Abstract
Commercial cultivars of canola with resistance to clubroot (Plasmodiophora brassicae) have been registered recently in Canada. However, little is known about how and when resistance is expressed. Time series assessments of root hair infection and cortical infection were made in four cultivars that differed in reaction to two pathotypes, P3 and P6 (Williams' system), of P. brassicae. The cultivars were ‘45H29’ (resistant), ‘InVigor 5030’ (moderately resistant), ‘46A76’ (susceptible) and ‘45H21’ (susceptible to P3, resistant to P6). For assessment of root hair infection (RHI), seedlings were harvested at 4, 8 and 12 days after inoculation (DAI). For assessment of cortical colonization (% root area occupied by the pathogen), plants were harvested at 16, 22 and 28 DAI. In compatible interactions (susceptible cultivar × virulent pathotype), RHI developed quickly and was slower in intermediate and incompatible interactions. The maximum RHI for both compatible and incompatible interactions was 65–70%; maximum RHI was slightly but significantly lower (59%) in the intermediate interaction type. At 28 DAI, cortical colonization was high in ‘46A76’ (P3 = 45%, P6 = 35%), intermediate in ‘InVigor 5030’ (P3 = 23%, P6 = 16%), and no colonization (0%) was observed for either pathotype in ‘45H29’. In ‘45H21’, cortical colonization by P3 was high (35%), but inoculation with P6 resulted in no colonization. In the compatible interactions, cortical colonization by P3 was consistently higher than by P6. There were small differences in the pattern of RHI associated with resistance, but resistance was most clearly expressed during secondary infection and development.
Résumé
Des cultivars commerciaux de canola, résistants à la hernie (Plasmodiophora brassicae), ont été enregistrés récemment au Canada. Toutefois, on ne sait pas vraiment comment et quand s'exprime la résistance. L'évaluation sur séries temporelles de l'infection de radicelles et de l'infection corticale a été effectuée chez quatre cultivars qui réagissaient différemment aux pathotypes P3 et P6 (système de William) de P. brassicae. Les cultivars étaient le ‘45H29’ (résistant), le ‘InVigor 5030’ (moyennement résistant), le ‘46A76’ (réceptif) et le ‘45H21’ (réceptif à l'égard de P3 et résistant à P6). Pour évaluer l'infection des radicelles (RHI), les semis ont été récoltés à 4, 8 et 12 jours après l'inoculation (JAI). Pour évaluer la colonisation corticale (pourcentage de la surface des racines envahie par l'agent pathogène), les plants ont été récoltés à 16, 22 et 28 JAI. Sur le plan des interactions compatibles (cultivar réceptif × pathotype virulent), la RHI s'est développée rapidement, mais plus lentement au cours des interactions intermédiaires et incompatibles. Le taux maximum de RHI pour les interactions compatibles et incompatibles était de 65 à 70 %. Quant aux interactions de type intermédiaire, le taux maximum était légèrement, mais significativement plus bas (59 %). À 28 JAI, le taux de colonisation corticale chez ‘46A76’ était intense (P3 = 45 %, P6 = 35 %), moyen chez ‘InVigor 5030’ (P3 = 23 %, P6 = 16 %) et nul (0 %) chez ‘45H29’ : aucun des deux pathotypes ne l'avait colonisé. Chez ‘45H21’, le taux de colonisation corticale par P3 était élevé (35 %), mais aucune colonisation n'a résulté de l'inoculation avec P6. Sur le plan des interactions compatibles, la colonisation par P3 était invariablement plus élevée que celle par P6. Il y avait de faibles différences dans le mode de la RHI associée à la résistance, par ailleurs celle-ci s'exprimait très nettement durant l'infection secondaire et son développement.
Introduction
Plasmodiophora brassicae Woronin is a soil-borne protist that causes clubroot disease (Woronin, Citation1878) on many members of the Brassicacae family. The pathogen's life cycle involves two infection phases in a susceptible host (Ingram & Tommerup, Citation1972). In the primary infection phase (root hair infection, RHI), resting spores in the soil germinate to produce primary zoospores that infect root hairs. Once inside the root hairs, the pathogen develops into primary plasmodia that produce zoosporangia. Mature zoosporangia liberate secondary zoospores that initiate the secondary infection phase by infecting the root cortex. Once inside the root, secondary zoospores develop into secondary plasmodia that produce resting spores. This process is accompanied by prolific cell division and hypertrophy in the host (Ludwig-Müller & Schuller, Citation2008), resulting in formation of club-shaped galls on the roots. The tissue disruption associated with large clubs inhibits nutrient and water transport within the plant, which can affect plant growth and production (Manzanares-Dauleux et al., Citation2006; Dixon, Citation2009).
In Canada, clubroot has historically been important only on Brassica vegetables, but the recent discovery and rapid spread of clubroot on canola (Brassica napus L.) in Alberta poses a threat to the canola industry in western Canada (Howard et al., Citation2010). The pathotypes that are present differ in various regions of Canada, but pathotype 3 (P3) predominates in Alberta, and P6 predominates in Ontario and British Columbia (Strelkov et al., Citation2006). A few fungicides (e.g. cyazofamid and fluazinam) are known to reduce clubroot severity in vegetable Brassicas (Tanaka et al., Citation1999; Pest Management Regulatory Agency, Citation2008), but they are not economical for use against clubroot on canola. Similarly, approaches such as liming are not economical in the extensive production system employed for canola in western Canada (Hwang et al., Citation2011c ).
The most effective approach for managing clubroot is through the use of resistant cultivars (Diederichsen et al., Citation2009). Clubroot-resistant cultivars of vegetable Brassicas reduce clubroot severity by inhibiting the primary or secondary phases of infection (Tanaka et al., Citation2006; Donald et al., Citation2008). The most widely used genes for clubroot resistance in Brassica vegetable crops were initially derived from European fodder turnip (B. rapa L.) (Diederichsen et al., Citation2009). However, resistance from this source has broken down quickly in several vegetable cultivars (Tanaka et al., Citation1998; Osaki et al., Citation2008), and in the winter rapeseed (B. napus) cultivar ‘Mendel’ in the UK (Harling & Oxley, Citation2007).
The timing of expression of resistance to clubroot has been examined in vegetable Brassicas such as radish (Raphanus sativus L.) (Kroll et al., Citation1983), Chinese cabbage (B. rapa L. subsp. pekinensis) (Tanaka et al., Citation2006) and cauliflower (B. oleracea var. botrytis L.) (Donald et al., Citation2008), as well as in Arabidopsis thaliana L. (Kobelt et al., Citation2000) and European Clubroot Differential-06 (ECD-06, B. napus) (Morgner, Citation1995). Most of these studies assessed the development of a single pathotype of P. brassicae in resistant vs. susceptible hosts, or two pathotypes of P. brassicae differing in pathogenicity on one cultivar (susceptible to one pathotype, resistant to the other). Primary infection has been reported to occur in every cultivar of a host species (and in many non-host species), independent of their susceptibility reaction. However, the extent of cortical infection and colonization often differs substantially among these hosts (Diederichsen et al., Citation2009), which may reflect differences in response among host genotypes, among pathotypes, or even among specific genes for resistance.
Breeding efforts have recently led to the commercialization of several resistant spring canola cultivars in Canada. The first cultivar released was ‘45H29’ (Pioneer Hi-Bred, ON, Canada). The source of the resistance genes used in these resistant cultivars is proprietary knowledge, and the durability of this resistance is not known. At present, almost no information is available on the mechanism of resistance in the resistant cultivars. Hwang et al. (Citation2011b ) demonstrated that root hair infection in the resistant cultivar ‘45H29’ occurred initially at a lower frequency than in a susceptible cultivar, but reached the same maximum level at 12 days after inoculation. The current study was initiated to determine when clubroot resistance against the two predominant pathotypes in Canada, P3 and P6, is expressed in canola cultivars. Canola cultivars representing a range of susceptibility and specificity to P3 and P6 (susceptible, moderately resistant and highly resistant) were selected based on the results of field trials in clubroot-infested sites in Ontario and Alberta (unpublished). The progression of primary and secondary infection and development of P. brassicae was examined using staining and light microscopy and symptom development was assessed in a growth room trial.
Materials and methods
Pathogen and plant materials
Clubbed roots of canola infected with pathotype 3 (P3) were collected from a commercial field near Edmonton, AB where P3 is predominant (provided by V. Manolii, University of Alberta, Edmonton, AB, Canada). Clubbed roots of cabbage infected with pathotype 6 (P6) were collected from the Muck Crops Research Station of the University of Guelph, Holland Marsh, ON, Canada where only P6 has been identified. At both sites, the roots were collected in the autumn of 2008 and stored at −4°C until required.
Four cultivars of canola were selected for the study to represent a range of reactions to P3 and P6, based on susceptibility in field trials at clubroot-infested sites in Alberta and Ontario. Three of the cultivars were from Pioneer Hi-Bred, Caledon, ON: ‘45H29’ – resistant (no clubbing) to both P3 and P6; ‘46A76’ – susceptible (severe clubbing) to both P3 and P6; and ‘45H21’ – susceptible to P3 and resistant to P6. The remaining cultivar was from Bayer CropScience, ON, Canada: ‘InVigor 5030’. It is considered to be moderately resistant (MR, less severe clubbing than in the susceptible reaction) to both P3 and P6. This ‘moderate resistance’ is similar to the term ‘partial resistance’ used by Alix and co-workers (Citation2007).
In this study, a compatible interaction refers to a cultivar × pathotype pairing where the cultivar is highly susceptible to the particular pathotype, resulting in rapid development of large clubs on the root. An incompatible interaction refers to a cultivar × pathotype interaction that results in no clubbing or very small and infrequent clubs. When the cultivar is moderately resistant (i.e. ‘InVigor 5030’) the interaction results in smaller clubs and a lower incidence of clubbed roots relative to a susceptible cultivar.
Primary infection
A trial to assess root hair infection and development of P. brassicae in each cultivar × pathotype interaction was conducted under controlled environment conditions following the method of Donald & Porter (Citation2004). Briefly, each seedling was grown in a 5-mL pipette tip filled with sterilized sand and held as a group of three in a 50-mL Falcon tube. The plants were maintained in a growth room at 25/20°C (day/night), with 16-h photoperiod and a light intensity of 300 μmol·m−2·s−1. Temperature and relative humidity were monitored using a HOBO datalogger (model U 10-003, Onset, Pocasset, MA). The plants were watered twice a day and fertilized with a nutrient solution composed of 0.025% NPK (20 : 20 : 20) and 0.025% MgSO4 two to three times per week. The water or fertilizer solution was adjusted to pH 6.0 using glacial acetic acid (95%, Acros Organics, NJ) before application.
Resting spores were extracted from the stored clubs and a suspension of 1 × 106 resting spores mL−1 of each pathotype was prepared. Each 7-day-old seedling was inoculated with 1 mL of the spore suspension applied at the base of the stem. The plants were watered twice a day with deionized water adjusted to pH 6.0 using glacial acetic acid, to create favourable conditions for clubroot infection and development. A non-inoculated control was treated with pH adjusted deionized water only.
The seedlings were harvested at 4, 8 and 12 days after inoculation (DAI) and assessed using a slight modification of the method of Sharma et al. (Citation2011a ). The roots were rinsed in running water, fixed in acetic acid: ethanol (1 : 1, v : v) solution, and stained with aniline blue solution (125 ppm) for 1 min. The percent root hair infection was assessed on 100 root hairs per seedling at 1 cm below the hypocotyls using a compound microscope at 250× magnification. To assess the progression of root hair infection, the incidence of plasmodia, sporangia and dehisced sporangia, which were deemed to be the most important developmental stages, was evaluated within each infected root hair. These developmental stages were differentiated based on morphological features and staining characteristics. A plasmodium is the amoeboid form of the pathogen that develops immediately following the initial infection of each root hair. When a mature plasmodium differentiates into sporangia, angular cleavage lines divide the cytoplasm into round sporangia. The presence of these angular lines was used to differentiate plasmodia (no lines) from immature sporangia. When mature, the cytoplasm of the sporangia differentiates into secondary zoospores that are subsequently released, leaving empty sporangial walls (dehisced sporangia) (Aist & Williams, Citation1971; Ingram & Tommerup, Citation1972). Plasmodia and sporangia stained as dark blue in aniline blue, while the dehisced sporangia appeared as clusters of light blue bubbles (rings) within root hairs.
The study was conducted in a factorial randomized complete block design with four replicates and three plants per experimental unit. The two factors were cultivar and pathotype. The entire trial was repeated.
Secondary infection
A time-series trial was conducted to quantify secondary infection and development over time in each cultivar × pathotype interaction. The plants were grown under the same temperature and light conditions as in the primary infection trial. Individual seedlings were grown in tall plastic pots (19-cm-high conetainers, Stuewe and Sons, Inc, Corvallis, OR) filled with autoclaved (121°C for 30 min) sand. A 5-mL suspension of 1 × 106 resting spores mL−1 was applied at the base of each 8-day-old seedling; the control received water only. The experiment was laid out in a split-plot design with four replications and one plant per replicate. The main plots were cultivar × pathotype and the subplots were sampling date (16, 22, 28 DAI). The entire trial was repeated.
The roots were harvested at 16, 22 and 28 DAI. Roots were prepared, sectioned, stained and assessed using the method of Sharma et al. (Citation2011b ). Briefly, a portion of the tap root, about 0.5 mm thick, was cut at 1 cm below the hypocotyl. The root hairs were removed from the root piece prior to sampling using a feather scalpel (Feather Safety Razor Co. Ltd., Osaka, Japan) and the root section was fixed in an acetone : ethanol solution (1 : 1, v : v) for 48 h. Samples were treated with neutral buffered formalin twice for 45 min and then dehydrated with an increasing isopropanol series (70, 95 and 100%) and embedded in paraffin. A 4-μm thick cross-section was obtained from each root section using a microtome (Leica 2255, Germany). The cross-sections from each of the four replicates (plants) of a treatment were placed on a single slide glass and stained in 0.5% methylene blue for 5 min. Five photos per section were taken at 250× magnification using a compound light microscope, and the proportion (%) of the total root area occupied by pathogen tissue in each field of view was quantified using image analysis (Assess software version 2.0, APS Press, Minneapolis, MN). A total of 20 fields of view per treatment were assessed in the trial. The entire trial was repeated.
Symptom development
To assess clubroot incidence and severity for each cultivar × pathotype interaction, a trial was conducted under the same conditions as in the secondary infection study except that the experiment was a factorial randomized complete block design with four replicates and 10 plants per experimental unit. The two factors were cultivar and pathotype, plus a non-inoculated control. At 6 weeks after inoculation, the plants were uprooted, the roots were washed, and clubroot severity was assessed using a 0–3 scale, where plants were divided into classes according to the extent of clubbing; 0 = no clubbing, 1 < 1/3 of root clubbed, 2 = 1/3 to 2/3 of roots clubbed, and 3 > 2/3 of roots clubbed (Xue et al., Citation2008 based on Kuginuki et al., Citation1999). The trial was repeated.
Clubroot incidence was calculated as the proportion (%) of plants showing clubroot symptoms. A disease severity index (DSI) was calculated using the following equation (Kobringer & Hagedorn, Citation1983):
The height (cm) of each plant at 6 wk after inoculation was measured from hypocotyl to plant apex and the mean height per replicate was calculated. The developmental stage of each plant was assessed as follows: vegetative (no reproductive structures), buds, flowers or pods, and the proportion of plants in each stage was calculated for each experimental unit.
Statistical analysis
Statistical analysis was performed using SAS software version 9.2. To minimize the potential heterogeneity of variance, an arcsine transformation was performed on the percent root hair infection data. However, for uniformity of presentation, non-transformed means are presented. A mixed model analysis of variance was conducted for total root hair infection, the proportion of infected root hairs containing specific developmental stages (plasmodia, sporangia, dehisced sporangia), clubroot incidence (%) and disease severity index. Cultivar, pathotype and their interaction were treated as fixed factors, and replicate and repetition as random factors. For the cortical infection study, cultivar, pathotype, sampling date and their interactions were fixed factors and replicate and repetition were random factors. For each of the variables, there was little or no effect of repetition, so the data were pooled across repetition for presentation. Means were separated using Tukey's test. Differences are significant at P ≤ 0.05 unless otherwise specified.
Single degree of freedom contrasts were utilized to examine several types of response to treatment in these trials. For primary infection, the largest differences in the pattern of response to cultivar and pathotype were between assessments at 4 and 12 DAI, so contrasts were used to assess disease progress between these two dates. Similarly, differences in root hair infection, cortical colonization and developmental stage of the pathogen were assessed among reaction types, i.e. compatible (‘45H21’ × P3, ‘46A76’ × P3 or P6), intermediate (‘InVigor 5030’ × P3 or P6), or incompatible (‘45H21’ × P6, ‘45H29’ × P3 or P6). Contrasts were also used to assess the impact of clubroot on plant height and growth stage among reaction types compared with non-inoculated controls, and to compare the effect of P3 and P6 across cultivars.
Results
Primary infection
There was a cultivar × pathotype × time interaction in analysis of variance for root hair infection (RHI) and for each of the developmental stages: plasmodia, sporangia and dehisced sporangia. The incidence of RHI across all of the interactions and dates of assessment was higher for P3 than for P6 at 4 DAI (51 vs. 47) and 8 DAI (60 vs. 56), but not at 12 DAI (64 vs. 62). The mean incidence increased steadily over time, from 50% at 4 DAI, to 58% at 8 DAI and 63% at 12 DAI.
Given the complex interactions in the dataset, clubroot reaction type (compatible, intermediate, incompatible) was selected to provide more meaningful grouping for data analysis. There were small differences in the pattern of increase among the reaction types. At 4 DAI, RHI was higher in the compatible interactions than in the intermediate and incompatible interactions (). There were no differences between the intermediate and incompatible interactions for the individual developmental stages or total RHI (). At 8 DAI, RHI had reached 68% (the maximum observed in the study) in the compatible interactions, but levels were slightly lower in the incompatible and intermediate interactions (data not shown). At 12 DAI, RHI in the compatible and incompatible interactions differed only slightly (68 vs. 63%), and both values were slightly higher than in the intermediate interaction (57%) (). The pattern of response over time among the developmental stages indicates that pathogen development occurred more slowly in the intermediate and incompatible interactions than in the compatible interaction ().
Table 1. Contrasts between the mean incidence (%) of developmental stages of Plasmodiophora brassicae during root hair infection (RHI) for three classes of clubroot reaction: compatible (C), intermediate (I) and incompatible (Inc), at 4 and 12 days after inoculation (DAI)
Secondary infection
No cortical colonization was observed at 16 DAI in any of the cultivar × pathotype interactions, so that sampling date was not included in the data analyses. For the remaining sampling dates, the impact of cultivar, pathotype, sampling date and their interactions were all significant for cortical colonization (). Therefore, the dataset was again grouped by clubroot reaction type for further analyses. No infection or pathogen development was observed in the root cortex at any sampling date in the incompatible interactions. At 22 DAI, colonization by the pathogen was observed in the cortical cells in all of the compatible and intermediate interactions (). Roots of plants with intermediate resistance bore primarily vegetative plasmodia, while in the compatible interactions, immature resting spores were also observed. Disorganization of tissue structure resulting from abnormal cell division (hyperplasia) and enlargement (hypertrophy) of cortical cells was observed in these interactions. At 28 DAI, most of the plasmodia had completed development into resting spores in the intermediate and compatible interactions.
Fig. 1. Area (%) of the root cortex colonized by Plasmodiophora brassicae pathotypes P3 and P6 in four canola cultivars at 22 and 28 days after inoculation (DAI). Note: no cortical infection was observed in the resistant ‘45H29’ or in ‘45H21’ inoculated with P6, so these interactions are not presented.
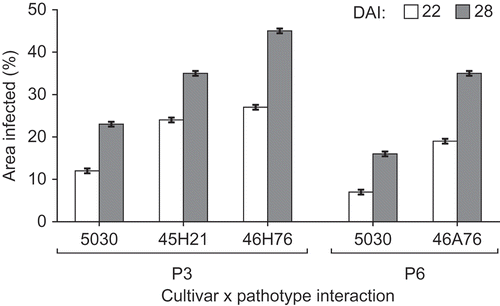
There was more cortical colonization in the compatible interaction than in the intermediate interaction at both 22 and 28 DAI, and the extent of colonization increased between the two sampling dates for both reaction types (). The mean cortical colonization in the compatible and intermediate interactions was slightly higher for P3 than for P6; 20% vs. 13% at 22 DAI and 34% vs. 25% at 28 DAI ().
Table 2. Contrasts between the mean area of the root cortex colonized by Plasmodiophora brassicae in three classes of clubroot reaction†: compatible (C), intermediate (I) and incompatible (Inc) at 22 and 28 days after inoculation (DAI)
Symptom development
The effects of cultivar, pathotype and their interaction on clubroot incidence and severity were all significant. Clubroot incidence was highest in the compatible interactions (mean = 95%), intermediate in the intermediate interactions (mean = 62%) and there were no clubroot symptoms in the incompatible interactions (mean = 0%). The pattern was similar for severity (mean = 79, 33 and 0%, respectively, ). Clubroot severity in the compatible and intermediate interactions was higher for P3 (94% and 40%, respectively) than for P6 (85% and 25%, respectively).
Table 3. Mean incidence and disease severity index (DSI) of clubroot in canola cultivars at 6 weeks after inoculation with Plasmodiophora brassicae pathotypes 3 (P3) and 6 (P6)
Shoot height and developmental stage
Plant height was reduced and development generally delayed (reduced incidence of flowers and pods, more plants still at the vegetative stage) in inoculated plants of all of the cultivars compared with the non-inoculated controls, irrespective of reaction type (). Inoculation with P3 reduced plant height and delayed development more than P6 (plant height: 26 vs. 60 cm, vegetative plants: 55 vs. 32%). Plants were stunted in the compatible interaction (23 cm) relative to the intermediate and incompatible interactions (28 and 33 cm, respectively) and plant development was delayed ().
Table 4. Effect of inoculation with Plasmodiophora brassicae pathotypes P3 and P6 (relative to a non-inoculated control) on plant height and growth stage (% of plants) of four canola cultivars at 6 weeks after inoculation, under controlled conditions
Table 5. Contrasts between means plant height and growth stage (% of plants) among reaction types†: compatible (C), intermediate (I) and incompatible (Inc)
Discussion
In time-course studies of four canola cultivars inoculated with two pathotypes of Plasmodiophora brassicae, small differences in the rates of RHI and pathogen development associated with primary infection were identified. However, the major difference in resistance reaction was expressed during secondary infection. No cortical infection or colonization was detected in incompatible interactions (‘45H21’ × P6, ‘45H29’ × P3 or P6). In contrast, the mean cortical area colonized was moderate (20%) in intermediate interactions (‘InVigor 5030’ × P3 or P6) compared with the compatible interactions (‘45H21 × P3’, ‘46A76’ × P3 or P6), where the area colonized was high (38%).
In the assessment of primary infection, RHI was present at 4 DAI in all of the reaction types, but was lower in the incompatible and intermediate interactions than in the compatible interactions. However, total RHI in the incompatible interactions increased over time, so that by 12 DAI it was similar (64%) to the compatible interaction (68%). A similar pattern was observed for individual developmental stages of the pathogen: development was initially slower in the incompatible and intermediate interactions than in the compatible interactions, but development progressed slightly more quickly in the incompatible interaction over time. This supports the results of previous studies on canola (Hwang et al., Citation2011b ) and Chinese cabbage (Tanaka et al., Citation2006), which showed that primary infection developed later in resistant (‘45H29’) than susceptible cultivars. In comparison, the RHI in intermediate interactions remained lower (57%) at 12 DAI than in the compatible or incompatible interactions. Similar to RHI, fewer (9%) sporangia dehisced in the intermediate interactions. We conclude that, across several canola genotypes and two pathotypes, RHI was slightly delayed in the incompatible interaction and slightly reduced in the intermediate interactions.
Even though differences in RHI in compatible and incompatible interactions were small, primary infection may play an important role in expression of resistance. In a recent study, high levels of cortical infection occurred in ryegrass inoculated with secondary zoospores of P. brassicae, but no cortical infection occurred in ryegrass where RHI occurred before exposure to secondary zoospores (Feng et al., Citation2012). Although primary infection is a non-specific phenomenon that can occur in both host species and in plants that do not develop any clubroot symptoms (Ludwig-Müller et al., Citation1999), this indicates that primary infection may have a role in recognition and induction of resistance. Another study showed that RHI is related to the levels of indole acetic acid (IAA), a phytohormone that influences clubroot development. In cabbage, the level of IAA initially increased in susceptible cultivars and then declined, but continued to increase even after 14 DAI in resistant cultivars (Ludwig-Müller et al., Citation1993).
In the present study, cortical infection was completely inhibited in all of the incompatible interactions. Cultivar ‘45H29’ was resistant to both P3 and P6 and ‘45H21’ was resistant to only P6. This differential response to pathotype indicates that the genetics of resistance to P3 and P6 differ in these two cultivars. However, the timing of action of the resistance genes in these cultivars appears to be similar, because the pathogen was completely inhibited at the cortical infection stage. This indicates that high levels of resistance in canola are expressed in a manner similar to that observed in other hosts (Kroll et al., Citation1983; Kobelt et al., Citation2000; Tanaka et al., Citation2006; Donald et al., Citation2008).
Morgner (Citation1995) monitored the histology of P. brassicae infections in roots of resistant B. napus (ECD-06), B. rapa (‘Chorus’), and B. oleracea (‘Böhmerwaldkohl’) and identified secondary stages, particularly the early secondary plasmodia, as present in the cortex 12 DAI, but no plasmodia or resting spores were detected at 22 DAI. Apparently, pathogen development was stopped before the completion of the life cycle. Similarly, Hwang et al. (Citation2011b ) reported the presence of secondary zoospores and secondary plasmodia in the resistant canola ‘45H29’, but we did not observe these stages in the current study. These differences might be attributable to different growth media and/or inoculum concentration in the two trials.
Complete inhibition of secondary infection by P. brassicae has been reported in several host species, including radish (Kroll et al., Citation1983), Chinese cabbage (Tanaka et al., Citation2006), cauliflower (Donald et al., Citation2008), ECD-06 (Morgner, Citation1995) and Arabidopsis (Kobelt et al., Citation2000). However, the mechanism of this resistance is not consistent across host species. Occurrence of a hypersensitive reaction to secondary infection has been observed in turnip (Dekuijzen, Citation1979) and Arabidopsis (Kobelt et al., Citation2000), but not in radish (Kroll et al., Citation1983), Chinese cabbage (Tanaka et al., Citation2006) or canola (Morgner, Citation1995). A study to determine if a hypersensitive response plays a role in incompatible reactions in canola would be useful and is currently underway.
The area of cortical infection in the intermediate interactions was much lower than in the compatible interactions. This is consistent with a study on radish, where moderate resistance delayed the development of secondary infection (Kroll et al., Citation1983). In a study on Arabidopsis, there were differences in regulation of arginine catabolism and polyamine metabolism (associated with cell proliferation and hypertrophy) between a susceptible and a moderately resistant ecotype (Jubault et al., Citation2008). Additional studies to assess the role of IAA and arginine catabolism and polyamine metabolism in clubroot resistance in canola may provide insights into how resistance functions in this important field crop.
Inoculation with P. brassicae reduced plant height and delayed plant development, irrespective of reaction type (compatible, intermediate, incompatible) or pathotype (P3, P6). This result supports a recent report of reduction in plant height and yield in both susceptible and resistant canola cultivars as a result of infection by P. brassicae (Hwang et al., Citation2011a ). In general, P3 was more aggressive than P6; it infected more root hairs, colonized a larger proportion of the root cortex, and produced more severe clubroot symptoms than P6 in both the compatible and intermediate interactions. Pathotype 3 also reduced plant height and development more than P6. These results support a previous report that P3 is more aggressive than the other pathotypes in Canada (Cao et al., Citation2009).
Previous studies on clubroot resistance either compared the reaction of a single pathotype of P. brassicae in a clubroot resistant and a susceptible host or two pathotypes of P. brassicae differing in their pathogenicity on a single cultivar (susceptible to one pathotype and resistant to the other), and little work had been done specifically on canola. The present study is the first to characterize and compare resistance patterns in a range of compatibility interactions in canola: highly resistant (‘45H29’), moderately resistant (‘InVigor 5030’), highly susceptible (‘46A76’) and pathotype-specific resistance (‘45H21’), as an initial step towards understanding the mechanism(s) of resistance in these new clubroot-resistant cultivars.
The present study demonstrated that resistance to clubroot was expressed predominantly against cortical infection in selected canola cultivars. Root hair infection and the release of secondary zoospores occurred in all of the cultivar × pathotype interactions, and differences in RHI associated with differences in resistance were small. The source(s) of the genes for clubroot resistance that have been incorporated into Canadian canola cultivars is not known. However, all of the highly resistant lines that were examined in the current trial exhibited a similar pattern of expression of resistance, and this was consistent with highly resistant cultivars of other host species. This indicates that studies on other hosts will likely be relevant to the situation on canola. Studies on a model crop may be extremely useful in elucidating the mechanism of clubroot resistance in canola and other Brassicas.
Acknowledgements
We thank Dr V. Manolii and Dr S. Strelkov, University of Alberta, Edmonton, AB, for providing inoculum of P3, and the Clubroot Risk Mitigation Initiative of Agriculture and Agri-Food Canada for providing financial support for the study.
References
- Aist , J.R. and Williams , P.H. 1971 . The cytology and kinetics of cabbage root hair penetration by Plasmodiophora brassicae . Can. J. Bot , 49 : 2023 – 2034 .
- Alix , K. , Lariagon , C. , Delourme , R. and Manzanares-Dauleux , M.J. 2007 . Exploiting natural genetic diversity and mutant resources of Arabidopsis thaliana to study the A. thaliana–Plasmodiophora brassicae interaction . Plant Breed , 126 : 218 – 221 .
- Cao , T. , Manolii , V.P. , Hwang , S.F. , Howard , R.J. and Strelkov , S.E. 2009 . Virulence and spread of Plasmodiophora brassicae [clubroot] in Alberta, Canada . Can. J. Plant Pathol , 31 : 321 – 329 .
- Dekuijzen , H.M. 1979 . Electron microscopic studies on the root hairs and cortex of a susceptible and a resistant variety of Brassica campestris infected with Plasmodiophora brassicae . Eur. J. Plant Pathol , 85 : 1 – 17 .
- Diederichsen , E. , Frauen , M. , Linders , E.G.A. , Hatakeyama , K. and Hirai , M. 2009 . Status and perspectives of clubroot resistance breeding in Crucifer crops . J. Plant Growth Regul , 28 : 265 – 281 .
- Dixon , G.R. 2009 . The occurrence and economical impact of Plasmodiophora brassicae and clubroot disease . J. Plant Growth Regul , 28 : 194 – 202 .
- Donald , E.C. and Porter , I.J. 2004 . A sand-solution culture technique used to observe the effect of calcium and pH on root hair and cortical stages of infection by Plasmodiophora brassicae. Australas . Plant Pathol , 33 : 585 – 589 .
- Donald , E.C. , Jaudzems , E.C. and Porter , I.J. 2008 . Pathology of cortical invasion by Plasmodiophora brassicae in clubroot resistant and susceptible Brassica oleracea hosts . Plant Pathol , 57 : 201 – 209 .
- Feng , J. , Xiao , Q. , Hwang , S.F. , Strelkov , S.E. and Gossen , B.D. 2012 . Infection of canola by secondary zoospores of Plasmodiophora brassicae produced on a nonhost (ryegrass) . Eur. J. Plant Pathol , 132 : 309 – 315 .
- Harling , R. and Oxley , S. 2007 . Clubroot disease of oilseed rape and other Brassicae crops . Tech. Note Scott. Agr. Coll. , (TN602). ISBN: 1854828908
- Howard , R.J. , Strelkov , S.E. and Harding , M.W. 2010 . Clubroot of cruciferous crops – new perspectives on an old disease . Can. J. Plant Pathol , 32 : 43 – 57 .
- Hwang , S.F. , Ahmed , H.U. , Strelkov , S.E. , Gossen , B.D. , Turnbull , G.D. , Peng , G. and Howard , R.J. 2011a . Seedling age and inoculum density affect clubroot severity and seed yield in canola . Can. J. Plant Sci , 91 : 183 – 190 .
- Hwang , S.F. , Ahmed , H.U. , Zhou , Q. , Strelkov , S.E. , Gossen , B.D. , Peng , G. and Turnbull , G.D. 2011b . Influence of cultivar resistance and inoculum density on root hair infection of canola (Brassica napus) by Plasmodiophora brassicae . Plant Pathol , 60 : 820 – 829 .
- Hwang , S.F. , Strelkov , S.E. , Gossen , B.D. , Turnbull , G.D. , Ahmed , H.U. and Manolii , V.P. 2011c . Soil treatments and amendments for amelioration of clubroot on canola . Can. J Plant Sci , 91 : 999 – 1010 .
- Ingram , D.S. and Tommerup , I.C. 1972 . The life history of Plasmodiophora brassicae Woron . Proc. R. Soc. Lond. [Biol] , 180 : 103 – 112 .
- Jubault , M. , Hamon , C. , Gravot , A. , Lariagon , C. , Delourme , R. , Bouchereau , A. and Manzanares-Dauleux , M.J. 2008 . Differential regulation of root arginine catabolism and polyamine metabolism in clubroot-susceptible and partially resistant Arabidopsis genotypes . Plant Physiol , 146 : 2008 – 2019 .
- Kobelt , P. , Siemens , J. and Sacristán , M.D. 2000 . Histological characterisation of the incompatible interaction between Arabidopsis thaliana and the obligate biotrophic pathogen Plasmodiophora brassicae . Mycol. Res , 104 : 220 – 225 .
- Kobringer , K.M. and Hagedorn , D.J. 1983 . Determination of bean root rot potential in vegetable production fields of Wisconsin's Central Sands . Plant Dis , 67 : 177 – 178 .
- Kroll , T.K. , Lacy , G.H. and Moore , L.D. 1983 . A quantitative description of the colonization of susceptible and resistant radish plants by Plasmodiophora brassicae . J. Phytopathol , 108 : 97 – 105 .
- Kuginuki , Y. , Yoshikawa , H. and Hirai , M. 1999 . Variation in virulence of Plasmodiophora brassicae in Japan tested with clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. ssp. pekinensis) . Eur. J. Plant Pathol , 105 : 327 – 332 .
- Ludwig-Müller , J. and Schuller , A. 2008 . What can we learn from clubroots: alterations in host roots and hormone homeostasis caused by Plasmodiophora brassicae . Eur. J. Plant Pathol , 121 : 291 – 302 .
- Ludwig-Müller , J. , Bendel , U. , Thermann , P. , Ruppel , M. , Epstein , E. and Hilgenberg , W. 1993 . Concentrations of indole-3-acetic acid in plants of tolerant and susceptible varieties of Chinese cabbage infected with Plasmodiophora brassicae Woron . New Phytol , 125 : 763 – 769 .
- Ludwig-Müller , J. , Bennett , R.N. , Kiddle , G. , Ihmig , S. , Rüppel , M. and Hilgenberg , W. 1999 . The host range of Plasmodiophora brassicae and its relationship to endogenous glucosinolate content . New Phytol , 141 : 443 – 458 .
- Manzanares-Dauleux , M.J. , Rocherieux , J. , Alix , K. , Glory , P. , Giboulot , A. Lariagon , C. 2006 . Deciphering the host mechanisms of resistance in the Brassicaceae/Plasmodiophora brassicae pathosystem . Acta Hort , 706 : 317 – 322 .
- Morgner , M. 1995 . Mikroskopische Untersuchungen der Reaktionen empfindlicher und resistenter Genotypen verschiedener Brassica-Arten gegenüber Plasmodiophora brassicae (Wor.) , Germany : PhD dissertation, Institut für Angewandte Genetik, Freie Universität Berlin .
- Osaki , K. , Tanaka , S. and Ito , S. 2008 . Pathogenicity of Plasmodiophora brassicae populations from small, spheroid, resistant-type clubroot galls on roots of clubroot-resistant cultivars of Chinese cabbage (Brassica rapa L. subsp. pekinensis) . J. Gen. Plant Pathol , 74 : 242 – 245 .
- Pest Management Regulatory Agency . 2008 . Registration Decision: Fluazinam , Health Canada . ISBN: 978-1-100-10510-9 (978-1-100-10511-6)
- Sharma , K. , Gossen , B.D. and McDonald , M.R. 2011a . Effect of temperature on primary infection by Plasmodiophora brassicae and initiation of clubroot symptoms . Plant Pathol , 60 : 830 – 838 .
- Sharma , K. , Gossen , B.D. and McDonald , M.R. 2011b . Effect of temperature on cortical infection by Plasmodiophora brassicae and clubroot severity . Phytopathology , 101 : 1424 – 1432 .
- Strelkov , S.E. , Tewari , J.P. and Smith-Degenhardt , E. 2006 . Characterization of Plasmodiophora brassicae populations from Alberta, Canada . Can. J. Plant Pathol , 28 : 467 – 474 .
- Tanaka , S. , Fujiyama , S. , Shigemori , S. , Nakayama , A. , Ito , S. and Kameya , I.M. 1998 . Pathogenesis of isolates of Plasmodiophora brassicae from Japan. 1. Race and pathogenesis in clubroot resistant cultivars . Proc. Assoc. Plant Prot. Kyushu , 44 : 15 – 19 .
- Tanaka , S. , Kochi , S. , Kunita , H. , Ito , S. and Kameya-Iwaki , M. 1999 . Biological mode of action of the fungicide, flusulfamide, against Plasmodiophora brassicae (clubroot) . Eur. J. Plant Pathol , 105 : 577 – 584 .
- Tanaka , S. , Mido , H. and Ito , S. 2006 . Colonization by two isolates of Plasmodiophora brassicae with differing pathogenicity on a clubroot-resistant cultivar of Chinese cabbage (Brassica rapa L. subsp. pekinensis) . J. Gen. Plant Pathol , 72 : 205 – 209 .
- Woronin , M.S. 1878 . “ Plasmodiophora brassicae, the cause of cabbage hernia. Translated by C. Chupp (1934) ” . In Phytopathological Classic No. 4 , St. Paul , MN : American Phytopathological Society .
- Xue , S. , Cao , T , Howard , R.J. , Hwang , S.F. and Strelkov , S.E. 2008 . Isolation and variation in virulence of single-spore isolates of Plasmodiophora brassicae from Canada . Plant Dis , 92 : 456 – 462 .