Abstract
Postbloom fruit drop (PFD) of citrus was observed for the first time after a widespread and severe outbreak occurred in Bermuda in the 1990s. Fruit losses from the disease were estimated at 25% to 35% in sweet orange, grapefruit, lemon and Tahiti lime. The causal agent of PFD has been reported as either Colletotrichum gloeosporioides or Colletotrichum acutatum. Bermuda isolates of Colletotrichum recovered from diseased orange trees produced lesions typical of PFD in detached petals of orange, lime and grapefruit, and in attached orange blossoms. The isolates produced few to no lesions of anthracnose on leaves of key lime seedlings. Koch's postulates were fulfilled following reisolation of morphologically identical fungi from inoculated tissues. The isolates were characterized using morphological (conidial size and shape and colony colour), physiological (growth rate at 24 °C and on a benomyl-amended medium), immunological (ELISA) and molecular (PCR amplification and sequencing of the ITS region) methods. Immunological and molecular techniques provided definitive identification of the isolates as Colletotrichum gloeosporioides. This report confirms that C. gloeosporioides can be the causal agent of PFD; therefore, identification of the causal agent to species by immunology, molecular analysis and fungicide sensitivity is suggested for new outbreaks of the disease.
Résumé
La coulure postfloraison (CPF) des agrumes a été observée pour la première fois aux Bermudes dans les années 90 à la suite d'une éruption grave et généralisée. Les pertes causées par la maladie chez l'oranger doux, le pamplemoussier, le citronnier et le limettier de Tahiti ont été de 25% à 35%. L'agent causal de la CPF était soit Colletotrichum gloeosporioides ou C. acutatum. Les isolats bermudiens de Colletotrichum collectés sur des orangers infectés ont causé les lésions typiques de la CPF sur des pétales prélevés de fleurs d'oranger, de limettier et de pamplemoussier ainsi que sur les fleurs d'oranger encore sur l'arbre. Les isolats ont provoqué quelques lésions (ou aucune) d'anthracnose sur les feuilles de semis de limettier. Les postulats de Koch ont été confirmés à la suite du réisolement de champignons morphologiquement identiques extraits de tissus inoculés. Les isolats ont été caractérisés par différentes méthodes morphologiques (forme et taille des conidies, couleur des colonies), physiologiques (taux de croissance à 24 °C sur un milieu de culture amendé avec du bénomyle), immunologiques (ELISA) et moléculaires (amplification et séquençage des régions de l'ITS par PCR). Les méthodes immunologiques et moléculaires ont permis d'attester que les isolats étaient de Colletotrichum gloeosporioides. Ce rapport confirme que C. gloeosporioides peut être l'agent causal de la CPF. Par conséquent, lors des prochaines éclosions de la maladie, l'identification à l'échelle de l'espèce de l'agent par analyse immunologique et moléculaire, ainsi que par sensibilité aux fongicides, est suggérée.
Introduction
Postbloom fruit drop (PFD) of citrus was first described in Belize (formerly British Honduras) in 1979 caused by the fungus Colletotrichum gloeosporioides (Penz.) Sacc. (Denham, Citation1979; Fagan, Citation1979). The disease has been detected in Argentina, Brazil, Mexico and the United States (Schwarz et al., Citation1978; Orozco Santos & Gonzalez Garza, Citation1986; McMillan & Timmer, Citation1989; Kuramae-Izioka et al., 1997). In Florida, the causal agents of PFD and a disease in key lime (Citrus aurantifolia L. Swingle) called key lime anthracnose (KLA) were initially identified morphologically and pathogenically as strains of C. gloeosporioides (Agostini et al., Citation1992). Genetic characterization of Colletotrichum isolates from Brazil suggested that PFD can be caused by both C. acutatum and C. gloeosporioides (Kuramae-Izioka et al., Citation1997). A report from south Brazil identified the fungus causing PFD as C. acutatum (Theodoro et al., Citation2003). In Florida, PFD was subsequently re-characterized through additional testing, including molecular analysis, as C. acutatum (Brown et al., 1996). The two species of Colletotrichum, C. acutatum and C. gloeosporioides, can differ in several aspects including morphology, molecular (ITS sequences) and fungicide sensitivity characteristics (Brown et al., Citation1996).
Postbloom fruit drop develops rapidly and symptoms usually occur within 2–3 days following infection when abundant moisture is prevalent during the bloom period. Early symptoms consist of brown to orange, water-soaked, elongated lesions on petals. These lesions enlarge and blighting of individual flowers and entire flower clusters occurs. Fruitlets drop off at their ovary bases leaving the basal disk and calyx (‘button’) firmly attached to its peduncle. These buttons may remain attached to the tree for a number of years, and are specifically diagnostic for PFD. In Florida, sporadic outbreaks of PFD, most severe in the 1990s, caused extensive losses, especially to sweet oranges [Citrus sinensis (L.) Osbeck], mandarin hybrids (C. reticulata Blanco × C. paradisi Macf.) and Tahiti limes (C. latifolia Tanaka) (McGovern & Rouse, Citation1994).
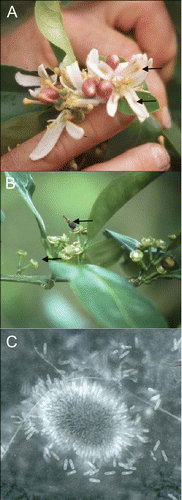
Citrus is widely grown in Bermuda in residential settings, with commercial production limited to one grove of about 20 trees because of the island's small size. During the 1990s, a new disease identical in symptomatology with PFD (blossom blight, fruit losses resulting from premature abscission of fruitlets, and persistent buttons) was widespread in citrus on the island (, B). Losses in fruit production on the island ranged from 25% to 36% in sweet orange, grapefruit, lemon and Tahiti lime (Bermuda Ministry of Agriculture and Fisheries, unreported data). Our objective was to identify and characterize the causal agent using morphological, physiological, immunological and molecular techniques.
Fig. 1. A, Elongated, brown lesions (arrows) typical of Postbloom Fruit Drop (PFD) observed in Tahiti lime flower petals in Bermuda. B, Persistent basal disks and calyces and mummified fruitlet (arrow) on orange tree affected by PFD in Bermuda. C, Acervulus and conidia produced in culture by C. gloeosporioides isolated from the mummified orange fruitlet in Bermuda.
Materials and methods
Pathogen isolation
Isolations were made from mummified orange fruitlets that remained attached to persistent buttons by plating symptomatic tissue on one-quarter strength acidified potato dextrose agar (APDA) following surface disinfestation in 0.5% NaOCl. Culture plates were incubated at 24 °C with a 12-hour photoperiod and the fungal isolates recovered were maintained on APDA plates by periodic transfers. Isolates were subcultured from a single spore and placed in 15% glycerol at −80 °C for long-term storage.
Isolates used in the study
Characterized isolates of C. acutatum and C. gloeosporioides from a variety of hosts (provided by D.O. TeBeest, University of Arkansas; M.J. Davis and D.E. Legard, University of Florida; and L.I. Rivera-Vargas, University of Puerto Rico) served as known standards for comparison to the isolates from citrus in Bermuda. A total of nine C. acutatum isolates were included: from strawberry (Fragaria x ananassa; Is96-23, Is96-25, and Is96-26), apple (Malus domestica; 1117, 1118), tomato (Solanum lycopersicum; 1335), key lime (KLF3-1) and orange (I98-115, LH-1). Six C. gloeosporioides isolates were obtained from: Tahiti lime (1145, 1146), mango (Mangifera indica; MD35, MD36) and avocado (Persea americana; MD22, MD23). Isolates were stored at −80 °C in 15% glycerol.
Pathogenicity tests
Pathogenicity of the isolates from Bermuda, O-A and O-C, was tested in a number of trials on several different citrus species and tissues: detached petals from three Citrus species, intact blossoms and buds on Valencia orange, and the foliage of key lime. The pathogenicity of the O-A isolate from Bermuda was tested on the swollen, but generally unopened, blossoms of Tahiti lime, grapefruit (Citrus paradisi) and orange. Blossoms were surface disinfested for 30 s in 0.5% NaOCl. Blossoms were rinsed for 2 min in sterile water and blotted dry. Four to five individual petals were then aseptically separated and placed in Petri dishes on filter paper moistened with sterile water. Inoculum was produced from 7- to 10-day-old PDA cultures and consisted of a conidial suspension in sterile water (4.0 × 106 conidia mL−1). A drop of the conidial suspension was placed per petal in three dishes. Dishes containing petals inoculated with sterile water were used as negative controls. Dishes were incubated at 28 °C and monitored for 3 days. The putative pathogen was reisolated on APDA following surface disinfestation and identified morphologically to fulfil Koch's postulates.
The pathogenicity of two Bermuda isolates was compared with a PFD isolate from Florida, C. acutatum I98-115, on intact blossoms and mature buds on a potted orange tree ‘Valencia’. One branch (2 to 4 flowers/buds) per isolate was inoculated with a conidial suspension in sterile water (1 ×105 conidia mL−1) obtained from 1 to 3-week-old APDA cultures. The conidial suspension, 1 mL flower−1, was applied with a calibrated, manual hydraulic sprayer. A non-inoculated control branch was sprayed with the same volume of sterile water. The tree was incubated at room temperature (21–24 °C), with each inoculated branch enclosed in a plastic bag to maintain a high relative humidity. Disease symptoms were recorded after 48 h, and symptomatic petals (three lesions per Colletotrichum isolate) were surface disinfested for 30 s, triple rinsed with sterile water, and plated on APDA to reisolate the putative pathogens, again to fulfil Koch's postulates.
The pathogenicity of the two Bermuda isolates was compared with two Florida isolates of C. acutatum, KLF3-1, known to cause key lime anthracnose, and a PFD isolate, I98-115, on key lime seedlings in two experiments. Three (experiment 1) or six (experiment 2) key lime seedlings per isolate were inoculated with a 1 × 105 conidia mL−1 suspension made from 1- to 3-week-old culture on APDA. The spore suspension, 2 to 2.5 mL per plant, was applied to the foliage with a calibrated, manual hydraulic sprayer. A non-inoculated control was sprayed with the same volume of sterile water. The seedlings were then incubated at room temperature (21–24 °C), under constant fluorescent lights, and inside plastic bags to maintain a high RH. Plants were monitored for 2 weeks, and symptomatic tissue was surface disinfested for 30 s (leaves) or 1 min (stems), triple rinsed with sterile water, and plated on APDA to reisolate the putative pathogens, once again to fulfil Koch's postulates.
Morphological characterization
Conidia (20–40 conidia per isolate) from 7- to 14-day-old acidified carnation leaf agar (ACLA) cultures of the two Bermuda isolates were examined morphologically. Conidial length and width in μm and the shape of both ends (rounded or fusoid) were recorded. Colony colour was observed from the top of 12-day-old PDA cultures grown at 24 °C under continuous light. Three culture plates were used per isolate to ensure consistency in morphological assessment.
Growth rate at 24 °C
The growth rate of Bermuda isolates and control isolates of Colletotrichum were compared at 24 °C. To determine the growth rate, a plug from the leading edge of a colony on PDA was used to inoculate a new PDA plate. The plate was incubated at the specified temperature, under constant light for 72 h, and the colony diameter was then measured. Each treatment was replicated three times, and the experiment was conducted twice.
Benomyl resistance – in vitro
The growth of the Bermuda isolates and control Colletotrichum isolates was measured on PDA amended with four concentrations of Benlate 50 WP (0, 10, 100 and 1000 mg L−1 a.i. benomyl). Isolates were considered resistant if any growth was observed at the highest rate and susceptible if no growth was observed at 10 mg L−1. Each treatment was replicated three times, and the experiment was conducted twice.
Enzyme-linked immunosorbent assay
A trapped antigen enzyme-linked immunosorbent assay (ELISA) was used to differentiate C. acutatum from C. gloeosporioides. This assay utilized the Colletotrichum acutatum IDENTIKIT (Neogen Europe Ltd – ADGEN Phytodiagnostics, Ayr, Scotland, UK) following the manufacturer's instructions. Samples tested consisted of conidial suspensions made from cultures grown on acidified carnation leaf agar. Results of the ELISA were read at 405 nm, with readings at 550 nm used as a reference point, utilizing the Easy Reader EAR 400AT plate reader (SLT Labinstruments, Australia).
Extraction of DNA for polymerase chain reaction (PCR) amplification of genomic DNA
The PCR results from the Bermuda isolates, O-A and O-C, were compared to reactions of known control isolates of C. acutatum and C. gloeosporioides and a no DNA (water) negative control. Genomic DNA was extracted from hyphae scraped from the surface of a 7–14 day APDA culture. The hyphae were ground in Doyle and Doyle extraction buffer (Doyle & Doyle, Citation1990) using glass beads (150–212 microns) and a pestle to release DNA. The supernatant was collected after centrifugation at 14,000 rpm for 10 min, and purified by chloroform extraction and isopropanol precipitation. The DNA was resuspended in sterile dH2O and stored at −30 °C.
Genomic DNA was assayed with two species-specific PCR reactions. The primer ITS4 (5'-TCCTCCGCTTATTGATATGC-3') was paired with the C. gloeosporioides specific primer CgInt (5'-GGCCTCCCGCCTCCGGGCGG-3') or the C. acutatum specific primer CaInt2 (5'-GGGGAAGCCTCTCGCGG-3') to produce bands of 450 bp or 490 bp, respectively (Mills et al., Citation1992; Brown et al., Citation1996). The PCR reactions were conducted in 50 μL volumes containing 1× Taq buffer (10 mM Tris-HCl, pH 9.0; 50 mM KCl), 0.2 mM of each dNTP (Promega, Madison, WI), 25 pmol of each primer, 1 U Taq polymerase (Fisher Scientific, Pittsburgh, PA or Sigma, St. Louis, MO), 1 mM Spermidine, and 1.5 or 3.75 mM MgCl2 (for the C. gloeosporioides or the C. acutatum specific reactions, respectively). The reactions were run on a MJ Research PTC100 thermocycler (Watertown, MA) and consisted of an initial 5 min, 95 °C denaturation, followed by 35 cycles: 45 s denaturation at 95 °C, 75 s annealing at 55 or 69 °C (for the C. gloeosporioides or the C. acutatum specific reactions, respectively), and 2 min plus 3 s per cycle elongation at 72 °C, and a final elongation of 5 min at 72 °C. Following PCR, the amplification product was stored at 4 °C until electrophoresis. The presence/absence of the amplified band was visualized by ethidium bromide staining after separation by electrophoresis on a 1.0% agarose gel in 1× TAE (0.04 M Tris, 1 mM EDTA adjusted to pH 8.0 with glacial acetic acid). The size of the amplified bands was estimated by comparison to a 1 KB ladder (Gibco BRL, Rockville, MD).
PCR amplification and sequencing of the internal transcribed spacer (ITS) region
Mycelia from 6-day-old one-quarter strength PDA cultures of each Bermuda isolate were transferred into wells of a 24-well plate, containing 2 mL pea broth (120 g frozen peas L−1, 5 g sucrose and amended with 100 mg L−1 ampicillin, 25 mg L−1 rifampicin and 25 mg L−1 PCNB) and cultured at 25 °C, with a 12 h photoperiod. Mycelial mats were collected, washed with sterile H2O and lyophilized prior to DNA extraction using the DNeasy Plant Mini Kit (Qiagen Inc., Valencia, CA). Genomic DNA was checked for quantity and purity using a NanoDrop2000 (Thermo Scientific Inc., USA) and stored at −20 °C.
Primers ITS-4 (5′-TCCTCCGCTTATTGATATGC-3′) (White et al., Citation1990) and ITS-6 (5′-GAAGGTGAAGTCGTAACAAGG-3′) (Cooke & Duncan, Citation1997) were used to amplify the ITS regions of ribosomal RNA (rRNA) of the isolates. PCR reactions were performed using Taq PCR Core Kit (Qiagen, Germany). Each 50 μL reaction contained 1× PCR buffer (Tris-Cl, KCl, (NH4)2SO4, 15 mM MgCl2; pH 8.7), 0.2 mM of each dNTP, 250 nM of each primer, 2 U Taq DNA polymerase, 2 μL template DNA and nuclease free water (Fisher Scientific, New Jersey, USA). The reactions were carried out in an Eppendorf Mastercycler® pro (Eppendorf, Germany) and consisted of an initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, extension at 72 °C for 45 s and a final extension at 72 °C for 7 min. The presence of an amplicon of the expected size was confirmed by agarose gel electrophoresis.
Amplicons of the PCR reaction were purified using QIAquick® PCR Purification Kit (Qiagen, USA) and submitted to the Interdisciplinary Center for Biotechnology Research (ICBR) at the University of Florida for sequencing using ABI 3130 DNA sequencers (Applied Biosystems). Individual sequence trace data were scored for quality using the PHRAP algorithm then edited using CodonCode Aligner (CodonCode Corporation, USA) and contigs identified using NCBI BLAST analysis.
Results and discussion
This study identified the pathogen causing PFD on orange in Bermuda as C. gloeosporioides and supported the findings of researchers in Brazil that PFD can be caused by C. gloeosporioides as well as C. acutatum (Kuramae-Izioka et al., Citation1997). Since other reports identified the causal agent of PFD in Florida and southern Brazil as C. acutatum, which is morphologically, molecularly and biologically distinct from C. gloeosporioides, a number of characteristics were used in this determination of species.
The fungi recovered from the mummified orange fruitlets can infect blossoms and present possible reservoirs of the pathogen for subsequent PFD outbreaks. In pathogenicity studies on detached petals, attached blossoms and buds, and key lime seedlings, Bermuda isolates caused symptoms of PFD. Bermuda isolate O-A caused orange to brown, elongated lesions typical of PFD after 24 h on detached petals of orange, Tahiti lime and grapefruit compared with non-inoculated petals which remained asymptomatic (). The isolates recovered from the symptomatic tissue were morphologically identical to isolate O-A, fulfilling Koch's postulates. On attached orange blossoms and buds, Bermuda Colletotrichum isolates O-A and O-C and the Florida PFD isolate I98-115 caused light brown petal lesions and complete petal drop within 48 hours. Reisolations made from all lesions (three per isolate) yielded cultures that were morphologically identical to the inoculated isolates, again fulfilling Koch's postulates.
Table 1. Comparison of the pathogenicity of Colletotrichum gloeosporioides isolates from orange affected by postbloom fruit drop (PFD) in Bermuda and C. acutatum isolates from Florida
On key lime seedlings, isolate KLF3-1, known to cause KLA, was easily distinguished from the Florida PFD isolate (I98-115), the two Bermuda isolates and the negative control (). Key lime seedlings inoculated with KLF3-1 were consistently infected (100% and 80% infection in experiments one and two, respectively). Younger leaves were blighted within 72 hours and the infection continued to spread several cm down the stem over 7 days. Isolates I98-115 and O-A infected key lime seedlings less consistently, and produced less severe symptoms. The percentage of seedlings infected was 67% and 40% with I98-115 and 100% and 0% with O-A in the first and second experiments, respectively. A few small leaf spots were observed but they were not typical of leaf or stem blight. Although the isolates from Bermuda are 100% identical by the molecular characterization (see below), the variability of the repeated experiments with the two isolates show the difficulty in some cases of repeating pathogenicity assays and obtaining the same results, probably due to variability in environmental conditions between the repeated experiments. However, since the Bermuda isolates produced only mild symptoms under conditions of high relative humidity and high inoculum density (105 conidia mL−1), they are unlikely to cause KLA under grove conditions. Isolates KLF3-1, I98-115 and O-A were all reisolated from symptomatic tissue. Bermuda isolate O-C was not confirmed to be pathogenic on the key lime seedlings.
In this research, neither colony colour nor conidial morphology could be used to clearly distinguish between C. acutatum and C. gloeosporioides (). The shape of the conidial ends appeared to be a good indicator of species identity among the controls in that C. gloeosporioides isolates had no more than 5% of conidia with a fusoid end while the percentage of conidia from the C. acutatum isolates with a fusoid end varied from 35 to 100%. However, no conidia of isolate O-A were observed to have fusoid ends (), while 40% of the conidia of isolate O-C had at least one fusoid end. The average conidial length and width for the two Bermuda isolates ranged from 13.7–13.9 and 3.9–4.2 μm, respectively, and overlapped with a number of both C. gloeosporioides and C. acutatum control isolates. The percentage of conidia with at least one fusoid end, the average conidial length and width, and the ratio of length/width for all of the isolates in this study were not statistical significant at P < 0.05 by ANOVA followed by Fisher's Protected LSD Test (SAS Institute, Cary, NC) (data not presented). Our findings that colony colour and conidial morphology are not always useful in distinguishing between species of Colletotrichum confirm previous reports (Freeman et al., Citation1998; Peres et al., Citation2002).
Table 2. Comparison of Colletotrichum gloeosporioides isolates from orange affected by postbloom fruit drop in Bermuda to control isolates of C. gloeosporioides and C. acutatum from various hosts
Since growth rate experiments were not significantly different, the data were pooled. The average radial growth rates of the isolates at 24 °C are presented in . The Bermuda isolates were grouped by growth rate with the control C. gloeosporioides isolates, which outgrew the control C. acutatum isolates at 24 °C. Our results support other reports indicating that a relatively faster growth rate can help distinguish C. gloeosporioides from the slower-growing C. acutatum (Agostini et al., Citation1992; Shi et al., Citation1996; Freeman et al., Citation1998).
Previous in vitro studies indicated that C. acutatum isolates are insensitive to benomyl, while C. gloeosporioides isolates are sensitive (Brown et al., Citation1996; Adaskaveg & Hartin, Citation1997). Occasionally, C. gloeosporioides can develop sensitivity to benomyl, possibly following fungicide exposure in the field (Bernstein et al., Citation1995; Adaskaveg & Hartin, Citation1997; Freeman et al., Citation1998). We also observed this differentiation, as all C. acutatum and two C. gloeosporioides isolates were able to grow on media amended with benomyl at all rates tested (ranging from 10 to 1000 μg mL−1; ). The remaining five control C. gloeosporioides isolates, and the Bermuda isolates, were sensitive (no growth occurred) with concentrations as low as 10 μg mL−1 benomyl.
All control isolates of C. acutatum had a positive reaction using the C. acutatum-specific ELISA, while all Bermuda isolates and all control isolates of C. gloeosporioides had a negative reaction. Following PCR amplification, the control isolates of C. acutatum produced a band of approximately 490 bp with the C. acutatum specific primer pair (CaInt2/ITS4) but did not produce a band with the C. gloeosporioides specific primer pair (CgInt/ITS4). The C. gloeosporioides control isolates and the Bermuda isolates had the opposite reaction pattern. They did not produce any band with CaInt2/ITS4, but produced a 450 bp band with CgInt/ITS4 (data not shown). Reactions are summarized by identification of Colletotrichum to species in . The DNA sequence from each Bermuda isolate, O-A and O-C (GenBank accession JN201890) amplified using primers ITS-4 and ITS-6 were identical and showed 100% identity to Colletotrichum gloeosporioides using NCBI BLAST.
These results identify C. gloeosporioides as the causal agent of PFD in Bermuda and are consistent with other reports that a number of different species of Colletotrichum can cause the same disease symptoms on the same host species (Smith & Black, Citation1990; Adaskaveg & Hartin, Citation1997; Freeman et al., Citation1998; Rivera-Vargas et al., Citation2006). Future phenotypic overlap among Colletotrichum species should be anticipated because of the existence of possible mechanisms for genetic exchange across fungal species such as conidial anastomosis and horizontal chromosome transfer (Roca et al., Citation2004; Mehrabi et al., Citation2011).
PFD was widespread in the western hemisphere including Belize, Bermuda, Brazil, Costa Rica, Mexico and the United States (Florida) in the 1990s and has become less of a problem in Florida in recent years, although it has not disappeared completely. However, the potential exists that PFD could re-emerge as a devastating disease of citrus (McGovern & Rouse, Citation1994; Timmer & Brown, Citation2000). The causal agent of future outbreaks of PFD should be characterized accordingly, using a number of approaches, including morphological and molecular techniques and sensitivity to fungicides, as described here.
References
- Adaskaveg , J.E. and Hartin , R.J. 1997 . Characterization of Colletotrichum acutatum isolates causing anthracnose of almond and peach in California . Phytopathology , 87 : 979 – 987 .
- Agostini , J.P. , Timmer , L.W. and Mitchell , D.J. 1992 . Morphological and pathological characteristics of strains of Colletotrichum gloeosporioides from citrus . Phytopathology , 82 : 1377 – 1382 .
- Bernstein , B. , Zehr , E.I. , Dean , R.A. and Shabi , E. 1995 . Characteristics of Colletotrichum from peach, apple, pecan, and other hosts . Plant Dis. , 79 : 478 – 482 .
- Brown , A.E. , Sreenivasaprasad , S. and Timmer , L.W. 1996 . Molecular characterization of slow-growing orange and key lime anthracnose strains of Colletotrichum from citrus as . C. acutatum. Phytopathology , 86 : 523 – 527 .
- Cooke , D.E.L. and Duncan , J.M. 1997 . Phylogenetic analysis of Phytophthora species based on the ITS1 and ITS2 sequences of ribosomal DNA . Mycol. Res. , 101 : 667 – 677 .
- Denham , T. G. 1979 . Citrus production and premature fruit drop disease in Belize . PANS , 25 : 30 – 36 .
- Doyle , J.J. and Doyle , J.L. 1990 . Isolation of plant DNA from fresh tissue . Focus , 12 : 13 – 15 .
- Fagan , H.J. 1979 . Postbloom fruit drop, a new disease of citrus associated with a form of . Colletotrichum gloeosporioides. Ann. Appl. Biol. , 91 : 13 – 20 .
- Freeman , S. , Katan , T. and Shabi , E. 1998 . Characterization of C olletotrichum species responsible for anthracnose diseases of various fruits . Plant Dis. , 82 : 596 – 605 .
- Kuramae-Izioka , E.E. , Lopes , C.R. , Souza , N.L. and Machado , M.A. 1997 . Morphological and molecular characterization of Colletotrichum spp. from citrus orchards affected by postbloom fruit drop in Brazil . Eur. J. Plant Pathol. , 103 : 323 – 329 .
- Mcgovern , R.J. and Rouse , R.E. 1994 . Grower survey of postbloom fruit drop incidence in southwest Florida . Proc. Fla. State Hort. Soc. , 107 : 92 – 94 .
- Mcmillan , R.T. JR and Timmer , L.W. 1989 . Outbreak of citrus postbloom fruit drop caused by Colletotrichum gloeosporioides in Florida . Plant Dis. , 73 : 81
- Mehrabi , R. , Bahkali , A.H. , Abd Elsalam , K.A. , Moslem , M. , Ben M'barek , S. Gohari , A.M. 2011 . Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range . FEMS Microbiol. Rev. , 35 : 542 – 554 .
- Mills , P.R. , Sreenivasaprasad , S. and Brown , A.E. 1992 . Detection and differentiation of Colletotrichum gloeosporioides isolates using PCR . FEMS Microbiol. Lett. , 98 : 137 – 144 .
- Orozco Santos , M. and Gonzalez Garza , R. 1986 . Caída de fruto pequeño y su control en naranja ‘Valencia’ en Veracruz . Aric. Tec. Mex. , 12 : 259 – 269 .
- Peres , N.A.R. , Kuramae , E.E. , Dias , M.S.C. and DE Souza , N.L. 2002 . Identification and characterization of Colletotrichum spp. affecting fruit after harvest in Brazil . J. Phytopathol. , 150 : 128 – 134 .
- Rivera-Vargas , L.I. , Lugo-Noel , Y. , Mcgovern , R.J. , Seijo , T.E. and Davis , M.J. 2006 . Occurrence and distribution of Colletotrichum spp. on mango (Mangifera indica L.) in Puerto Rico and Florida, U.S.A . Plant Pathol. J. , 5 : 191 – 198 .
- Roca , M.G. , Davide , L.C , Davide , L.M. , Mendez-Costa , M.C. , Schwan , R.F. and Wheals , A.E. 2004 . Conidial anastomosis fusion between Colletotrichum species . Mycol. Res. , 108 : 1320 – 1326 .
- Schwarz , R.E. , Klein , E.H.J. and Monsted , P. Fungal infection of citrus flowers: probable cause of abnormal fruit drop in the Panama mist zone of Misiones, Argentina . Proceedings of the Third International Congress of Plant Pathology . August 16–23 1978 . Munich : Federal Republic of Germany (p.130). Purey . Abstr
- Shi , Y. , Correll , J.C. , Guerber , J.C. and Rom , C.R. 1996 . Frequency of Colletotrichum species causing bitter rot of apple in the Southeastern United States . Plant Dis. , 80 : 692 – 696 .
- Smith , B.J. and Black , L.L. 1990 . Morphological, cultural, and pathogenic variation among Colletotrichum species isolated from strawberry . Plant Dis. , 74 : 69 – 76 .
- Theodoro , G.F. , Peres , N.A.R. and Verona , L.A.F. 2003 . Outbreak of postbloom fruit drop of citrus, caused by Colletotrichum acutatum, in Santa Catarina State, southern Brazil . New Disease Rep. , 8 : 4
- Timmer , L.W. and Brown , G.E. 2000 . “ Biology and control of anthracnose diseases of citrus ” . In Colletotrichum: Host Specificity, Pathology, and Host–Pathogen Interaction , Edited by: Freeman , S. , Prusky , D. and Dickman , M. 317 – 336 . St. Paul , , Minn : APS Press .
- White , T.J. , Bruns , T. , Lee , S. and Taylor , J.W. 1990 . “ Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics ” . In PCR Protocols: A Guide to Methods and Applications , Edited by: Innis , M.A. , Gelgard , D.H. , Snisky , J.J. and White , T.J. 315 – 322 . New York : Academic Press .