ascospores and pycnidiospores of the blackleg disease causal agent . J. Aus. Inst. Agric. Sci. , 43 : 79 – 80 .
- Xi , K. , Morall , R.A.A. , Gugel , R.K. and Verma , P.R. 1991 . Latent infection in relation to the epidemiology of blackleg of spring rapeseed . Can. J. Plant Pathol. , 13 : 321 – 331 .
- Zhou , Y. , Fitt , B.D.L. , Welhm , S.J. , Gladders , P. , Sansford , C.E. and West , J.S. 1999 . Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK . Eur. J. Plant Pathol. , 105 : 715 – 728 .