Abstract
A leaf blight on eucalyptus, caused by Pestalotiopsis virgatula, was found on a Eucalyptus camaldulensis plantation for the first time at Chiang Mai Province, Thailand in 2011. The fungus was isolated and its pathogenicity confirmed. Pathogenicity tests showed that P. virgatula could infect E. camaldulensis, which developed the same symptoms under artificial inoculation conditions as those observed in the field. The fungus was identified based on morphological and culture characteristics as P. virgatula. Identifications were confirmed using comparisons of DNA sequences of internal transcribed spacers (ITS) regions 1 and 2, including 5.8S rDNA (ITS1-5.8S-ITS2). This is the first report of eucalyptus leaf blight disease caused by P. virgatula.
Résumé
La brûlure helminthosporienne de l'eucalyptus, causée par Pestalotiopsis virgatula, a été découverte dans une plantation d'Eucalyptus camaldulensis pour la première fois en 2011, en Thaïlande, dans la province de Chiang Mai. Le champignon a été isolé et sa pathogénicité, confirmée. Les tests de pathogénicité ont montré que P. virgatula pouvait infecter E. camaldulensis qui développait les mêmes symptômes au champ que lorsqu'il était inoculé artificiellement. En se basant sur les caractéristiques morphologiques et culturales, le champignon a été identifié comme P. virgatula. L'identification a été confirmée en comparant des séquences de l'ADN des régions 1 et 2 de l'espaceur transcrit interne, y compris la région 5.8S de l'ADNr (ITS1-5.8S-ITS2). Il s'agit de la première mention de la brûlure helminthosporienne de l'eucalyptus causée par P. virgatula.
Introduction
Eucalyptus, a fast growing deciduous tree, is able to grow under a wide range of conditions and is a valuable resource for foresters and farmers (Old et al., Citation2003; Brooker & Kleinig, Citation2006). The most common species planted in Thailand is Eucalyptus camaldulensis Dehnh. while others such as E. alba Reinw. ex Blume., E. deglupta Blume., E. globulus Labill., E. grandis W. Hill. ex Maiden., E. multiflora Poir. and E. urophylla S.T. Blake. are also grown (Old et al., Citation2003; Barney, Citation2005). In addition to industrial size plantations, commune, farm-scale plantings make a significant contribution to household incomes. Areas planted with eucalyptus have increased in Thailand and as more plantations are established on less suitable sites, disease problems have increased. The aim of this study was to determine the identity and pathogenicity of fungi causing eucalyptus leaf blight disease in Chiang Mai Province, Thailand.
Disease symptoms and pathogen description
Eucalyptus leaves showing blight symptoms were collected from E. camaldulensis plantations in Chiang Mai Province, Thailand (18°47′74′N, 98°57′41′E) in November 2011. The initial symptoms were brown, oval or irregular-shaped lesions, 0.9–1.3 × 1.6–5.2 cm (n = 30), on the leaf margin or leaf tip. Lesions enlarged and coalesced causing diseased leaves to become blighted and desiccated (). In a humid environment, black, sessile and discoid conidiomata developed and exuded a spore mass that turned black (–e). Diseased leaves eventually dropped off. Acervuli measuring 200–350 μm in diameter, occasionally up to 500 μm, were visible on the surface of the leaf lesions (). Conidiogenous cells were discrete or integrated, lageniform to ampulliform or subcylindrical, colourless, smooth-walled, 12.5–18.7 × 3.7–6.2 μm (). The five-celled conidia were clavate fusiform, the apical and basal cells were hyaline, while the three median cells (the upper two were slightly darker than the lower one) were olivaceous. Conidia (n = 50) were 19.8–21.2 × 5.8–6.7 μm, with typically three apical appendages averaging 15.7–17.5 μm in length. The basal appendage was 3.6–4.0 μm long (). The fungus was initially identified as Pestalotiopsis virgatula (Kleb.) Stey (Keith, Citation2008) based on these observations.
Fig. 1. a, Natural symptoms of Eucalyptus camaldulensis leaf blight caused by Pestalotiopsis virgatula. b–e, The exuded spore mass on leaves. f, Acervulus of P. virgatula on leaf. g, Conidiogenous cells in acervulus. h, Fusiform five-cell conidia on E. camaldulensis leaf. i, Colonies of P. virgatula grown for 4 days at 25°C on PDA (left, surface view and right, reverse view). j–k, Conidia on PDA culture. Bars: a–b = 5 cm; c = 100 μm; d–f, h = 50 μm; g, j–k = 10 μm and i = 1 cm.
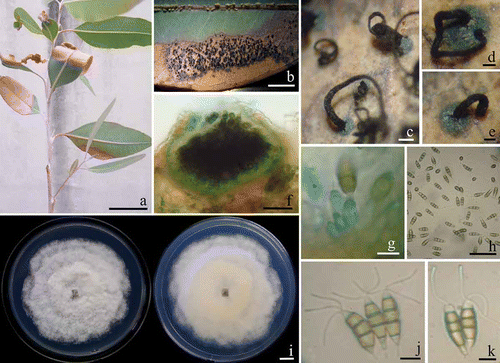
Fungal isolation and morphology
Five symptomatic leaves were kept in plastic box with wet filter paper to induce sporulation. Spore masses were then suspended in 250 μL of sterilized distilled water on sterilized glass slides and dropped on 2% (w/v) water agar containing 0.5 mg/L of chloramphenicol. After 24 h incubation at 25 °C, individual germinating spores was selected and transferred directly to potato dextrose agar (PDA) according to the procedures by Choi et al. (Citation1999) and subculturing onto PDA. Fungal colonies on PDA grew to 70–75 mm diameter in one week at room temperature (25 ± 2 °C) with an even to undulating, glabrous, colourless margin; immersed mycelium was pale buff (), aerial mycelium pure white and woolly-cottony. Acervuli formed on the aerial mycelium and contained black, slimy spore masses. The conidia were fusiform, five-cell, straight or slightly curved. The cells comprised three colored median cells and hyaline apical and basal cells with appendages (–k).
Molecular identification
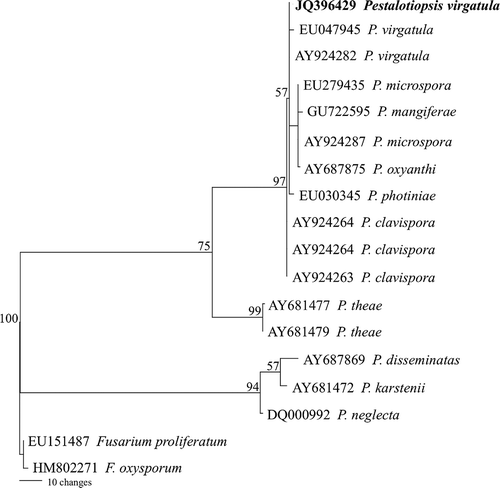
For phylogenetic analysis, genomic DNA of the fungus was extracted using the SDS-CTAB method described by Suwannarach et al. (Citation2010) and the ITS1-5.8S-ITS2 region was amplified using primers ITS4 and ITS5 under the following thermal conditions: 95°C for 2 min, 30 cycles of 95°C for 30 s, 50°C for 30 s, 72°C for 1 min and a cycle of 72°C for 10 min. Three PCR products of size 600 bp were directly sequenced. The partial ITS sequence, containing 548 bp, was deposited in GenBank as JQ396429. BLAST searches of the database showed that the pathogen had 99 and 100% similarity with P. virgatula EU047945 and AY924282, respectively. For the phylogenetic analysis, a multiple sequence alignment was carried out using the alignment subroutines in Clustal X (Thompson et al., Citation1997) and a maximum-parsimony phylogenetic analysis using the PAUP beta 10 software versions 4.0 (Swofford, Citation2002). A maximum-parsimony tree is shown in The results confirmed that the fungus was P. virgatula.
Fig. 2. Maximum parsimonious tree of the internal transcribed spacer (ITS)1, 5.8S ribosomal RNA gene and ITS 2 sequence alignments of 18 isolates. Fusarium proliferatum and F. oxysporum were used to root the tree. Branches with bootstrap values ≥ 50% are shown at each branch and bar represents 10 showed substitutions per nucleotide position.
Pathogenicity test
To confirm pathogenicity, five healthy E. camaldulensis leaves were sterilized with 2% sodium hypochlorite, rinsed with sterile distilled water and then placed in a plastic box. A spore suspension of the fungus was adjusted to 12 × 106 spores/mL and dropped on the leaves. After 15 days, the symptoms described above were observed on all inoculated E. camaldulensis leaves. The same fungus was re-isolated from diseased leaves, confirming Koch's postulates. The test was repeated twice.
In Thailand, Mycosphaerella has been the only known cause of leaf blight prior to this research (Cheewangkoon et al., Citation2008). Eucalyptus plantations in southern China have a leaf blight caused by Calonectria spp. (Chen et al., Citation2011). In addition, Cylindrocladium and Kirramyces species have also been reported as eucalyptus leaf blight pathogens (Old et al., Citation2003). However, P. virgatula has previously been described as a fruit rot pathogen of rambutan in Hawaii (Keith, Citation2008). Here we report that the leaf blight disease of E. camaldulensis, caused by P. virgatula, is a new disease in Thailand.
Acknowledgements
This work was supported by grants from The Office of Higher Education Commission, Thailand under the National Research University (A1) Program, Chiang Mai University and Thailand Research Fund (IUG5380003). We are grateful to Mr. Keegan Kennedy for improving the English text.
References
- Barney , K. 2005 . At the supply edge: Thailand's forest policies, plantation sector, and commodity export links with China , Washington , DC : Forest Trends .
- Brooker , M.I.H. and Kleinig , D.A. 2006 . Field guide to eucalyptus Volume 1. South-Eastern Australia , Melbourne : Bloomings Books .
- Cheewangkoon , R. , Crous , P.W. , Hyde , K.D. , Groenewald , J.Z. and To-anan , C. 2008 . Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand . Persoonia , 21 : 77 – 91 .
- Chen , S.F. , Lombard , L. , Roux , J. , Xie , Y.J. , Wingfield , M.J. and Zhou , X.D. 2011 . Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China . Persoonia , 26 : 1 – 12 .
- Choi , Y.W. , Hyde , K.D. and Ho , W.W.H. 1999 . Single spore isolation of fungi . Fungal Divers. , 3 : 29 – 38 .
- Keith , L.M. 2008 . First report of Pestalotiopsis virgatula causing pestalotiopsis fruit rot on rambutan in Hawaii . Plant Dis. , 92 : 835
- Old , K.M. , Wingfield , M.J. and Yuan , Z.Q. 2003 . A manual of diseases in Eucalyptus in South-East Asia , Bogor : Centre for International Forestry Research (CIFOR) .
- Suwannarach , N. , Bussaban , B. , Hyde , K.D. and Lumyong , S. 2010 . Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota . Mycotaxon , 114 : 15 – 23 .
- Swofford , D.L. 2002 . PAUP*: Phylogenetic analysis using parsimony (*and other methods), beta version 4.0b10 , Sunderland : Sinauer .
- Thompson , J.D. , Ginson , T.J. , Plewniak , F. , Jeanmougin , F. and Higg , D.G. 1997 . The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools . Nucleic Acids Res. , 25 : 4876 – 4882 .