Abstract
The infection process of Discula destructiva Redlin on Cornus florida L. leaves was studied using histological and microscopic techniques. Penetration of fungal hyphae through natural openings and wounds was not observed, while direct penetration without appressorium formation was demonstrated 3 days after inoculation (DAI). Leaves inoculated with D. destructiva developed symptoms of dogwood anthracnose after 7 to 8 days. At 8 DAI, hyphae were observed in aggregations located between the cuticle and epidermis and also growing intracellularly towards epidermal, palisade, parenchymal and spongy mesophyll cells. At 16 DAI, typical chlorotic and necrotic halos, with a red to purple external border, were formed on the inoculated leaves. Within leaf tissues, at 16 DAI, chloroplasts were intact but decompartmentalized and infection sites were clearly defined. Sporulation and ruptured acervuli (cuticle ruptured and spores released) were first detected at 20 DAI, and had fully developed to rupture the plant cuticle on both adaxial and abaxial leaf surfaces by 24 DAI.
Résumé
Le processus d'infection de Discula destructiva Redlin sur les feuilles de Cornus florida L. a été étudié au moyen de techniques histologiques et microscopiques. La pénétration des hyphes fongiques par des ouvertures naturelles ou des blessures n'a pas été observée, tandis que la pénétration directe, sans formation d'appresseurs, a été démontrée trois jours après l'inoculation (JAI). Les feuilles inoculées avec D. destructiva ont affiché des symptômes de l'anthracnose du cornouiller au bout de sept à huit jours. À huit JAI, des hyphes étaient observables en agrégats localisés entre la cuticule et l’épiderme ainsi que croissant intracellulairement dans le mésophylle (épiderme, parenchyme palissadique et parenchyme spongieux). À 16 JAI, des halos chlorotiques et nécrotiques caractéristiques, comportant une bordure externe variant du rouge au pourpre, s’étaient formés sur les feuilles inoculées. Dans les tissus, à 16 JAI, les chloroplastes étaient intacts, mais décloisonnés et les sites d'infection étaient clairement délimités. La sporulation et les acervules perforés (perforation de la cuticule et libération des spores) ont été détectés initialement à 20 JAI et étaient en mesure de perforer la cuticule des faces ventrales et dorsales des feuilles 24 JAI.
Introduction
Flowering dogwood (Cornus florida L.) is a small tree reaching 10 metres in height and is found throughout much of the eastern United States (Bailey et al., Citation1989; Dirr, Citation1990). Foliage, fruit and seeds of flowering dogwood provide nutrition to more than 50 wildlife species active in the forest understorey (Rossell et al., Citation2001). It is also valued as an ornamental tree in managed landscapes (Bailey et al., Citation1989; Windham et al., Citation1991).
In the late 1970s, high dogwood mortality associated with anthracnose symptoms was reported across the northeastern United States (Hiers & Evans, Citation1997; Carr & Banas, Citation1999; Jenkins & White, Citation2002). In 1980s, the causal agent was identified as a Discula species (Daughtery et al., Citation1988), perhaps in combination with other environmental phenomena (Pirone, Citation1980). Redlin (Citation1991) named the dogwood anthracnose pathogen Discula destructiva Redlin. Several Cornus species are susceptible to dogwood anthracnose, including C. controversa Hemsl. Ex Prain, C. florida L., C. kousa ‘Chinensis’ Buerger ex Mia., C. nuttallii Audubon ex Torr. & A. Gray, and C. sericea L. (Windham et al., Citation1993; Ranney et al. Citation1995; Brown et al., Citation1996). The most obvious disease symptom in flowering dogwood is leaf blight, which begins as a red to purple external bordered lesion with chlorotic and necrotic centres. Infections in young branches may spread downwards resulting in sunken stem cankers. Multiple stem cankers may girdle cambial tissues, thereby contributing to tree death (Daughtery et al., Citation1988; Bailey et al., Citation1989; Redlin Citation1991).
Interactions between D. destructiva and C. florida have been investigated by light microscopy (Redlin, Citation1992, Hiers & Evans, Citation1997) and scanning electron microscopy (Brown et al., Citation1990, Redlin, Citation1991, Citation1992). The morphology of acervular conidiomata of D. destructiva on leaf disks of C. florida has been described and evidence for direct hyphal penetration of dogwood leaves without requiring a wound or stomata were provided (Graham et al., Citation1991). Although studies suggested D. destructiva invaded tissues both inter- and intracellularly (Walkinshaw & Anderson, Citation1991), the sequence of infection events remains inconclusive. To understand the mechanisms of resistance to dogwood anthracnose evident among some C. florida cultivars and unnamed selections in further studies, it is essential to characterize early stages of pathogenesis, including conidial germination, infection structure formation, penetration and ramification. The objective of this research was to describe the sequence of events in the infection process of D. destructiva in C. florida.
Materials and methods
Leaf collection
Fully expanded healthy flowering dogwood leaves were collected from Cornus florida L. ‘Cloud 9’, a dogwood anthracnose susceptible cultivar (Ranney et al., Citation1995), which was growing in a greenhouse at the University of Tennessee at Knoxville. Leaves were washed with sterile distilled water (100 mL per leaf) three times and cut into 1-cm-diameter disks that were placed adaxial surface up on two layers of P8-creped filter paper (Fisher Scientific, Pittsburgh, PA) which were moistened with 5 mL sterile distilled water in 9-cm diameter Petri dishes (Fisher Scientific, Pittsburgh, PA).
Isolate collection and identification
Ten isolates of D. destructiva were obtained from leaves and twigs of diseased trees located at the University of the South (Sewanee, TN) in March 2008. Samples were collected from naturally propagated trees in a forest environment, placed in labelled sterile polyethylene bags and transported in coolers to the laboratory.
Moisture chambers were prepared using Petri dishes containing creped filter paper that was moistened with sterile distilled water. About 40 1-cm long stems and 20 1-cm2 leaf pieces were held in moisture chambers at 20 °C in darkness for up to 2 weeks until cirri appeared on acervular conidiomata. Spore morphology was used to identify fungi as D. destructiva (Redlin, Citation1992). Discula destructiva isolates were transferred using sterilized insect needles and allowed to grow on dishes containing potato dextrose agar (PDA; Difco Laboratories, Detroit, MI) amended with 30 mg L−1 each of chlorotetracycline and streptomycin sulphate (ICN Biochemicals, Cleveland, OH). Cultures were incubated at 20 °C with a 12 hour light : dark cycle until sufficient fungal growth had occurred. All isolates were confirmed to be D. destructiva using the gallic acid test as described by Trigiano et al. (Citation1991), wherein D. destructiva could be differentiated from other Discula species because they turned the gallic acid medium brown. In addition, fungal DNA of one isolate (U3-16) was extracted using the DNeasy Plant Mini DNA isolation kit (Qiagen, German) and quantified with a spectrophotometer (Nanodrop Technologies). DNA was further PCR amplified using internal transcribed spacer (ITS) primers (ITS1, ITS4 and ITS5) (White et al., Citation1990) following initial denaturation steps of 2 min at 94 °C; 40 cycles of 30 s at 94 °C, 1 min at 57 °C, and 1.5 min at 72 °C, and a final extension of 7 min at 72 °C. The PCR products were purified using QIAquick® PCR Purification Kit (Qiagen, Germany) and sequenced using capillary electrophoresis DNA analyser ABI 3100. Assembled sequences were checked using Basic Local Alignment Search Tool (BLAST) to confirm identity of D. destructiva.
Inoculation procedure
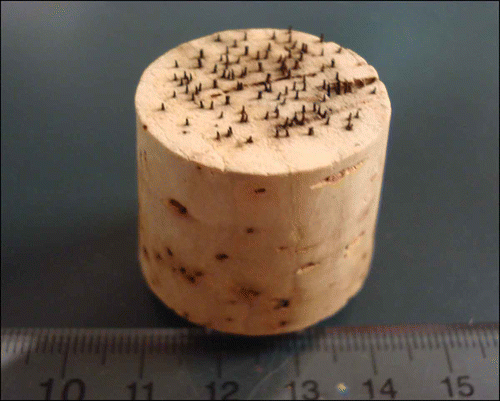
A single isolate of D. destructiva (U3-16) was selected from the isolates collected at Sewanee, TN and used as the inoculum throughout all experiments. An agar plug (0.5 cm × 0.5 cm × 0.5 cm) was placed on a piece of 8-cm diameter sterilized dogwood leaf in a 9-cm diameter Petri dish. The dogwood leaf was autoclaved at 121 °C for 1 hour and again after 24 hours to kill bacteria on the leaf surface. Leaf pieces were placed on PDA with the adaxial surface up. Approximately 50 Petri dishes were prepared. Three weeks later, conidiomata containing conidial masses were observed underneath trichomes all over the leaf. Conidia were collected by submerging leaf pieces in sterile distilled water and gently rubbing the leaf surface with a sterilized ‘7’ shaped glass rod. Conidial suspensions were adjusted to 8 ×104 conidia mL−1, and then applied to leaf surfaces of fresh, pre-wounded dogwood leaf disks with the adaxial surface up in Petri dishes using a mist fingertip sprayer. The spore density on inoculated leaf disks was maintained at 200–300 conidia cm−2 by adjusting the number of sprays during the inoculation. Wounding of leaf disks was achieved using a tool fabricated from a cork stopper embedded with #1 insect pins in a random pattern (). After inoculation, Petri dishes were sealed with Parafilm (Pechiney Plastic Packaging, Menasha, WI) and incubated at 20 °C with a 12-hour light : dark cycle.
Figure 1. Experimental wounding tool – a cork stopper embedded with #1 insect pins (16 pins/cm2) – used to abrade adaxial leaf surfaces.
In order to examine the vigour and germination percentage of conidia, 100 conidia on three randomly chosen leaf disks were counted to assess whether or not germination had occurred by 1, 2 and 3 days after inoculation (DAI), respectively. To include a control treatment which was conducted to make sure most of the spores had the vigour to germinate, conidial suspensions were also spread on PDA using the same protocol as for the leaf disks and 100 conidia on three randomly chosen Petri dishes were assessed for germination at 1, 2 and 3 DAI, respectively.
Tissue preparation for histology
Ten leaf disks were chosen per sampling period at 2, 4, 8, 16, 20 and 24 DAI. Disks were trimmed into smaller rectangular pieces (c. 7 × 3 mm), then fixed in 50% FAA solution (Fisher Scientific, Pittsburgh, PA) in glass tubes for 2 days, dehydrated in a series of 50, 75, 85, 95% and absolute alcohol (Decon Labs, Inc., King of Prussia, PA), and embedded in paraffin (Paraplast® Oxford Labware St. Louis, MO). Serial 10 μm thick cross-sections were cut using a rotary microtome. Slides were prepared by washing with detergent (Clorox, Clorox Professional Products Company, Oakland, CA) and rinsed with distilled water. Cross-sections were expanded on a puddle of distilled water maintained at 42 °C, and affixed to glass slides without adhesive, by draining off water and drying for 12 h at 42 °C. Sections were deparaffinized through three changes of Microclear™ (Micron Diagnostics, Inc., Baltimore, MD) for 5 min each and rehydrated by moving slides through a series of absolute ethanol, 95, 85, 70, 50 and 30% ethanol, and two deionized water rinses each lasting 5 min. The staining series consisted of 0.01% aqueous safranin O stain (Sciencelab.com, Inc., Houston, TX) for 24 h, followed by two 1-min deionized water rinses, 1% crystal violet (Sciencelab.com, Inc., Houston, TX) for 1 min, followed by 5-min deionized water rinses. Sections were dehydrated using a graded series of ethanol in reverse order of the rehydration series previously described and then stained with fast green (Sciencelab.com, Inc., Houston, TX) for 1 min, followed by 1-min and 5-min absolute ethanol rinses. Sections were then rinsed three times in Microclear™ with 10-min submersion per rinse. Coverslips were attached to slides with Permount (Fisher Scientific, Pittsburgh, PA) and slides were dried for 24 h at 42 °C. Sections were examined with a compound microscope (Nikon Instruments, Melville, NY). Approximately 10 slides, each with 20 leaf sections, were viewed for each sampling time.
Tissue preparation for scanning electron microscope
Early stages of pathogenesis of the disease were examined using a scanning electron microscope (SEM). The tissue preparation protocol was used as described by Zachariah & Pasternak (Citation1970), wherein random leaf disks were chosen at 1, 2 and 3 DAI and fixed with chilled (4 °C) 3% glutaraldehyde (Sigma-Aldrich, St. Louis, MO) in 0.05 M phosphate buffer (Fisher Scientific, Pittsburgh, PA), pH 6.8, and Histochoice® (Amresco, Solon, OH) for 1 h at 20 °C, followed by three washes each for 10 min, in 0.05 M phosphate buffer, pH 6.8. Secondary fixation was in 2% osmium tetroxide (Sigma-Aldrich, St. Louis, MO) dissolved in 0.05 M phosphate buffer, pH 6.8, for 1 h at 20 °C. Fixed samples were dehydrated in a graded ethanol series (25, 50, 70, 95, 100% ethanol and 100% dry ethanol, 30 min in each). Samples were air dried, mounted on aluminium stubs and sputter-coated with gold-palladium. Samples were examined by a FEI Quanta 200 scanning electron microscope (SEM) operating at an accelerating voltage of 10 keV. Five samples for each sampling time were examined using SEM.
Some paraffin-embedded samples (three samples for each sampling period) were also examined using SEM. These serial 10-μm thick cross-sections were attached to special 1-cm diameter thin slides, deparaffinized through three changes of Microclear™ for 10 min each, air dried and sputter-coated with gold-palladium.
Results and discussion
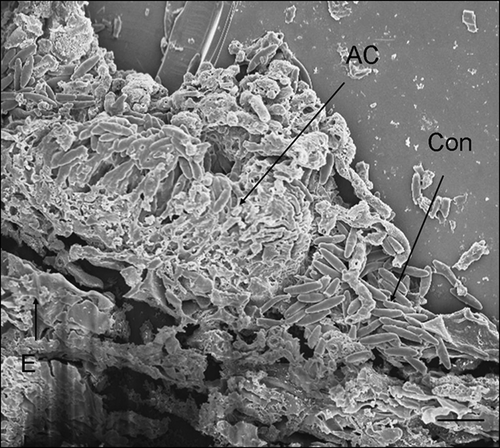
Conidia were considered germinated when germ tubes were more than half the length of the spore. One day after inoculation (DAI), germ tube primordia were observed with some conidia (). However, a majority (78%, ) had not reached the half length threshold. At 2 DAI, most conidia (91%, ) had germinated (). At 3 DAI, hyphae were observed on the leaf surface () and direct penetration of D. destructiva hyphae without appressorium formation was evident (). Hyphae accumulated near epidermal cells prior to sporulation and these hyphal masses were usually located beneath the base of trichomes, confirming previous descriptions of Walkinshaw & Anderson (Citation1991). At 8 DAI, hyphae had accumulated between the cuticle and epidermis and had grown intracellularly in epidermal cells, palisade parenchyma, and spongy mesophyll cells (, B). At 16 DAI, chloroplast dye colour had shifted from light to bright red, which suggests that the chloroplasts were decompartmentalized and the infection sites were easily delimited. By contrast, in healthy tissues, dyed chloroplasts appeared light blue (). Hyphae were observed near or inside most decompartmentalized cells (). Acervulus primordia had developed by 20 DAI () and acervuli were fully developed at 24 DAI (). Although the leaf disks were inoculated only on the adaxial surface, acervuli were observed on both adaxial and abaxial leaf surfaces. Acervuli were mostly formed at the bases of trichomes and were observed in both necrotic and living tissue. Sporulation through ruptured acervuli was observed at 20 DAI.
Figure 2. Scanning electron micrographs of Cornus florida ‘Cloud 9’ leaves with Discula destructiva conidia. A, Conidia (Con) begin to germinate on the leaf surface underneath the trichome (T) 1 day after inoculation (DAI). B, Germinated conidia with germ tube (GT) on leaf surface 2 DAI. C, Hypha (HY) growing from conidia 3 DAI. Bar = 2 μm.
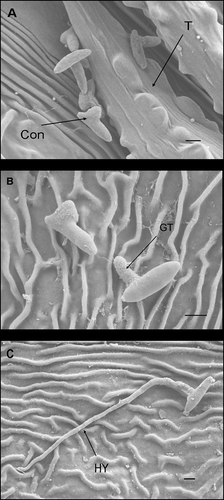
Figure 3. Histogram of conidial germination percentage at 1, 2 and 3 days after inoculation (DAI) on potato dextrose agar (PDA) and Cornus florida ‘Cloud 9’, respectively. Sample size n = 100 and bar = 1 SD.
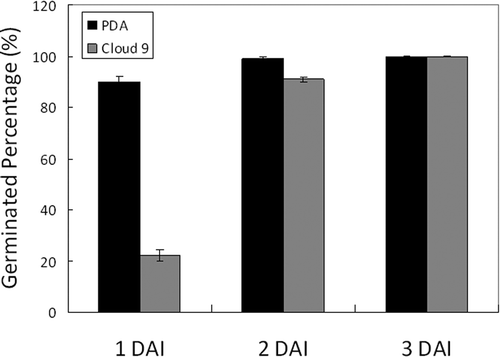
Figure 4. Scanning electron micrograph of a Discula destructiva hypha (HY) directly penetrating through the Cornus florida ‘Cloud 9’ leaf cuticle (C) and cell wall (CW) into epidermal cells (E) at 3 DAI. Bar = 2 μm.
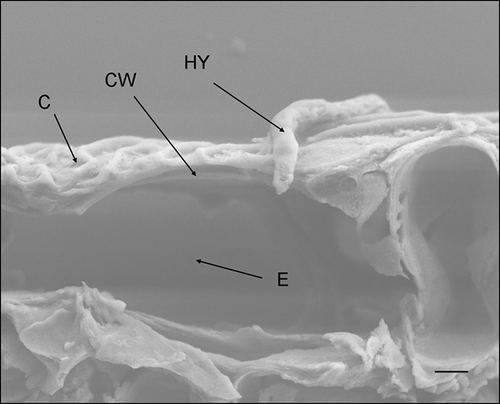
Figure 5. A, B, Light micrographs and scanning electron micrographs, respectively, of cross-sectioned Cornus florida ‘Cloud 9’ leaves inoculated with Discula destructiva conidia at 8 DAI. A, Hypha (HY) is growing intracellularly within epidermal cells (E). Bar = 10 μm. B, Hypha (HY) is growing in epidermal cells. Leaf orientation is adaxial surface up. Bar = 2 μm.
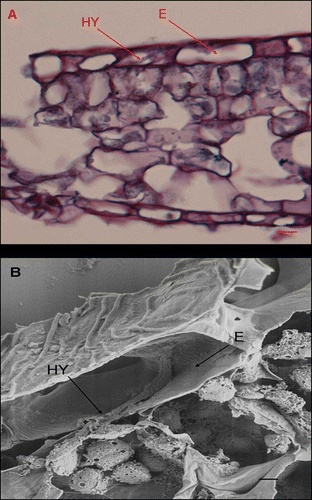
Figure 6. Light micrographs of Cornus florida ‘Cloud 9’ leaves inoculated with Discula destructiva conidia at 16 DAI. A, Diseased infection sites (IS) and healthy tissue (HT). B, Typically diseased, palisade parenchyma (PP) were stained dark red using safranin O. Bar = 20 μm.
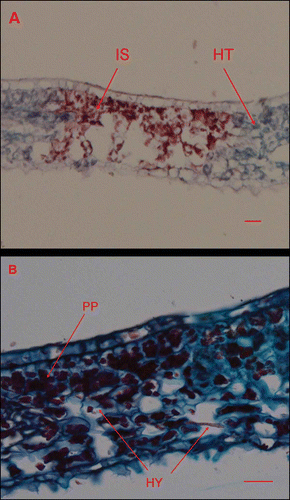
Figure 7. Scanning electron micrograph of Cornus florida ‘Cloud 9’ leaf inoculated with Discula destructiva conidia at 20 DAI. Developing acervulus (AC) with conidia underneath the cuticle and above the epidermal cell layer (E). C, Cuticle; Con, Conidia. Bar = 10 μm.
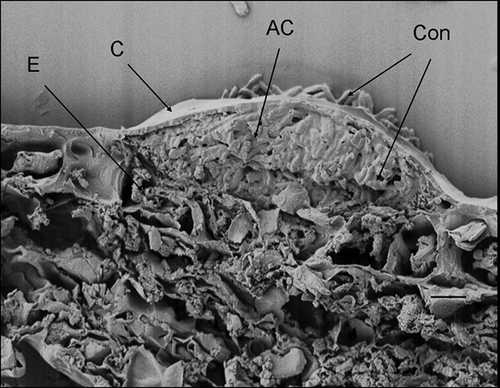
Figure 8. Scanning electron micrograph of Discula destructiva acervulus (AC) fully developed on Cornus florida ‘Cloud 9’ leaf at 24 DAI. Note the crushed epidermal cell layer (E). Con, Conidia. Bar = 10 μm.
Four toxins, 4-hydroxy-3-(3’-methyl-2’-butenyl)benzoic acid, 4-hydroxybenzoic acid, (+)-6-hydroxymellein and (−)-isosclerone, have been isolated from culture filtrates of Discula isolates recovered from infected dogwoods (Venkatasubbaian & Chilton, Citation1991). These toxins may be important in the pathogenesis of D. destructiva as evidenced by chloroplasts being damaged by hyphal contact with cells containing chloroplasts (). At the end of pathogenesis, plant tissues were disintegrated (). Toxins produced by necrotrophic pathogens are capable of breaking down host plant tissues, thereby releasing nutrients, and this could explain the above observations (Agrios, Citation1997; van Kan, Citation2006).
Early stages of pathogenesis leading to dogwood anthracnose symptoms included conidial germination, penetration, and colony development by the pathogen and host responses to infection as described above. The sequence of infection events for D. destructiva on flowering dogwood has now been clearly described and mechanisms of resistance which interfere with the pathogen's ability to infect dogwoods will be investigated in later studies.
Acknowledgements
This research was financially supported by Tennessee Ag-Research and by USDA-ARS 58-6404-7-213. We are also grateful to Dr Denita Hadziabdic for her assistance with DNA extraction, ITS amplification, cloning, sequencing and alignment.
References
- Agrios , G.N. 1997 . Plant pathology , 4th , San Diego , CA : Academic Press .
- Bailey , K.R. , Brown , E.A. II and Usda Forestry Service . 1989 . Growing and maintaining healthy dogwoods. USDA Forest Service Southern Region . Forestry Rep. R8-FR , 14 : 1
- Brown , D.A. , Windham , M.T. , Graham , E.T. and Trigiano , R.N. 1990 . Infection of flowering dogwood (Cornus florida) by the anthracnose fungus . Discula spp. Phytopathology , 80 : 1068 Abstr
- Brown , D.A. , Windham , M.T. and Trigiano , R.N. 1996 . Resistance to dogwood anthracnose among Cornus species . J. Arbor , 22 : 83 – 85 .
- Carr , D.E. and Banas , L.E. 1999 . Dogwood anthracnose (Discula destructiva): Effects of and consequences for host (Cornus florida) demography . Amer. Midl. Nat , 143 : 169 – 177 .
- Daughtery , M.L. , Hibben , C.R. and Hudler , G.W. 1988 . Cause and control of dogwood anthracnose in northeastern United States . J. Arboric. , 14 : 159 – 164 .
- Dirr , M.A. 1990 . Manual of woody landscape plants; their identification, ornamental characteristics, culture, propagation and uses , 4th , Champaign , IL : Stipes Publishing Company .
- Graham , E.T. , Windham , M.T. , Malueg , K.R. and Brown , D.A. 1991 . A histological study of dogwood anthracnose pathogenesis . Phytopathology , 81 : 1202 – 1203 .
- Hiers , J.K. and Evans , J.P. 1997 . Effects of anthracnose on dogwood mortality and forest composition of the Cumberland Plateau (U.S.A.) . Conserv. Biol. , 11 : 1430 – 1435 .
- Jenkins , M.A. and White , P.S. 2002 . Cornus florida L. mortality and understory composition changes in western Great Smoky Mountains National Park . J. Torrey Bot. Soc. , 129 : 194 – 206 .
- Pirone , P.P. 1980 . Parasitic fungus affects region's dogwood . New York Times, Feb. 24, Section , 2 : 34 – 37 .
- Ranney , T.G. , Grand , L.F. and Knighten , J.L. 1995 . Susceptibility of cultivars and hybrids of kousa dogwood to dogwood anthracnose and powdery mildew . J. Arbor , 22 : 11 – 16 .
- Redlin , S.C. 1991 . Discula destructiva sp. nov., cause of dogwood anthracnose . Mycologia , 83 : 633 – 642 .
- Redlin , S.C. 1992 . Scanning electron microscopy of the conidioma of Discula destructiva (Coelomycetes) . Mycologia , 84 : 257 – 260 .
- Rossell , I.M. , Rossell , C.R. , Hining , K.J. and Anderson , R.L. 2001 . Impacts of dogwood anthracnose (Discula destructiva Redlin) on the fruits of flowering dogwood (Cornus florida L.): Implications for wildlife . Amer. Midl. Nat. , 146 : 379 – 387 .
- Trigiano , R.N. , Gerhaty , N.E. , Windham , W.T. and Brown , D.A. 1991 . A simple assay for separating fungi associated with dogwood anthracnose . Proc. Southern Nurseryman's Assoc. Res. Conf. , 36 : 209 – 211 .
- Van Kan , J.A.L. 2006 . Licensed to kill: the lifestyle of a necrotrophic plant pathogen . Trends Plant Sci. , 11 : 247 – 253 .
- Venkatasubbaian , P. and Chilton , W.S. 1991 . Toxins produced by the dogwood anthracnose fungus Discula sp . J. Nat. Prod , 54 : 1293 – 1297 .
- Walkinshaw , C.H. and Anderson , R.L. 1991 . Histology of Cornus florida L. leaves infected naturally and artificially by Discula sp . U.S. Dept. Agric. Forest. Serv. Res. Note. SE- , 360 : 1 – 4 .
- White , T.J. , Bruns , T. , Lee , S. and Taylor , J.W. 1990 . PCR protocols: a guide to methods and application , San Diego , CA : Academic Press .
- Windham , M.T. , Trigiano , R.N. and Brown , D.A. 1993 . Dogwood anthracnose resistance of Cornus species . Amer. Nurseryman. , 178 : 91
- Windham , M.T. , Windham , A.S. and Langdon , K. 1991 . “ A comparison of factors that affect the incidence of dogwood anthracnose on forest and urban environments ” . In Proceedings of the Dogwood Anthracnose Working Group Athens , Georgia
- Zachariah , K. and Pasternak , J. 1970 . Processing soft tissues for scanning electron microscopy. Simplification of the freeze drying procedure . Stain Tech , 45 : 43