Abstract
The infection process of two pathogenic races of Colletotrichum truncatum on lentil during the biotrophic phase was studied using the susceptible lentil cultivar ‘Eston’ and ‘CDC Robin’ which has partial resistance to race 1. Isolates of both races successfully invaded the host tissue of both cultivars through direct penetration aided by appressoria. Overall, conidial germination and appressorial formation on detached leaflets of both cultivars were higher for three isolates of the more virulent race 0 than for three isolates of race 1. Higher appressorium formation of race 0 isolates compared with race 1 isolates was observed on detached ‘CDC Robin’, but not on ‘Eston’ leaflets. Conidial germination and appressorium formation of race 1 isolates on detached leaflets were greater on ‘Eston’ than on ‘CDC Robin’. These differences in conidial germination and appressorium formation may contribute to higher aggressiveness of race 0 isolates. Penetration success, however, was not associated with race identity and any differences observed were isolate-specific. The size of primary hyphae assessed at 48 and 60 h post-inoculation (hpi) varied among isolates, but again was not associated with race. Comparison of the detached leaflets with a whole plant assay using two of the six isolates revealed differences in development, indicating that the process of leaf detachment may influence fungal development and host response. Overall, there was no clear evidence for differential development of the two races during the biotrophic phase, indicating that events during the critical switch to necrotrophy or thereafter may be more significant for determining race-specific phenotypes.
C. Armstrong-Cho and J. Wang contributed equally to this work.
Résumé
Les processus d'infection de deux races pathogènes de Collectotrichum truncatum chez la lentille durant la phase biotrophe ont été étudiés en utilisant le cultivar réceptif ‘Eston’ et le cultivar ‘CDC Robin’, partiellement résistant à la race 1. Les isolats des deux races ont facilement envahi les tissus hôtes des deux cultivars par pénétration directe à l'aide des appresseurs. Dans l'ensemble, le taux de germination des conidies et de formation des appresseurs sur des folioles prélevées sur les deux cultivars étaient plus élevés chez trois isolats de la race 0, plus virulente, que chez trois isolats de la race 1. On a observé un taux plus élevé de formation des appresseurs des isolats de la race 0 que de la race 1 sur des folioles prélevées sur ‘CDC Robin’, mais par sur celles d'‘Eston’. Le taux de germination des conidies et de formation des appresseurs des isolats de la race 1 sur des folioles prélevées d'‘Eston’ était plus élevé que sur celles de ‘CDC Robin’. Ces différences sur le plan de la germination des conidies et de la formation des appresseurs peuvent contribuer à la plus grande virulence des isolats de la race 0. Toutefois, le degré de pénétration n'a pas été associé à la race et les différences observées étaient particulières aux isolats. La grosseur des hyphes primaires, évaluée de 48 à 60 heures après inoculation, variait chez les isolats, mais, de nouveau, n'était pas associée à la race. Un test comparatif fait sur des folioles prélevées et sur une plante entière, effectué avec deux des six isolats, a révélé des différences sur le plan de la croissance, indiquant que le fait d'utiliser des folioles prélevées peut influencer le développement fongique et la réponse de l'hôte. Dans l'ensemble, il n'y avait aucun signe manifeste d'un développement différentiel des deux races durant la phase biotrophe, ce qui indique que les évènements ayant cours durant le passage crucial à la phase nécrotrophe, ou après, peuvent concourir davantage à déterminer des phénotypes spécifiques de la race.
Introduction
Species in the genus Colletotrichum are fungal pathogens of a wide range of economically important crops grown around the world. Apart from its economic importance in agriculture, the genus has also been identified as an interesting model system for the study of host–pathogen interactions because of several advantages. For example, different species of Colletotrichum have developed different invasion strategies, the pathogen can be easily cultured in vitro, and the teleomorph of several species has been described, allowing for classical genetic studies. In addition, transformation protocols have been developed for several species, and at least one species, C. higgensianum Sacc. is pathogenic on the model plant Arabidopsis thaliana (L.) Heynh. (Perfect et al., Citation1999; O'Connell et al., Citation2004).
Most Colletotrichum species follow one of two infection strategies to invade plants, either through intracellular hemibiotrophic infection or subcuticular intramural infection (reviewed by Bailey et al., Citation1992). In both cases, the pathogen usually produces melanized appressoria as a prerequisite for direct penetration into host tissue. Intracellular hemibiotrophic infection is characterized by the formation of large primary hyphae within epidermal cells. The morphology of these specialized hyphae varies among Colletotrichum species, and they may be restricted to single cells, or expand through plasmodesmata into neighbouring cells. This phase of infection is symptomless and biotrophic, until thinner secondary hyphae are differentiated from primary hyphae and grow into adjacent cells where they cause cell collapse (reviewed by Bailey et al., Citation1992; Perfect et al., Citation1999; O'Connell et al., Citation2000). In the case of subcuticular intramural infection, hyphae initially spread below the cuticle and around the host epidermal cell walls, resulting in the disintegration of cell walls. Once hyphae have grown extensively through cell walls, and between and within the host cells, plant tissues are heavily damaged. The biotrophic stage is almost negligible.
Colletotrichum truncatum (Schwein.) Andrus and Moore has been reported from many leguminous crops including lentil, pea, fababean, soybean, sweet pea, chickpea and cowpea (Weidemann et al., Citation1988; Boyette, Citation1991; Adebitan et al., Citation1996; Gossen et al., Citation2009), and is also a pathogen of some weeds, including hemp sesbania (Boyette, Citation1991), wild vetch (Bailey et al., Citation2003), Florida beggarweed (Cardina et al., Citation1988), jimsonweed, dogbane, cocklebur (Hartman et al., Citation1986) and scentless chamomile (Graham et al., Citation2006). However, a recent detailed phylogenetic study on Colletotrichum species with curved conidia indicated that several of these C. truncatum isolates, including lentil isolates, may be more closely related to species other than C. truncatum as originally described (Damm et al., Citation2009). Colletotrichum truncatum from lentil was described as the causal organism of anthracnose of lentil in Canada in 1988 (Morrall, Citation1988). Ford et al. (Citation2004) showed that lentil isolates formed a clade distinct from other C. truncatum isolates based on 18–25 S rDNA sequence data, although lentil isolates were more closely related to those than to C. destructivum O'Gara or C. trifolii Bain. Damm et al. (Citation2009) proposed that C. truncatum ex lentil belongs to the C. destructivum clade, but probably not to C. destructivum itself.
The initial infection process of C. truncatum has been studied in detail on pea with pea isolates (O'Connell et al., Citation1993), and on soybean (Manandhar et al., Citation1985), and to some degree on lentil with lentil isolates (Chongo et al., Citation2002). In pea cells, highly branched primary hyphae were observed within 72 h post-inoculation (hpi), after which time secondary hyphae developed (O'Connell et al., Citation1993). A similar infection process was described for lentil where primary hyphae were observed 24 hpi, and first lesions became visible 72 hpi (Chongo et al., Citation2002). On soybean, in contrast, infection hyphae developed between the cuticle and the outer epidermal cell walls, and inter- and intracellular hyphae were visible within 2 days (Manandhar et al., Citation1985).
Anthracnose of lentil is a major disease on the Canadian prairies (Tullu et al., Citation2003). Characteristic symptoms are defoliation, stem girdling, plant wilting and, in severe cases, death after the vascular tissue of stems is impaired (Morrall, Citation1988; Buchwaldt et al., Citation1996). Buchwaldt et al. (Citation2004) identified two pathogenic races, referred to as race Ct0 and race Ct1, using seven differential lentil accessions. Whereas partial resistance to race Ct1 was identified in cultivated lentil, resistance to the more aggressive race Ct0 has only been found in other Lens species, particularly L. ervoides (Tullu et al., Citation2006). Genetic studies indicated that resistance to race Ct1 was controlled by the single recessive gene lct-1 in cultivar ‘Indianhead’, and by the single dominant genes LCt-2 and LCt-3 in lentil accessions PI320937 and PI3456329, respectively (Buchwaldt et al., Citation2001; Tullu et al., Citation2003). To simplify the nomenclature of the races, and avoid confusion with isolate identification numbers, resistance genes or potential avirulence gene designations, we will refer to these races as race 0 and race 1 henceforth.
As part of a larger study aimed at understanding the genetic nature of virulence in the two pathogenic races of C. truncatum from lentil, detailed cytological studies of the infection process during the biotrophic phase of both races were conducted. The objective was to determine whether there were qualitative and/or quantitative differences in the development of the two races on and in lentil leaf tissues of susceptible and partially resistant lentil cultivars that would warrant a study of gene expression during this phase of infection.
Materials and methods
Selection of isolates and culture maintenance
Isolates from lentil included the race 1 isolates CT-15, CT-21 and CT-35, and the race 0 isolates CT-20, CT-30 and CT-34. Isolates CT-34 (sender ID 95A8) and CT-35 (sender ID 95B36) originated from Manitoba, and were received from Agriculture and Agri-Food Canada, Saskatoon Research Centre (Buchwaldt et al., Citation2004). The remaining four isolates were arbitrarily selected field isolates from Saskatchewan. Prior to experimentation, all isolates were passed through plants of the fully susceptible lentil cultivar ‘Eston’, re-isolated and single-spored. Race identification was confirmed through pathogenicity testing on the fully susceptible lentil ‘Eston’ and the race 1-resistant cultivar ‘CDC Robin’ (details not presented, ). Preliminary spore germination studies on glass slides were conducted and revealed no inherent difference in germination rates among these isolates (data not presented).
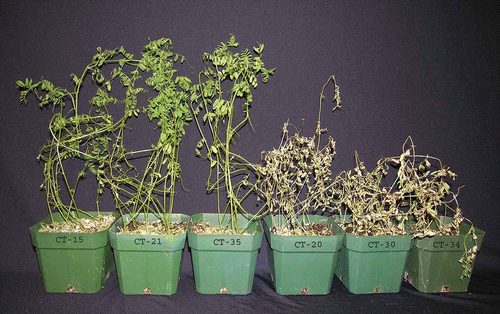
Fig. 1. Response of lentil cultivar ‘CDC Robin’ with partial resistance to race 1 isolates 10 days post-inoculation with Colletotrichum truncatum isolates CT-15, CT-21 and CT-35 (all race 1), and CT-20, CT-30 and CT-34 (all race 0).
Conidial suspensions for inoculation were prepared from 7- to 10-day-old cultures grown on oatmeal agar (30 g blended quick oats [The Quaker Oats Company, Ontario, Canada], 8.8 g agar [Difco, Becton, Dickinson and Company, Franklin Lakes, NJ, USA], 1 L distilled water) and incubated in an incubator (Sanyo Versatile Environmental Test Chamber Model MLR-350H, Sanyo Electric Co., Ltd., Gunma, Japan) at 22 °C with a 12 h photoperiod. The cultures were washed with sterile distilled water and the resulting conidial suspensions were diluted to a final concentration of 1 × 105 conidia mL−1as determined with a haemocytometer.
Plant material
Lentil ‘CDC Robin’ and ‘Eston’ were used in the experiments. ‘CDC Robin’ is a small seeded cultivar with brown seed coats and red cotyledons (Vandenberg et al., Citation2002), and ‘Eston’ is a small green cultivar with yellow cotyledons (Slinkard & Bhatty, Citation1981). ‘CDC Robin’ has partial resistance to race 1 of C. truncatum derived from lentil cultivar ‘Indianhead’ but is susceptible to isolates of race 0. ‘Eston’ is susceptible to both races of C. truncatum (). For example, extensive pathogenicity testing showed that isolate CT-21 (race 1) caused disease severities of 91% on leaves and 81% on stems of ‘Eston’, but only 47% on leaves and 15% on stems of ‘CDC Robin’. Isolate CT-30 (race 0) caused 93% disease severity on leaves and 85% on stems of ‘Eston’, and 93% on leaves and 86% on stems of ‘CDC Robin’ (S. Banniza, unpublished data).
Seeds of the two cultivars were planted at 8 per 95 mm × 95 mm plastic pot filled with soil-less mixture (Terra-Lite Redi-Earth®, Scotts-Sierra Horticultural Products Co., Marysville, Ohio, USA). Plants were thinned to six per pot 2 weeks after seeding. Ten pots per cultivar were prepared and maintained in a growth chamber (Model PGV 56, Conviron, Winnipeg, MB) at 22 °C/16 °C day/night with a 16 h photoperiod. A complete fertilizer solution (20-20-20 NPK + micronutrients) was applied at a concentration of 3 g L−1 water 2 weeks after seeding and then once a week. Three-week-old plants were used in all experiments.
Conidial germination and appressorium formation on the surface of detached lentil leaflets
Six leaflets of each cultivar were picked randomly and placed in a single Petri dish lined with sterile, moistened filter paper for inoculation with six isolates (one leaflet per cultivar per isolate). Leaflets of the same cultivar were placed together. Three Petri dishes were prepared this way at each time, and values were averaged. The experiment was repeated three times, representing three replicates blocked over time, and the entire experiment was repeated once. For each set of Petri dishes, preparation and inoculation of the six isolates were conducted in a random sequence to reduce experimental error which might be caused by different time periods between the preparation of conidium suspensions and inoculation for each isolate. Using a point-inoculation method, a droplet of 10 μL of conidial suspension was placed in the centre of each leaflet. Inoculated leaflets were incubated for 12 h at 22 °C and approximately 92% RH under continuous light in an incubator (Sanyo). Leaflets were air dried following the 12 h incubation, and stained with aniline blue without prior clearing (Gurr, BDH laboratory supplies, Poole, UK). Investigations of conidial germination and appressorium formation were carried out under the light microscope (Nikon Microphot FXA, Japan) at × 100 magnification.
Penetration success of isolates into detached lentil leaflets
For detailed cytological studies of the infection process into detached lentil leaf tissue, four replicate leaflets of each cultivar were placed in a Petri dish lined with moistened filter paper to be inoculated with one isolate. Six Petri dishes were prepared for each isolate for a time course analysis with incubation periods ranging from 18 to 30 h post-inoculation (hpi). Samples were incubated at 22 °C and approximately 92% RH under continuous light in an incubator (Sanyo). Penetration success was assessed every two hours through destructive sampling. Samples were cleared in a solution of 3 : 1 glacial acetic acid : 95% ethanol. The leaflets were rehydrated with a decreasing ethanol series (95%, 70%, 50%, 30%, 0%) and stained with lacto-acid fuchsin (0.05% acid fuchsin [Acros Organics, New Jersey, USA] in 20% lactic acid). For each leaflet, 50 appressoria were evaluated for the presence of infection vesicles or primary hyphae using brightfield and DIC microscopy (Zeiss Axioskop 40, Nikon Eclipse 80i) at × 200 magnification. The experiment was conducted twice.
Development of primary hyphae in detached and attached lentil leaflets
A second set of Petri dishes containing four replicate detached lentil leaflets per isolate and cultivar was prepared and inoculated as described above, and incubated for 48 and 60 h to quantify the size of primary hyphae (PH) using brightfield and DIC microscopy. Samples were cleared as described before and the area of a plant epidermal cell occupied by PH was estimated visually for 20 PH per leaflet for each isolate and incubation period. The experiment was conducted twice.
Isolates CT-34 (race 0) and CT-35 (race 1) were selected for further studies on attached leaflets. In order to successfully use the point inoculation method, single whole plants from each cultivar were gently removed from pots, the roots were wrapped in moist paper towel, and one ‘Eston’ and one ‘CDC Robin’ plant were placed horizontally on moistened paper towel in a transparent plastic container (Rubbermaid® brand, Rubbermaid Commercial Products, Saratoga Springs, NY, USA). Ten leaflets per plant were fixed to the bottom of the containers with tape. A total of six containers were prepared this way, three for each isolate. The secured leaflets were inoculated as described for detached leaflets. After inoculation, a plastic film and a lid were used to cover the container to maintain high humidity. Containers were incubated for 48 h under the same conditions described for Petri dishes above. The per cent area of the plant epidermal cells occupied by PH was visually estimated from 20 PH of three leaflets from each cultivar. The experiment was conducted twice.
Statistical analysis
All statistical analyses were conducted in SAS (Version 9.1, SAS Institute Inc., Cary, NC). All data were tested for homogeneity of variance, and if required, heterogeneous variances were modelled using the mixed procedure in SAS.
Conidial germination and appressorium formation were analyzed as a split-plot experiment considering isolate and cultivar as fixed factors and repeat and repeat × cultivar as random factors. As block effects in this first experiment were not significant (P = 0.3079), all subsequent experiments were conducted and analyzed as completely randomized experiments to simplify experimental set-up and data analysis. Differences among isolates were determined based on Fisher's least significant difference (LSD), and differences between the races were analyzed through linear contrasts.
The temporal progression of penetration and the development of PH by C. truncatum into detached lentil leaflets were analyzed through repeated measures analysis using the mixed model procedure of SAS (Littell et al., Citation2006). A first-order autoregressive covariance structure with heterogeneous variances which had the best fit among several tested was specified for this analysis. Isolates, cultivars, incubation period and their interactions were considered fixed factors whereas all other factors were random. Differences among isolates were determined based on LSDs. Percentage penetration into leaf tissue was modelled through simple linear regression for each isolate on each of the two cultivars, and linear contrasts were used to determine differences in intercepts and slopes between race 1 and race 0 isolates.
Data of plant epidermal cell area occupied by PH (attached and detached) were analyzed with the mixed model procedure for 48 h and 60 h incubation period separately. As before, isolates and cultivars were considered fixed factors whereas all other factors were random.
Results
Conidial germination and appressorium formation on the surface of detached lentil leaflets
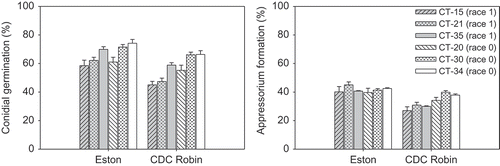
Conidial germination and appressorium formation of six isolates were investigated on the surface of detached uncleared leaflets of ‘CDC Robin’ and ‘Eston’ at 12 hpi. Isolates and cultivars had highly significant effects on conidial germination and appressorium formation (P < 0.0001). The isolate × cultivar interaction was significant for per cent appressorium formation (P = 0.0001), but not for conidial germination (P = 0.0557). Contrast analysis revealed that conidial germination of race 0 isolates was significantly higher than that of race 1 isolates on ‘Eston’ (P = 0.0334) as well as on ‘CDC Robin’ (P < 0.0001) (). On ‘CDC Robin’, race 0 isolates had significantly higher percentages of appressorium formation (37%) than race 1 isolates (29%) (P < 0.0001), whereas there was no difference between the races on ‘Eston’ (). All race 1 isolates had significantly higher germination and appressorium formation on ‘Eston’ compared with those on ‘CDC Robin’ whereas these were similar for all three race 0 isolates on both cultivars. Isolate by isolate comparison revealed also that race 1 isolate CT-35 had significantly higher conidial germination and appressorium formation than race 1 isolates CT-21 and CT-15 on ‘CDC Robin’, and of CT-15 on ‘Eston’, but was similar in these features to all three race 0 isolates. Similarly, race 0 isolate CT-20 only had significantly higher conidial germination and appressorium formation compared with CT-15 on ‘CDC Robin’, but had consistently lower germination and fewer appressoria compared to race 0 isolate CT-30 on both cultivars.
Penetration success of isolates into detached lentil leaflets
Following germination of conidia, germ tubes and appressoria had developed on the leaflet surface, and infection vesicles (IVs) were observed inside epidermal cells of both cultivars underneath penetration sites starting at 20 hpi (, ). In most cases, IVs appeared to be directly connected to the underside of appressoria. Sometimes appressoria were not found above IVs, probably because these appressoria were washed away during the clearing process. The development of IVs and PH increased steadily up to about 50% at 30 hpi, but appeared to level off on ‘Eston’ for most isolates whereas this was not the case on ‘CDC Robin’. PH were either unbranched or had two to several lobes, were restricted to a single epidermal cell, and were never observed to expand into neighbouring cells ().
Fig. 3. Stages in the infection process of Colletrotrichum truncatum on lentil leaflets. Conidia germinate and produce appressoria (a). Upon penetration of the host tissue (b), an infection vesicle is produced (arrow) and (c) elongates into a primary hypha (ph). As the primary hypha continues to grow in the epidermal host cell it becomes branched and lobed (d). By the time secondary hyphae (sh) grow rapidly through the surrounding host tissue, the primary hypha has filled the host epidermal cell (e). Appressoria are denoted with an *. Bars = 10 μm.
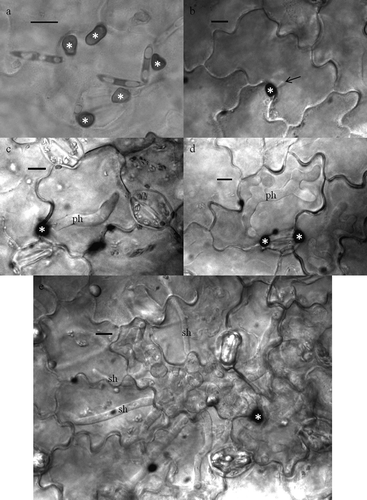
Fig. 4. Penetration (as per cent of appressoria) of six isolates of Colletotrichum truncatum belonging to two races into detached leaflets of lentil cultivars ‘Eston’ and ‘CDC Robin’.
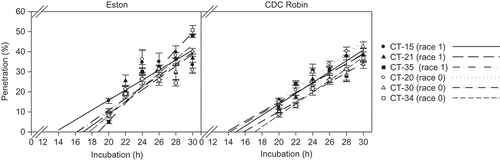
Cultivar, isolate, incubation time and their interactions all had significant effects on penetration of detached lentil leaflets (P ≤ 0.0088). Interestingly, overall penetration success was higher on ‘CDC Robin’ than on ‘Eston’ (P = 0.0088) although means comparisons revealed that this was isolate-dependent. For example, there was no difference between cultivars in the penetration success of race 0 isolate CT-30 whereas CT-20 consistently penetrated leaflets of ‘CDC Robin’ more frequently than those of ‘Eston’ except after 22 and 24 hpi (). Comparison of slopes and intercepts for race 1 and race 0 isolates did not reveal significant differences (‘Eston’: P = 0.5369 for slopes, P = 0.4522 for intercepts; ‘CDC Robin’: P = 0.1843 for slopes, P = 0.0697 for intercepts), indicating that isolates of both races had similar penetration success on both cultivars.
Development of primary hyphae in detached and attached lentil leaflets
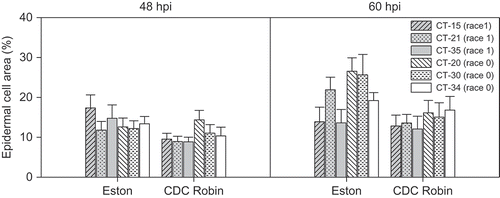
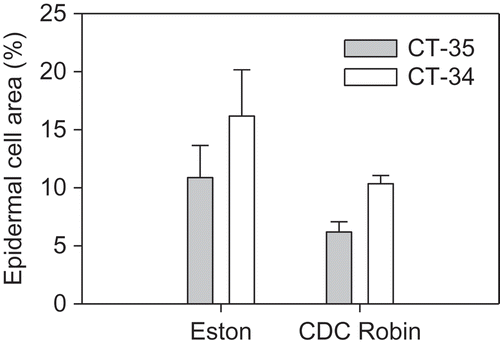
There was no effect of isolate (P > 0.5) or cultivar (P > 0.1) at 48 or 60 hpi on the size of PH in detached leaflets of lentil. Linear contrasts also showed that there was no effect of races (P > 0.07) on the size of PH that occupied between 9 to 17% of an epidermal cell at 48 hpi and increased up to 27% at 60 hpi (). PH generated by isolates CT34 and CT-35 were similar in size at both incubation times in detached leaflets. In contrast, on attached leaflets, PH generated by CT-34 at 48 hpi occupied a significantly larger area of epidermal cells than PH of CT-35 on both ‘Eston’ (P = 0.0002) and ‘CDC Robin’ (P < 0.0001) ().
Discussion
Experiments were conducted to determine whether there were differences between the two races of C. truncatum during the first 60 h of the infection process on detached, and to a lesser extent, attached leaflets of lentil cultivars ‘Eston’ (susceptible) and ‘CDC Robin’ (partial resistance to race 1). Our hypothesis was that both races would infect ‘Eston’ leaflets at a similar pace and to a similar extent, whereas infection by race 1 isolates would be inhibited compared with race 0 isolates on ‘CDC Robin’ during the biotrophic infection stage.
Successful infection of lentil by C. truncatum is dependent upon germination, and most critically, the differentiation of appressoria on the plant surface. Conidial germination of race 0 isolates was significantly higher than that of race 1 isolates at 12 hpi on both ‘Eston’ and ‘CDC Robin’ leaflets. This contrasts to germination results on glass slides where germination was similar for all isolates, suggesting that host-specific cues resulted in differential germination rates. If conidial germination was considered an indicator of fitness, race 0 isolates would have a comparative advantage over race 1 isolates, potentially resulting in higher infection rates and more disease. On ‘CDC Robin’, but not on ‘Eston’, the percentage of appressoria formed by race 0 isolates was significantly higher than that of race 1 isolates. Invasion of soybean leaves by C. truncatum was shown to occur with the help of an appressorium or through direct penetration by the germ tube (Manandhar et al., Citation1985) whereas direct penetration has never been observed on lentil. Melanized appressoria are essential for invasion in this host–pathogen system, thus a delay or suppression of appressorium formation of race 1 isolates on ‘CDC Robin’ may be one aspect of the resistance mechanism involved, and supports the hypothesis of differential interactions. However, isolate-by-isolate comparisons showed that not all race 1 isolates were clearly distinguishable from race 0 isolates based on these features, indicating that there is overlap between the two groups, and that other factors are likely involved in determining race-identity.
Studying the infection process of two races of C. trifolii on alfalfa, Mould et al. (Citation1991) found that race identity did not influence conidial germination or appressorium development. No differences in the initial infection process, including conidial germination and appressorium formation, were observed when inoculating C. gloeosporioides (Penz.) Penz. & Sacc. on susceptible and resistant cultivars of muscadine grape (Daykin & Milholland, Citation1984). Conidial germination and appressorium formation of C. orbiculare Berk. & Mont. was higher on Nicotiana tabacum L. than N. benthamiana L. at 12 hpi although lower lesion numbers on N. tabacum suggested a lower success rate in actually invading the host (Shen et al., Citation2001).
Several isolates of C. truncatum were more successful at invading detached leaflets of ‘CDC Robin’ than those of ‘Eston’, but this was not associated with race identity, nor was the size of PH developing in the epidermal cells different between the races. Detached leaflet tests observing PH development were compared with experiments using whole plants for two of the six isolates. Results in this study showed different responses based on the type of assay as larger PH were observed for the race 0 isolate CT-34 compared with the race 1 isolate CT-35 on attached leaflets of both cultivars, but not on detached leaflets. Differences between detached leaf and intact plant assays were also reported when comparing gene expression, notably PR1 and PDF1.2 in leaves of Arabidopsis after inoculation with C. higginsianum (Liu et al., Citation2007). Expression of these genes was compromised in detached leaves and senescence was induced through the detachment process which in turn promoted disease symptom development.
In lentil leaflets, the development of PH of C. truncatum was always restricted to a single epidermal cell. Other species of Colletotrichum, e.g. C. orbiculare on N. tabacum (Shen et al., Citation2001), produce PH that will extend into several neighbouring epidermal cells. A restriction in growth of the PH through hypersensitive cell death was not evident in the current experiments. This is in contrast to several other host–pathogen systems involving hemibiotrophic Colletotrichum species. For example, on sorghum, isolates of C. sublineolum Sacc. & Trotter failed to initiate PH in incompatible interactions, the invaded cell appeared dead after 42 h, and further colonization of the tissue was suppressed (Wharton & Julian, Citation1996). Incompatible strains of C. lindemuthianum (Sacc. & Magnus) Briosi & Cavara were arrested in their development in a resistant common bean line through the hypersensitive reaction (O'Connell et al., Citation1985). Hypersensitive cell death can thus be an effective strategy for a host plant to prevent invasion by a hemibiotrophic pathogen by withdrawing the nutrient basis provided by the living host cell. However, the resistance mechanism(s) deployed in lentil is clearly different and less successful, as inoculation of a race 1 isolate on ‘CDC Robin’ still results in a compatible interaction and symptom development. It has been speculated that the biotrophic phase of hemibiotrophic Colletotrichum spp. may be essential for successful infection of host tissue by preventing early recognition of the pathogen during the initial phase of establishment in the host tissue (Münch et al., Citation2008). Indeed, although mutants lacking the ability to switch to the necrotrophic phase have been recovered and shown to be non-pathogenic (Dufresne et al., Citation2000; Thon et al., Citation2002), no mutants lacking the biotrophic phase have been reported.
Isolates from both races followed the typical infection process on both lentil cultivars, as had been described before for this and several other Colletotrichum species (e.g. Wharton & Julian, Citation1996; Latunde-Dada et al., Citation1997; Shen et al., Citation2001; Chongo et al., Citation2002; Latunde-Dada & Lucas, Citation2007). The length of the biotrophic phase often constitutes the most significant difference among species. Detached leaflet assays in this study showed that secondary hyphae became visible at 48 h post-inoculation, signalling the end of the biotrophic phase of C. truncatum, without clear differences between the two races (data not presented). An incubation period of at least 72 h had previously been reported by Chongo et al. (Citation2002) who studied the infection process on lentil cultivar ‘Eston’ and the resistant line PI 320937. A short biotrophic phase of 48 h was also reported for C. destructivum on Medicago sativa (Latunde-Dada et al., Citation1997) whereas on Vigna unguiculata this phase lasted for 72 h (Bailey et al., Citation1990). A much longer biotrophic phase of 168 h was observed during the infection of turfgrass by C. graminicola (Khan & Hsiang, Citation2003).
In conclusion, differences between the two pathogenic races described for C. truncatum during the early stages of infection appear to be primarily restricted to conidial germination and the formation of appressoria. Results also suggest that there is very little evidence for clear differential interactions of race 1 and race 0 isolates with the partially resistant cultivar ‘CDC Robin’ and the fully susceptible cultivar ‘Eston’ during the biotrophic phase of infection, as race 0 isolates appeared to be more successful than race 1 isolates on both cultivars. Based on these results, a gene expression study has been initiated, focusing on the period following the biotrophic phase where the pathogen switches from biotrophic to destructive necrotrophic growth (Bhadauria et al., Citation2011). Currently, potential effector candidates are being evaluated for differential expression in isolates of both races. In addition, a population of a cross between a race 1 and a race 0 isolate is being assessed to shed light on the genetic control of race identity in this lentil pathogen.
Acknowledgements
Financial support from the Natural Sciences and Engineering Research Council of Canada is gratefully acknowledged. We would also like to acknowledge technical support by Stephanie Boechler and Anthea Cabral. Thank you to Dr Troy Harkness and Chelsea Stahl for providing DIC microscope access and expertise.
Notes
C. Armstrong-Cho and J. Wang contributed equally to this work.
References
- Adebitan , S.A. , Fawole , B. and Hartman , G.L. 1996 . Effect of plant spacing and cropping pattern on brown blotch (Colletotrichum truncatum) of cowpea . Trop. Agr. , 73 : 275 – 280 .
- Bailey , J.A. , Nash , C. and O'Connell , C.J. 1990 . Infection process and host specificity of a Colletotrichum species causing anthracnose disease of cowpea . Vigna unguiculata. Mycol. Res. , 94 : 810 – 814 .
- Bailey , J.A. , O'Connell , R.J. , Pring , R.J. and Nash , C. 1992 . “ Infection strategies of Colletotrichum species ” . In Colletotrichum: biology, pathology and control , Edited by: Bailey , J.A. and Jeger , M.J. 88 – 120 . Wallingford , UK : CABI .
- Bailey , K.L. , Gossen , B.D. , Gugel , R.K. and Morrall , R.A.A. 2003 . Diseases of field crops in Canada , 193 – 194 . University of Saskatchewan, Saskatoon: University Extension Press .
- Bhadauria , V. , Banniza , S. , Vandenberg , A. , Selvaraj , G. and Wei , Y. 2011 . EST mining identifies proteins putatively secreted by the anthracnose pathogen . Colletotrichum truncatum. BMC Genomics , 12 : 327 – 342 .
- Boyette , C.D. 1991 . Host range and virulence of Colletotrichum truncatum, a potential mycoherbicide for hemp sesbania (Sesbania exaltata) . Plant Dis. , 75 : 62 – 64 .
- Buchwaldt , L. , Anderson , K.L. , Morrall , R.A.A. , Gossen , B.D. and Bernier , C.C. 2004 . Identification of lentil germ plasm resistant to Colletotrichum truncatum and characterization of two pathogen races . Phytopathology , 94 : 236 – 243 .
- Buchwaldt , L. , Morrall , R.A.A. , Chongo , G. and Bernier , C.C. 1996 . Windborne dispersal of Colletotrichum truncatum and survival in infested lentil debris . Phytopathology , 86 : 1193 – 1198 .
- Buchwaldt , L. , Vandenberg , A. , Tullu , A. and Bernier , C.C. Genetics of resistance to anthracnose (Colletotrichum truncatum) in lentil . Proceedings of the 4th European Conference on Grain Legume Research . Edited by: AEP . pp. 242 Krakow , , Poland : Pais .
- Cardina , J. , Littrell , R.H. and Hanlin , R.T. 1988 . Anthracnose of Florida beggarweed (Desmodium tortuosum) caused by . Colletotrichum truncatum. Weed Sci. , 36 : 329 – 334 .
- Chongo , G. , Gossen , B.D. and Bernier , C.C. 2002 . Infection by Colletotrichum truncatum in resistant and susceptible lentil genotypes . Can. J. Plant Pathol. , 24 : 81 – 85 .
- Damm , U. , Woudenberg , J.H.C. , Cannon , P.F. and Crous , P.W. 2009 . Colletotrichum species with curved conidia from herbaceous hosts . Fungal Divers. , 39 : 45 – 87 .
- Daykin , M.E. and Milholland , R.D. 1984 . Histopathology of ripe rot caused by Colletotrichum gloeosporioides on muscadine grape . Phytopathology , 74 : 1339 – 1341 .
- Dufresne , M. , Perfect , S. , Pellier , A.L. , Bailey , J.A. and Langin , T.A. 2000 . GAL4-like protein is involved in the switch between biotrophic and necrotrophic phases of the infection process of Colletotrichum lindemuthianum on common bean . Plant Cell , 12 : 1579 – 1590 .
- Ford , R. , Banniza , S. , Photita , W. and Taylor , P.W.J. 2004 . Morphological and molecular discrimination of Colletotrichum truncatum causing anthracnose on lentil in Canada . Australas. Plant Path. , 33 : 559 – 569 .
- Gossen , B.D. , Anderson , K.L. and Buchwaldt , L. 2009 . Host specificity of Colletotrichum truncatum from lentil . Can. J. Plant Pathol. , 31 : 65 – 73 .
- Graham , G.L. , Peng , G. , Bailey , K.L. and Holm , F.A. 2006 . Effect of dew temperature, post-inoculation condition, and pathogen dose on suppression of scentless chamomile by . Colletotrichum truncatum. Biocont. Sci. Tech. , 16 : 271 – 280 .
- Hartman , G.L. , Manandhar , J.B. and Sinclair , J.B. 1986 . Incidence of Colletotrichum spp. on soybeans and weeds in Illinois and pathogenicity of Colletotrichum truncatum . Plant Dis. , 70 : 780 – 782 .
- Khan , A. and Hsiang , T. 2003 . The infection process of Colletotrichum graminicola and relative aggressiveness on four turfgrass species . Can. J. Microbiol. , 49 : 433 – 442 .
- Latunde-Dada , A.O. and Lucas , J.A. 2007 . Localized hemibiotrophy in Colletotrichum: cytological and molecular taxonomic similarities among C. destructivum, C. linicola . C. truncatum. Plant Pathol. , 56 : 437 – 447 .
- Latunde-Dada , A.O. , Bailey , J.A. and Lucas , J.A. 1997 . Infection process of Colletotrichum destructivum O'Gara from lucerne (Medicago sativa L.) . Eur. J. Plant Pathol. , 103 : 35 – 41 .
- Littell , R.C. , Milliken , G.A. , Stroup , W.W. , Wolfinger , R.D. and Schabenberger , O. 2006 . SAS for mixed models (2nd ed.). Cary, NC: SAS Institute Inc. ,
- Liu , G. , Kennedy , R. , Greenshields , D.L. , Peng , G. , Forseille , L. , Selvaraj , G. and Wei , Y. 2007 . Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against hemibiotrophic Colletotrichum spp . Mol. Plant Microbe Inter. , 20 : 1308 – 1319 .
- Manandhar , J.B. , Kunwar , I.K. , Singh , T. , Hartman , G.L. and Sinclair , J.B. 1985 . Penetration and infection of soybean leaf tissues by Colletotrichum truncatum . Glomerella glycines. Phytopathology , 75 : 704 – 708 .
- Morrall , R.A.A. 1988 . A new disease of lentil induced by Colletotrichum truncatum in Manitoba . Plant Dis. , 72 : 994
- Mould , M.J.R. , Boland , G.J. and Robb , J. 1991 . Ultrastructure of the Colletotrichum trifolii – Medicago sativa pathosystem. I. Pre-penetration events . Physiol. Mol. Plant Pathol. , 38 : 179 – 194 .
- Münch , S. , Lingner , U. , Floss , D.S. , Ludwig , N. , Sauer , N. and Deising , H.B. 2008 . The hemibiotrophic lifestyle of Colletotrichum species . J. Plant Physiol. , 165 : 41 – 51 .
- O'Connell , R. , Herbert , C. , Sreenivasaprasad , S. , Khatib , M. , Esquerre-Tugaye , M.T. and Dumas , B. 2004 . A novel Arabidopsis–Colletotrichum pathosystem for the molecular dissection of plant–fungal interactions . Mol. Plant Microbe Inter. , 17 : 272 – 282 .
- O'Connell , R. , Perfect , S. , Hughes , B. , Carzaniga , R. , Bailey , J. and Green , J. 2000 . “ Dissecting the cell biology of Colletotrichum infection processes ” . In Colletotrichum: host specificity, pathology, and host–pathogen interaction , Edited by: Prusky , D. , Freeman , S. and Dickman , M. B. 57 St. Paul , MN : APS Press .
- O'Connell , R.J. , Bailey , J.A. and Richmond , D.V. 1985 . Cytology and physiology of infection of Phaseolus vulgaris by Colletotrichum lindemuthianum . Physiol. Mol. Plant Pathol. , 27 : 75 – 98 .
- O'Connell , R.J. , Uronu , A.B. , Waksman , G. , Nash , C. , Keon , J.P.R. and Bailey , J.A. 1993 . Hemibiotrophic infection of Pisum sativum by . Colletotrichum truncatum. Plant Pathol. , 42 : 774 – 783 .
- Perfect , S.E. , Hughes , H.B. , O'Connell , R.J. and Green , J.R. 1999 . Review: Colletotrichum: a model genus for studies on pathology and fungal–plant interactions . Fungal Genet. Biol. , 27 : 186 – 198 .
- Shen , S. , Goodwin , P.H. and Hsiang , T. 2001 . Infection of Nicotiana species by the anthracnose fungus . Colletotrichum orbiculare. Eur. J. Plant Pathol. , 107 : 767 – 773 .
- Slinkard , A.E. and Bhatty , R.S. 1981 . Eston lentil . Can. J. Plant Sci. , 61 : 733 – 734 .
- Thon , M.R. , Nuckles , E.M. , Takach , J.E. and Vaillancourt , L.J. 2002 . CPR1: a gene encoding a putative signal peptidase that functions in pathogenicity of Colletotrichum graminicola to maize . Mol. Plant Microbe Inter. , 15 : 120 – 128 .
- Tullu , A. , Buchwaldt , L. , Lulsdorf , M. , Banniza , S. , Barlow , B. and Slinkard , A.E. 2006 . Sources of resistance to anthracnose (Colletotrichum truncatum) in wild Lens species . Genet. Resour. Crop Evol. , 53 : 111 – 119 . et al.
- Tullu , A. , Buchwaldt , L. , Warkentin , T. , Taran , B. and Vandenberg , A. 2003 . Genetics of resistance to anthracnose and identification of AFLP and RAPD markers linked to the resistance gene in PI 320937 germplasm of lentil (Lens culinaris Medikus) . Theor. Appl. Genet. , 106 : 428 – 434 .
- Vandenberg , A. , Kiehn , F.A. , Vera , C. , Gaudiel , R. , Buchwaldt , L. and Dueck , S. 2002 . CDC Robin lentil . Can. J. Plant Sci. , 82 : 111 – 112 . et al.
- Weidemann , G.J. , Tebeest , D.O. and Cartwright , R.D. 1988 . Host specificity of Colletotrichum gloeosporioides f. sp. aeschynomene and C. truncatum in the leguminosae . Phytopathology , 78 : 986 – 990 .
- Wharton , P.S. and Julian , A.M. 1996 . A cytological study of compatible and incompatible interactions between Sorghum bicolor . Colletotrichum sublineolum. New Phytol. , 134 : 25 – 34 .