Abstract
Fusarium foetens was recently reported on greenhouse Hiemalis begonia (Begonia × hiemalis Fotsch) in Canada, causing disease symptoms which consist of a dull green leaf colour, wilting, and vascular necrosis. During our greenhouse trials, a concentration of 100 conidia mL−1 added to the growing substrate was sufficient to cause severe disease symptoms. F. foetens symptoms were observed as early as 2 weeks after inoculation, for highly susceptible Hiemalis begonia cultivars (i.e. ‘Berseba’ red, ‘Golden Edith’ yellow and red, ‘Rainbow Spectrum’ white, ‘Berseba’ hot pink). Disease symptoms were observed 3 weeks following inoculation for moderately susceptible Hiemalis begonia cultivars (i.e. white ‘Netja’, ‘Binos’ pink, ‘Batik’ orange, ‘Solenia’ velvet red, ‘Rainbow Spectrum’ pink chablis and ‘Netja’ dark pink), and after 4 weeks for slightly susceptible cultivars (i.e. ‘Rainbow Spectrum’ and ‘Rainbow Spectrum Vivian’). The Hiemalis begonia cultivar ‘Rainbow Spectrum Camilla’ was ranked as moderately resistant. Other begonia types such as Rex begonia hybrid (Begonia rex-hybrid Putz.), Fibrous begonia (Begonia semperflorens Hook.), and Tuberous begonia (Begonia tuberhybrida Voss), which exhibited symptoms 12 weeks following F. foetens inoculation, were considered resistant. Additional ornamental species (i.e. petunia ‘Polo Rose Flare’, impatiens ‘Balfieplos’ and poinsettia ‘Prestige’) inoculated with F. foetens did not develop visible disease symptoms, suggesting that F. foetens may be relatively host specific to begonia.
Résumé
Au Canada, on a récemment détecté, sur le bégonia hiemalis (Begonia × hiemalis Fotsch) cultivé en serre, Fusarium foetens qui provoque les symptômes suivants: des feuilles d'un vert terne, de la flétrissure et de la nécrose vasculaire. Durant nos essais en serre, des conidies ajoutées au milieu de croissance, à raison de 100 conidies/ml−1, ont suffi pour provoquer de graves symptômes. Chez les cultivars hautement réceptifs à l'égard de la maladie (‘Berseba’ rouge, ‘Golden Edith’ jaune et rouge, ‘Rainbow Spectrum’ blanc, ‘Berseba’ rose vif), les symptômes de F. foetens ont été observés à peine deux semaines après l'inoculation. Les symptômes ont été observés trois semaines après l'inoculation chez les cultivars de bégonia hiemalis moyennement réceptifs (‘Netja’ blanc, ‘Binos’ rose, ‘Batik’ orangé, ‘Solenis’ rouge velours, ‘Rainbow Spectrum’ chablis rosé, ‘Netja’ rose foncé). Quatre semaines après l'inoculation, les symptômes sont apparus sur les cultivars légèrement susceptibles (‘Rainbow Spectrum’ et ‘Rainbow Spectrum Vivian’). Le cultivar ‘Rainbow Spectrum Camilla’ a été classé moyennement résistant. D'autres types de bégonias, comme le bégonia Rex hybride (Begonia rex-hybrid Putz.), le bégonia à souche fibreuse (Begonia semperflorens Hook.) et le bégonia tubéreux (Begonia tuberhybrida Voss), qui ont affiché des symptômes 12 semaines après l'inoculation, ont été considérés comme résistants. D'autres variétés ornementales (pétunia ‘Polo Rose Flare’, impatiens ‘Balfieplos’, poinsettia ‘Prestige’), inoculées avec F. foetens, n'ont pas développé de symptômes visibles, ce qui suggère que F. foetens peut être relativement spécifique du bégonia.
Introduction
Begonias represent one of the 10 largest angiosperm genera (> 1500 species) in the flowering plant family Begoniaceae. Hiemalis begonias (Begonia × hiemalis Fotsch), also known as Elatior hybrids or Winter Begonias, will bloom at any time of the year and are well suited as winter indoor plants. Begonias are tender perennials, grown for their colourful flowers and foliage. They are indigenous to tropical climates, so potted begonias are produced and sold as annuals in Ontario. Most begonias can be grown outdoors in containers, garden beds or hanging baskets, or indoors as houseplants. They are Ontario's seventh most important potted flowering plant crop.
Fusarium foetens (Schroers et al.) was first reported to cause a wilt in Elatior hybrid begonias in nurseries in the Netherlands in 2000, and has since been reported in Germany in 2001 (Schroers et al., Citation2004), Connecticut, USA in 2003 (Elmer & Vossbrinck, Citation2004), Japan in 2005 (Sekine et al., Citation2008), Canada in 2010 (Tian et al., Citation2010) and New Hampshire, USA in 2010 (W. Elmer, personal communication). Fusarium foetens causes serious losses during greenhouse production of Hiemalis begonias (Elmer & Vossbrinck, Citation2004; Schroers et al., Citation2004). For example, during the spring of 2010, 5–10% of the potted Hiemalis begonias grown in a commercial greenhouse in southern Ontario, Canada, developed wilt symptoms which were suspected to be caused by F. foetens. Fusarium foetens was first recovered from an Elatior hybrid begonia crop in Canada produced using ebb-and-flow sub-irrigation (Tian et al., Citation2010). The current popular method of potted begonia production uses ebb-and-flow sub-irrigation in troughs that allows for recirculation of the nutrient solution; however, high susceptibility of begonias to water-borne pathogens makes this production practice risky for spreading disease during begonia production. In addition to Hiemalis begonias, it is unknown whether other Begonia species or ornamental crops are susceptible to F. foetens.
The objectives of this research were to: (i) describe in vitro colony characteristics of F. foetens and symptoms caused by this pathogen on Hiemalis begonias under greenhouse production conditions; (ii) examine the effect of F. foetens inoculum density on disease expression; (iii) screen different cultivars of Hiemalis begonias for susceptibility to the disease; and (iv) evaluate the susceptibility of other ornamentals common to commercial greenhouses in Ontario.
Materials and methods
Pathogen morphology and identification
The F. foetens GB1 strain employed in this study was isolated from a naturally infected begonia (Begonia × hiemalis) plant grown in a greenhouse in Ontario during the spring of 2010. The pathogen was identified by morphological features and molecular methods (Tian et al., Citation2010). To describe variation of in vitro culture characteristics of F. foetens, the isolates were cultured on the following media: synthetic nutrient-poor agar (SNA), potato-dextrose agar (PDA, Difco), and oatmeal agar (OA), in plastic Petri dishes, 9-cm diameter, and incubated in the dark at 21 °C. Colony diameters were measured daily and growth rates determined.
Plant growth and propagation
Hiemalis begonias used in inoculation studies were obtained from a commercial greenhouse in southwest Ontario and consisted of 4-week-old rooted plugs at the three leaf stage. The plugs were transplanted into 1 L plastic pots (12-cm diameter) filled with BM6 potting mix medium (Canadian Sphagnum Peat Moss, Berger/Berger peatmoss, Quebec, Canada), and maintained on greenhouse benches with a drip irrigation system (one emitter per plant, flow rate of 4 L h−1). Experiments were conducted in the greenhouse complex at the University of Guelph for 12 weeks with 22–24 °C day/18–20 °C night temperatures. The used nutrient solution had a pH of 5.5 ± 0.5 and electrical conductivity (EC) of 1.0–1.5 ms cm−1 to suit the begonia plants. The nutrient solution that drained from pots was discarded.
Effects of inoculum density on infection of Hiemalis begonias
For inoculation studies, the F. foetens GB1 strain was cultured on potato carrot agar (PCA) (Dhingra & Sinclair, Citation1985) for 5 days at 22–25 °C. Macroconidia were washed from colonies grown on PCA plates with sterile water, quantified microscopically using a haemocytometer and serially diluted to achieve concentrations of 0, 102, 103, 104, 105 and 106 conidia mL−1. Plugs of Hiemalis begonias ‘Golden Edith’ were transplanted into 12-cm pots filled with a BM6 potting mix and allowed to grow for 10 weeks. Each pot was drenched with 25 mL of the conidial suspension. The pots were then arranged on greenhouse benches using a randomized complete block design. There were five replicate plants per treatment. Plants were rated for disease severity after 6 weeks using a scale of 1–5 (Elmer, Citation2008). The experiment was repeated once.
Host susceptibility
Thirteen cultivars of Begonia × hiemalis, three begonia species and three non-begonia species were evaluated for their sensitivity to F. foetens (). Begonia cultivars included ‘Golden Edith’ yellow and red, ‘Berseba’ red, ‘Binos’ pink, white ‘Netja’, ‘Berseba’ hot pink, ‘Netja’ dark pink, ‘Batik’ orange, ‘Rainbow Spectrum’ white, ‘Rainbow Spectrum’ salmon pink, ‘Rainbow Spectrum’ pink chablis, ‘Rainbow Spectrum Vivian’, ‘Rainbow Spectrum Camilla’ and ‘Solenia’ velvet red begonia. In addition, Rex begonia hybrid (Begonia rex-hybrid Putz.), Fibrous begonia ‘Prelude White’ (Begonia semperflorens Hook.), and Tuberous begonia ‘Non-stop Apricot’ (Begonia tuberhybrida Voss) were included. Petunia ‘Polo Rose Flare’ (Petunia multiflora), impatiens ‘Balfieplos’ (Impatiens walleriana), poinsettia ‘Prestige’ (Euphorbia pulcherrima) were also evaluated. Plants were transplanted into 12-cm plastic pots and grown for an additional 2 weeks in the greenhouse prior to inoculation.
Table 1. Susceptibility rating of the begonia and non-begonia species inoculated with Fusarium foetens
In the first experiment, the 13 Hiemalis begonia cultivars were inoculated with F. foetens by drenching each pot with 50 mL of 106 conidia mL−1 (harvested from PCA culture with autoclaved water). There were five replicate plants per cultivar along with an equal number of non-inoculated controls. Disease severity was recorded using the scale of 1–5 (Elmer, Citation2008), 8 weeks after inoculation.
In the second experiment, Rex begonia, Fibrous begonia and Tuberous begonia were artificially inoculated with F. foetens by drenching pots with 50 mL 106 conidia mL−1. There were five replicate plants per species. Disease severity was evaluated after 12 weeks.
At the end of the experiments, the roots and stems were visually examined for vascular discolouration. Samples of infected tissues were placed on agar media described above and the resulting cultures were examined microscopically for morphological characteristics according to Schroers et al. (Citation2004).
For DNA extraction, isolates were incubated on cellophane sheets on PDA for 1 week at room temperature. Mycelia were scraped from the cellophane and used fresh in 1.5 mL microcentrifuge tubes. DNA was extracted with the Dneasy Plant Mini Kit (Qiagen, Mississauga, ON) following the manufacturer's instructions. Extracted DNA was stored at −20 °C for polymerase chain reaction (PCR) amplification. PCR amplification with primer pairs EF1 and EF2 that amplify a region of the elongation factor 1 gene was conducted according to the procedure described by Geiser et al. (Citation2004).
The third experiment involved the examination of petunia, impatiens and poinsettia. Five plants per species were inoculated with F. foetens as described above. The disease severity was evaluated at weekly intervals over a period of 12 weeks. For the above experiments, treatments were arranged in a randomized complete block design with five replicate plants per treatment.
Fusarium detection in nutrient solutions
Re-circulated nutrient solution samples were collected from four commercial Hiemalis begonia greenhouse operations in southern Ontario. One begonia grower subjected the re-circulated solution to a UV treatment before reusing it for irrigation. Samples were also obtained from one gerbera greenhouse producer in southern Ontario. Each sample was diluted to 10−1, 10−2, 10−3, 10−4 and then 0.1 mL of these solutions was spread on Tryptic soy agar (TSA) and PDA with 100 mg streptomycin and 50 mg tetracycline. After 5 days, presence of Fusarium and microbial colonies on the Petri dishes were examined microscopically.
Results and discussion
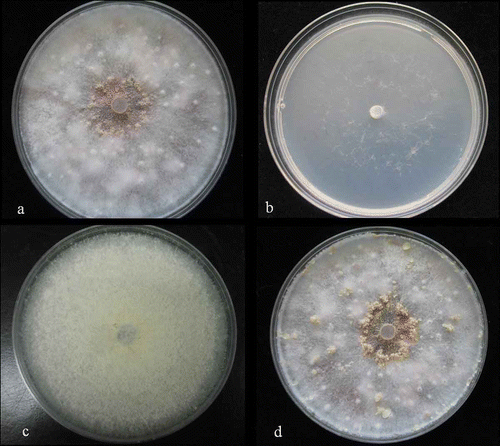
The F. foetens strain originally isolated from infected Hiemalis begonia was used. The average radial growth rate of the GB1 isolate of F. foetens on SNA was 5.0 mm per day, 4.9 mm per day on OA and 5.2 mm per day on PDA. Over the period of 3–7 days, colony diameters reached 29, 38, 48, 58 and 69 mm on SNA, 30, 41, 50, 60 and 71 mm on OA and 31, 38, 51, 61 and 70 mm on PDA (–c). There was a distinct pungent colony odour on PDA and OA. Light orange sporodochia containing a mass of conidia were produced on OA, and were up to 6 mm in diameter, and hemispherical to dome shaped. Stromata also formed on OA at 28 days (). PCR amplification and sequencing results showed that a partial EF1 sequence had a 100% match with F. foetens (Geiser et al., Citation2004).
Fig. 1. Growth of Fusarium foetens on different agar media. a, On OA after 14 days. b, On SNA after 14 days. c, On PDA after 14 days. d, On OA after 28 days.
Foliage of Hiemalis begonia ‘Golden Edith’ became soft and turned to dull green () after 14 days, and leaves wilted after 21 days (). A few days later, a yellowing of the vascular system at the base of stems at the soil line developed that became darker and water soaked, and eventually became necrotic (). Large masses of sporodochia of F. foetens were observed on the flowers (), leaves () and wilted stems (). In later stages of infection, the affected stems, flowers and leaves wilted, withered and died.
Fig. 2. Symptoms of Fusarium foetens wilt on Hiemalis begonia ‘Golden Edith’ post inoculation. a, Dull-green leaves. b, Wilted leaves. c, Brown stem rot. d, Wilting of flowers. e, Veins in wilted stems containing a mass of sporodochia. f, Stem rot with a mass of sporodochia.
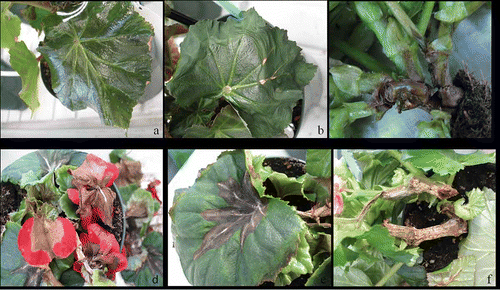
Typical symptoms observed in infected plants developed in all Hiemalis begonias upon inoculation with F. foetens. Inoculated plants showed yellowing and dwarfing, followed by wilting and vascular collapse. The bases of the stems were covered by masses of sporodochia. The inoculated fungus was consistently recovered from discoloured tissues. To date, no forma specialis of F. oxysporum has been encountered attacking Begonia × hiemalis (Armstrong & Armstrong, Citation1981).
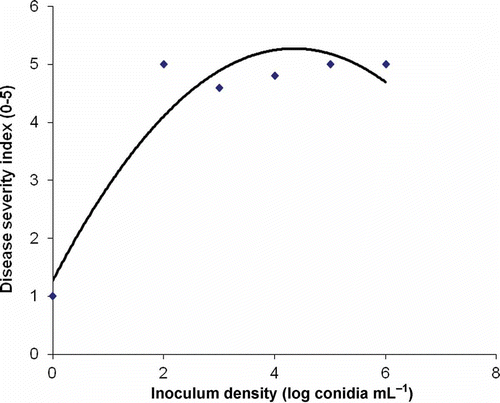
When a range of inoculum densities was added to soil containing Hiemalis begonia, a polynomial fit best described the relationship between the log inoculum density and disease severity (). Symptoms appeared in all inoculated plants and all plants showed high disease severity when inoculated with 102, 103, 104, 105, 106 conidia mL−1 (log 2 to 6). This suggested that severe symptoms develop at very low inoculum densities of 100 conidia mL−1. These results are consistent with those of Wohanka (Citation2003) and Elmer (Citation2008). Elmer (Citation2008) suggested that the rapid spread of the pathogen on plants resulted from the re-use of contaminated irrigation water. However, fusarium wilt of begonia by F. foetens in Ontario develops rapidly in greenhouses that employ drip irrigation that does not involve reuse of the irrigation water. This suggests that F. foetens, once introduced into a greenhouse in infected plants, can likely spread via conidia produced in sporodochia on infected stems, leaves and flowers and possibly by aerially transmission from plant to plant.
Fig. 3. Effect of inoculum density (X) of Fusarium foetens on the disease severity index (Y) on Hiemalis begonia 5-weeks post inoculation (Y = −0.2119x2 + 1.8429x + 1.69; R2 = 0.8988; P < 0.001).
Experiments with various species and cultivar groups of begonia revealed that a number of cultivars were highly susceptible to F. foetens. After artificial inoculation, typical symptoms developed within 2–11 weeks, depending on the species and the cultivars (). The most susceptible group was Begonia × hiemalis with the cultivars ‘Berseba’ red, ‘Golden Edith’ yellow and red, ‘Rainbow Spectrum’ white, ‘Berseba’ hot pink, and white ‘Netja’ these Hiemalis begonia cultivars began to show the dull green symptom on leaves 2 weeks after inoculation. The cultivars ‘Binos’ pink, ‘Batik’ orange, ‘Solenia’ velvet red, ‘Rainbow Spectrum’ pink chablis, and ‘Netja’ dark pink showed symptoms 3 weeks post-inoculation. ‘Rainbow Spectrum’ salmon pink and ‘Rainbow Spectrum Vivian’ started to exhibit symptoms after 4 weeks. The cultivar ‘Rainbow Spectrum Camilla’ showed symptoms after 7 weeks suggesting that it is moderately resistant to F. foetens. All of the tested Hiemalis begonia cultivars had disease severity ratings between four and five, except ‘Rainbow Spectrum Camilla’, which had a rating of two.
Hiemalis begonias became infected without any wounding of roots or stems. The first symptoms were observed as early as 14 days after inoculation. After that, further symptoms developed rapidly. The fungus killed all inoculated susceptible plants within two months, demonstrating that it is a primary pathogen of Hiemalis begonias. While most Fusarium species are soil-borne (Smith et al., Citation1988), there are no data showing that F. foetens could be introduced on soil adhering to roots of imported begonia plants, which could serve as a pathway for introduction, or from infected cuttings.
Apart from the indicated begonias, thus far, the fungus has only been reported on the cultivars ‘Bacchus’, ‘Baladin’, ‘Balamon’, ‘Barkos’, Bastos, ‘Bazan’, ‘Bellona’, ‘Berseba’, ‘Fayal’ and ‘Fuga’ (Schroers et al., Citation2004). All of the tested Hiemalis begonia cultivars ‘Bente’, ‘Berseba’, ‘Camill’, ‘Dina’, ‘Ema’, ‘Nadine’ and ‘Tes’ had disease severity ratings of between four and five (Elmer, Citation2008). Brand & Wienberg (Citation2005) reported that the most sensitive group was Hiemalis begonia ‘Bellona’. Lorraine begonia ‘Kardinal’ as well as Begonia tuberhybrida ‘Champagner’ developed delayed symptoms; Begonia semperflorens hybrids and wild species (B. cinnabarina, B. schmidtiana, B. boliviensis, B. partita) did not show any symptoms.
Fusarium foetens symptoms developed on B. rex, B. semperflorens as well as B. tuberhybrida, but were delayed in comparison to the Hiemalis begonias. Tuberous begonia showed leaf wilting and leaf drop. Stems started rotting and showed stunted growth symptoms 7 weeks after inoculation, and plants wilted and died. All three begonia disease severity ratings were five at 12 weeks from the inoculation. This is the first report of F. foetens infecting B. rex, B. semperflorens or B. tuberhybrida.
Rex begonia hybrid exhibited necrotic dying of the leaf edges, stem base wilting and showed stunted growth 11 weeks after inoculation. Fibrous and tuberous begonia exhibited leaf drop, branch wilting and a wilted stem base 11 weeks after inoculation. All the Rex begonia hybrid, fibrous begonia and tuberous begonia plants eventually succumbed to the disease. Fusarium foetens was re-isolated and identified from all begonia plants independent of the disease symptoms. The identity of F. foetens strains 2011M-186, 2011M-187, 2011M-188 were confirmed by Dr T. Barasubiye at DAOM, Ottawa and the cultures were deposited with the National Fungal Identification Service.
The petunia, impatiens and poinsettia species tested did not develop any visible disease symptoms. Since F. foetens was not pathogenic to poinsettia, petunia or impatiens, this suggests that F. foetens may be relatively host specific to begonia.
Recirculated nutrient solutions were sampled from five commercial growers. There was no Fusarium spp. found in the nutrient solutions sampled from either of the greenhouses, although fungi (0.1 CFU mL−1) and bacteria (47.7 CFU mL−1) were detected. Thus, the origin of F. foetens in these greenhouses remains unknown. Most likely, pathogen introductions originated from infected cuttings.
At an early stage of the disease, F. foetens is difficult to detect based on visual inspection. In this study, only 13 cultivars were evaluated but most were susceptible. In some cultivars, such as ‘Rainbow Spectrum’ salmon pink and ‘Rainbow Spectrum Vivian’, symptoms were delayed by 2 weeks, in comparison to the most sensitive ones, and plants started to exhibit symptoms 4 weeks after inoculation. ‘Rainbow Spectrum Camilla’ was moderately resistant to F. foetens, exhibiting symptoms only 7 weeks after inoculation.
Although F. foetens has caused severe problems on Hiemalis begonia in southern Ontario greenhouses, there are few control options available to producers. Currently, there are no effective chemical fungicides for this disease. Further research is needed to identify ways to prevent the spread of F. foetens within and among greenhouses in Ontario, control crop losses after infection is detected, identify chemicals which control the spread of F. foetens and determine how an infected greenhouse can be sanitized after a F. foetens infection. In addition, the F. foetens susceptibility of additional begonia species and cultivars needs to be identified in order to protect greenhouse begonia crops in Ontario.
Acknowledgements
We thank Ontario Centres of Excellence Agriculture and Agri-Food Canada's Agri-science cluster initiative for providing financial support to this research and Denis Gaudet for his inputs. Thanks also to Quarry Ridge Growers for providing experimental materials and advice. We also thank Dr Sue W. Si and Dr T. Barasubiye for the informative discussion on using molecular methods in identifying the pathogen.
References
- Armstrong , G.M. and Armstrong , J.K. 1981 . “ Formae speciales and races of Fusarium oxysporum causing wilt disease ” . In Fusarium: Disease, Biology, and Taxonomy , Edited by: Nelson , P.E. , Toussoun , T.A. and Cook , R.J. 391 – 399 . University Park , PA : Pennsylvania State University Press .
- Brand , T. and Wienberg , J. 2005 . Susceptibility of various begonia to Fusarium foetens . Gesunde Pflanzen , 57 : 27 – 29 .
- Dhingra , O.D. and Sinclair , J.B. 1985 . Basic Plant Pathology Methods , Boca Raton , FL : CRC Press, Inc .
- Elmer , W.H. 2008 . Preventing spread of fusarium wilt of Hiemalis begonias in the greenhouse . Crop Prot , 27 : 1078 – 1083 .
- Elmer , W.H. and Vossbrinck , C. 2004 . First report of a wilt disease of Hiemalis Begonias caused by Fusarium foetens in the United States . Plant Dis , 88 : 1287
- Geiser , D.M. , Jiménez-Gasco , M.M. , Kang , S. , Makalowska , I. , Veeraghavan , N. Ward , T.J. 2004 . FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium . Eur. J. Plant Pathol , 110 : 473 – 479 .
- Schroers , H.J. , Baayen , R.P. , Meffert , J.P. , Gruyter , J. , de, Hooftman , M. and O'donnell , K. 2004 . Fusarium foetens, a new species pathogenic to begonia elatior hybrids (Begonia × hiemalis) and the sister taxon of the Fusarium oxysporum species complex . Mycologia , 96 : 393 – 406 .
- Sekine , T. , Kanno , H. and Aoki , T. 2008 . Occurrence of leaf and stem rot caused by Fusarium foetens on begonia elatior hybrids (Begonia × hiemalis) . Jpn. J. Phytopathol , 74 : 164 – 166 .
- Smith , I.M. , Dunez , J. , Lelliott , R.A. , Phillips , D.H. and Archer , S.A. 1988 . European Handbook of Plant Diseases , Oxford : Blackwell Scientific Publications .
- Tian , X. , Dixon , M. and Zheng , Y. 2010 . First report of Hiemalis begonias wilt disease caused by Fusarium foetens in Canada . Plant Dis , 94 : 1261
- Wohanka , W. 2003 . Untersuchungen zur Ausbreitung einer neuen Fusariose an Elatiorbegonien bei Anstaubewasserung mit Langsamfiltration . Mitteilungen der Deutschen Phytomedizinischen Gesellschaft , 33 : 69 – 70 .