Abstract
In this study, microbial isolations were made from symptomatic Rhododendron plants from a large Finnish nursery, known to be harbouring Phytophthora based on PCR screenings. The nearby waterways were also sampled. A diversity of common Nordic plants was screened for Phytophthora susceptibility. Isolates recovered from Rhododendron plants included P. ramorum, P. cactorum, P. plurivora, P. pini and Pestalotiopsis sp. Baits floated in water samples from nearby waterways did not become infected with Phytophthora. In infection trials, all Phytophthora detected here were pathogenic to Rhododendron but nonpathogenic to Pinus sylvestris and Quercus robur. Phytophthora plurivora infected Betula pendula, Alnus glutinosa, Picea abies, Viburnum lentago, Vaccinium myrtillus, V. uliginosum, V. angustifolium and Fragaria × ananassa, the latter four species being new host records for this pathogen. Phytophthora ramorum caused small lesions on B. pendula, A. glutinosa and V. uliginosum, and more serious symptoms in Rhododendron, Viburnum lentago, V. lantana, Vaccinium myrtillus and V. angustifolium. Phytophthora pini was pathogenic to most plants tested, including Rhododendron, V. lentago and P. abies. In spite of an annual eradication programme in the nursery, P. ramorum was detected in annual samples taken during 2004–2010. Microsatellite analysis revealed that all isolates of P. ramorum belonged to the EU1 lineage.
Résumé
Dans cette étude, des isolements microbiens ont été obtenus à partir de rhododendrons symptomatiques provenant d'une grande pépinière finlandaise où Phytophthora avait été détecté par criblage par PCR. Des échantillons d'eau avaient également été prélevés dans les voies navigables voisines. Diverses plantes nordiques communes ont été criblées pour en évaluer la vulnérabilité à Phytophthora. Les isolats obtenus des rhododendrons incluaient P. ramorum, P. cactorum, P. plurivora, P. pini et Pestalotiopsis sp. Phytophthora n'a pas infecté les appâts placés dans les échantillons d'eau des voies navigables. Durant les tests d'infection, tous les isolats de Phytophthora qui ont été détectés ont infecté Rhododendron, mais pas Pinus sylvestris ni Quercus robur. Phytophthora plurivora a infecté Betula pendula, Alnus glutinosa, Picea abies, Viburnum lentago, Vaccinium myrtillus, V. uliginosum, V. angustifolium et Fragaria × ananassa, les quatre dernières espèces étant de nouvelles mentions d'hôte pour cet agent pathogène. Phytophthora ramorum a causé de petites lésions chez B. pendula, A. glutinosa et V. uliginosum, ainsi que des symptômes plus graves chez Rhododendron, Viburnum lentago, V. lantana, Vaccinium myrtillus et V. angustifolium. Phytophthora pini a infecté la plupart des plantes testées, y compris Rhododendron, V. lentago et P. abies. Malgré le programme annuel d'éradication appliqué à la pépinière, P. ramorum a été détecté dans les échantillons collectés annuellement de 2004 à 2010. L'analyse des microsatellites a révélé que tous les isolats de P. ramorum appartenaient à la lignée EU1.
Introduction
International trade and travel have facilitated the spread of harmful organisms around the world. Human-mediated movement of plants and plant products is now generally accepted to be the primary mode of introduction of pathogens and pests (Brasier, Citation2008). Species of Phytophthora are among the most common pathogens spreading in this manner and are known in nurseries across Europe and North America (Themann et al., Citation2002; Jung & Blaschke, Citation2004; Moralejo et al., Citation2009a ; Yakabe et al., Citation2009; Hulvey et al., Citation2010; Goss et al., Citation2011). The nursery trade has most likely played a key role in recent outbreaks of Sudden Oak Death (caused by Phytophthora ramorum De Cock & Man in 't Veld) in coastal Northwest USA, lethal disease of alders (caused by P. alni Brasier & S.A. Kirk) in most of Europe in addition to several other hosts (P. kernoviae Brasier, Beales & S.A. Kirk) in the UK, and the introduction of Port Orford Cedar Root Disease (caused by P. lateralis Tucker & Milbrath) into Europe (Rizzo et al., Citation2002; Brasier et al., Citation2004a , Citation2005; Jung & Blaschke, Citation2004; Robin et al., Citation2010). The expected climate change will also likely promote the spread and establishment of pathogen species into new areas (Brasier, Citation1996; Venette & Cohen, Citation2006; Sturrock et al., Citation2011).
Relatively few species of Phytophthora have so far been detected in Finnish nurseries. Of those, Phytophthora cactorum (Lebert and Cohn) J. Schröt is one that causes disease in woody and herbaceous plants throughout the world (Erwin & Ribeiro, Citation1996). In Finland, strains of P. cactorum have affected commercial production of strawberries (Fragaria × ananassa Duch.) and caused stem lesions and top dying in silver birch seedlings (Betula pendula Roth.) for over 20 years (Hantula et al., Citation1997, Citation2000).
Phytophthora ramorum was described in 2001 (Werres et al., Citation2001) and later identified as the causal agent of Sudden Oak Death (SOD) in California, Oregon and southwestern Canada (Rizzo et al., Citation2002, Citation2005; Davidson et al., Citation2005; Kliejunas, Citation2010). In Europe, P. ramorum has so far been detected in Belgium, Czech Republic, Denmark, Finland, France, Italy, Ireland, Norway, Poland, UK, Serbia, Slovenia, Spain, Sweden and Switzerland (EPPO, Citation2010) and recent isolations have been made in Estonia (Prof. M. Hanso, pers. comm.). The European findings are mainly from nurseries, gardens and parks, and mostly on rhododendron, viburnum (Viburnum spp.) and camellia (Camellia spp.). Phytophthora ramorum was also infrequently isolated from mature trees in Europe, but before 2009 always in the proximity of understorey rhododendrons (Anonymous, Citation2004a , Citation2004b ; Brasier et al., Citation2004b ). However, in 2009, P. ramorum was reported from a semi-natural environment in Europe, causing widespread dieback and mortality on introduced Japanese larch trees [Larix kaempferi (Lanb.) Carriere] in southwestern England, and later in 2010 on the same tree species in Wales, Northern Ireland and the Republic of Ireland (Brasier & Webber, Citation2010; Forestry Commission, Citation2010). Isolates of P. ramorum examined to date comprise three distinct lineages: the European EU1 and the North American NA1 and NA2 (Ivors et al., Citation2004, Citation2006; Grünwald et al., Citation2009). The European populations of P. ramorum consist solely of EU1 genotypes and represent predominantly the A1 mating type (Werres & DeMerlier, Citation2003; Grünwald et al., Citation2009; Vercauteren et al., Citation2010). The NA1 lineage responsible for SOD is the most widespread genotype in US nurseries (Ivors et al., Citation2006). NA2 isolates have been found in relatively few US nurseries, but it is the most common lineage in Canadian ornamentals (Goss et al., Citation2011).
In Finland, ornamentals are produced in roughly 800 nurseries encompassing c. 560 ha of greenhouse production area (TIKE Information Centre of Ministry of Agriculture and Forestry, Citation2011). Many of the cultivated ornamental species are known hosts of Phytophthora spp., such as azalea (Azalea spp.), rhododendron, lilac (Syringa spp.), rose (Rosa spp.), maple (Acer spp.), Viburnum spp., barberry (Berberis spp.), dogwood (Cornus spp.) and willow (Salix spp.). In 2004, the Finnish Food Safety Authority (EVIRA) found P. ramorum for the first time on rhododendrons in a domestic ornamental nursery. It was detected through the observation of typical symptoms and species-specific PCR (Lilja et al., Citation2007). In view of the damage occurring in the USA and the recent outbreak in the GB, there is a growing concern that the pathogen might spread into natural ecosystems also elsewhere in Europe.
The aims of this study were to: (i) determine the Phytophthora species present in symptomatic rhododendron plant lots found to be PCR-positive for P. ramorum; (ii) determine the possible escape of Phytophthora spp. from a nursery by sampling the nearby waterways; (iii) determine the origin(s) of isolated strains of P. ramorum; and (iv) screen a diversity of common Nordic plants for Phytophthora susceptibility.
Materials and methods
Sampling and isolation
EVIRA annually inspects c. 120 Finnish nurseries where possible host plants of P. ramorum are produced. The initial PCR-screenings using P. ramorum-specific primers were carried out according to the protocol of the European and Mediterranean Plant Protection Organization (EPPO) for regulated pests (EPPO 03/10563). We isolated microbes from 10–20 symptomatic rhododendron seedlings, which were collected during the inspections and represented sampling lots found to be PCR-positive for P. ramorum. Since only one nursery was found to be infested by the pathogen, all of our samples originated from the same nursery located in southwestern Finland. Isolations were made from samples collected during 2004–2007, 2009 and 2010. The viability of P. ramorum and the presence of other species were determined by plating sections from the margin of infected and healthy tissue onto modified PARP-agar (Erwin & Ribeiro, Citation1996).
Baiting and identification
To investigate the possible escape of Phytophthora from the nursery, 1 L water samples were taken from water bodies close to the nursery in April 2009. Two locations were sampled at a nearby lake, three locations at a nearby stream and one from a ditch alongside the nursery fields. Each sample was immediately distributed into plastic pots (25 × 25 × 10 cm) and placed in randomized order on a table at 22 ± 2 °C. Washed and surface-sterilized (sprayed with 70% ethanol and air-dried) green apples (‘Golden Delicious’) and juvenile rhododendron (Rhododendron catawbiense Michx) leaves were used as baits, with three apples and leaves floating in each box.
Isolates on MEA (5 g L−1 malt extract and 10 g L−1 agar, Difco, USA) were first sorted according to colony morphology. For morphological and genetic comparisons, we relied on a single reference strain (CBS 101326) of P. ramorum and reference publications. Also, sequences of β-tubulin coding region and the internal transcribed spacer 1 (ITS 1) of isolates representing different morphotypes of Phytophthora were determined and compared with sequences in GenBank using the BLAST program (Altschul et al., Citation1997).
DNA techniques
DNA was isolated from cultures representing different Phytophthora species and isolates as described by Vainio et al. (Citation1998). Primer pair Oom(Ph)-Btub-up901 and Oom-Btub-lo1401R (Bilodeau et al., Citation2007) was used to amplify a large part of the β-tubulin coding region. The reaction conditions were according to the manufacturer of the Dynazyme II DNA-polymerase, except that primers were at 25 μM. Samples were denaturated at 95 °C for 10 min, after which 37 amplification cycles were carried out (30 s denaturation at 95 °C, 45 s annealing at 53 °C, 2 min extension at 72 °C). The ITS1 region was amplified with primers ITS6 and ITS7 (Cooke et al., Citation2000) according to the temperature cycle given in Cooke et al. (Citation2000). The High Pure PCR purification kit (Roche, Germany) was used to purify amplification products prior to sequencing. Sequence analyses were conducted using Therm EXCEL™ II DNA sequencing kit-LC (for 66 cm gels) (Epicentre®) with labelled primer pairs Oom(Ph)-Btub-up901/Oom-Btub-lo1401R and ITS6/ITS7. Sequences were determined using an automated sequencer (LI-COR global edition IR2 system: LI-COR Inc. USA) following the manufacturer's instructions. BLAST searches were used to find similar sequences in GenBank.
Suitability of the Random Amplified Microsatellite (RAMS) technique was tested as an identification tool for P. ramorum genotypes by comparing them to reference isolates representing EU1, NA1 and NA2 lineages. RAMS loci were amplified from six P. ramorum isolates using RAMS primers DDB(CCA)5, VDD(TCC)5, VDV(CT)7C and DBV(CAT)5 (Hantula et al., Citation1996; Vainio & Hantula, Citation1999; Rytkönen et al., Citation2008, Citation2011). The temperature cycles consisted of 10 min denaturation at 95 °C and 37 cycles of amplification (30 s denaturation at 95 °C, 45 s annealing, 2 min primer extension at 72 °C). The annealing temperatures for the primers were 64 °C for DDB(CCA)5 and 50 °C for VDV(CT)7C, DBV(CAT)5 and VDD(TCC)5.
Finally, microsatellite loci 18, 64, 82a (Ivors et al., Citation2006), PrMS43 (Prospero et al., Citation2007), 82b, ILVOPrMS145a and ILVOPrMS145c (Vercauteren et al., Citation2010) were characterized to determine the microsatellite multilocus genotype of each P. ramorum isolate. Loci were amplified following the protocol of Vercauteren et al. (Citation2010) using universal fluorescent primers in a multiplex PCR reaction.
Pathogenicity
The pathogenicity of selected Phytophthora isolates was tested using the stem-wound inoculation method, which is shown to correlate with field symptoms in several hosts of P. ramorum (Hansen et al., Citation2005). Plant stem tissue was wounded with a surgical blade before adding the inoculum and a moist cotton pad, and wrapping with parafilm. In most cases, the wound was a leaf scar (a leaf or a needle was removed), but in some cases the plant was wounded with a sharp stick (Hantula et al., Citation2000). The inoculum was an agar plug cut from the margin of 2-week-old culture of Phytophthora spp. on malt extract agar (MEA) or from a pure MEA plate (control). The parafilm and the cotton were removed 3–4 days after inoculation. For the inoculations, we used isolates collected from rhododendrons in 2004 and 2007. The inoculated plants were 1-year-old container-grown seedlings of silver birch, common alder [Alnus glutinosa (L.) Gaertner], Norway spruce [Picea abies (L.) Karst.], Scots pine (Pinus sylvestris L.), 3-year-old sheepberry viburnum (Viburnum lentago L.), wayfaring tree viburnum (V. lantana L.) and 6-year-old English oaks (Quercus robur L.). Also 1.5-year-old micropropagated plants were inoculated, including rhododendron ‘Elvira’ Tigersted (Repens-group), blueberry (Vaccinium myrtillus L.), bog bilberry (V. uliginosum L), lowbush blueberry (V. angustifolium L.) and 2-month-old strawberry runner plants (‘Jonsok’). Each seedling was separately replanted in 5–8 dl of Sphagnum peat growth medium (Vapo). The number of seedlings inoculated with each isolate varied (6–15). In each trial, seedlings were arranged after inoculation randomly in a greenhouse maintained at 20–24 °C. Each plant species was grown separately. The health condition of seedlings was assessed for 4 weeks using a rating of 1 to 4 where: 1 = no lesion, 2 = lesion covering less than half of the stem perimeter, 3 = lesion spread over half of the stem perimeter but not girdling the stem, 4 = lesion girdling the stem. Since the disease development on strawberry seedlings was different, the condition of plants was marked with: 1 = healthy, 2 = moderate disease symptoms, 3 = severe disease symptoms and 4 = nearly dead or dead plant.
Statistical analysis
Statistical analysis of differences in pathogenicity between different species and/or control relied on a likelihood ratio test for a multinomial response at the 95% confidence level. The null hypothesis in this setting is that the probability distribution of the four ratings is equal among a given set of species, and the alternative is that for at least one species the distribution is different. The test statistic is D = −2ln(R) where
where in turn f i is the total number of observations in category i, f ji is the number of observations related to species j in category i, p i and p ji are the corresponding symptom class frequencies f i/n and f ji/n j, respectively, and finally n j is the total number of observations related to species j, which sum to n, the total number of observations. The test statistic follows asymptotically the chi squared distribution with 3(j−1) degrees of freedom. This test is equivalent to a chi squared independence test. The frequency of serious symptoms (rating 3 or 4) was used as a more simple one-dimensional measure of pathogenicity. The maximum likelihood estimate of this is (f j3 + f j4)/n j, using the notation above.
Results
Identity of the isolates
Only one of the nurseries inspected by EVIRA 2004–2010 was found to harbour P. ramorum. In this nursery, PCR screening with P. ramorum-specific primers gave a positive result every year. It was also possible to isolate mycelial cultures of P. ramorum from the rhododendron samples every year except 2007 (). Other Phytophthora species isolated were P. cactorum, P. plurivora T. Jung & T.I. Burgess and P. pini L.H. Leonian (). The most common fungus isolated was Pestalotiopsis sp. which was not identified to species level. Only a few lesions formed on the baits floating in the water samples originating from the nearby waterways, and only Pythium spp. was isolated from the lesions.
Table 1. Microbes isolated from symptomatic rhododendrons in a Finnish nursery in 2004, 2005, 2006, 2007, 2009 and 2010
The morphological characteristics i.e. the dimensions and presence of oogonia, chlamydospores and sporangia of isolated Phytophthora spp. corresponded with descriptions given in the literature (Erwin & Ribeiro, Citation1996; Werres et al., Citation2001; Jung & Burgess, Citation2009; Hong et al., Citation2011). The β-tubulin and ITS 1 sequences of our P. cactorum isolates were identical to the most common P. cactorum sequences in GenBank. Isolates of P. ramorum were identical with P. ramorum CBS 109279 and CBS 101327. Originally, isolates Ph411, Ph412, Ph481 and Ph515 were identified by their common β-tubulin sequence, which was identical to P. inflata strain IMI 342898 (Lilja et al., Citation2007). However, ITS 1 sequences and the recent taxonomic revision of the P. citricola species complex (Jung & Burgess, Citation2009) allowed their more precise identification as P. plurivora and P. pini (one isolate). Incidentally, P. pini is considered to be a synonym of P. citricola I (Hong et al., Citation2011). The GenBank accession numbers for the Phytophthora sequences determined in this study are given in .
Table 2. GenBank accession numbers for the DNA sequences of Phytophthora isolates used in this study
RAMS fingerprinting of the P. ramorum isolates with four primers DDB(CCA)5, VDD(TCC)5, VDV(CT)7C and DBV(CAT)5 resulted in identical fingerprints in all of the isolates (data not shown). Thus, the method was not successful in separating EU and NA lineages of P. ramorum. Finally, microsatellite analysis of seven loci (18, 64, 82a, 82b, PrMS43, ILVOPrMS145a and ILVOPrMS145c) revealed that all P. ramorum isolates belonged to the EU1 lineage. One of seven markers used was polymorphic in the Finnish population of P. ramorum and only two intra-lineage genotypes (MG groups) were identified. The main EU1 genotype (EU1MG1) dominates the population [isolates Ph390 (2004), Ph405 (2005), Ph409 (2005), Ph455 (2009) and Ph456 (2009)], and genotype EU1MG18 (isolate Ph426) was only found in 2006.
Pathogenicity
All isolates caused clear lesions on rhododendron cultivar ‘Elvira’, although lesions caused by two P. cactorum isolates (Ph407 and Ph440) were relatively small ( e). Phytophthora plurivora was able to infect most of the tested host plants including silver birch ( a), common alder ( b), grey alder ( c), Norway spruce ( d), sheepberry ( f), wayfaring tree ( g), blueberry ( h), bog bilberry ( i), lowbush blueberry ( j) and strawberry ( k).
Fig. 1a–k. The percentage of seedlings in different health classes four weeks after inoculation with different isolates of Phytophthora cactorum (CAC), P. plurivora (PLU), P. ramorum (RAM) or P. pini (PIN) from rhododendron. Wound inoculated plant species were (a) silver birch, (b) common alder, (c) grey alder, (d) Norway spruce, (e) rhododendron, (f) sheepberry, (g) wayfaring tree, (h) blueberry, (i) bog bilberry, (j) lowbush blueberry and (k) strawberry. Controls were inoculated with pure ME agar plug.
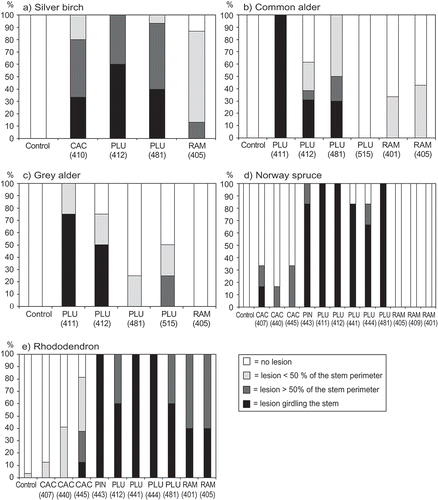
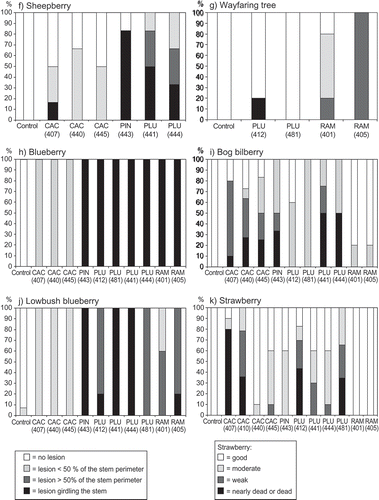
Phytophthora ramorum was able to cause lesions on silver birch ( a), common alder ( b) and bog bilberry ( i), but the most serious symptoms were observed on rhododendron ( e), wayfaring tree ( g), blueberry ( h) and lowbush blueberry ( j). Phytophthora pini caused large lesions in all species it was tested, excluding strawberry, on which it was only slightly pathogenic ( d, e, f, h, i and j). None of the four Phytophthora species produced symptoms on Scots pine or Pedunculate oak in our trials. At least one isolate of each species except P. ramorum caused lesions on strawberry. The pathogenicity of P. cactorum isolates to strawberry varied considerably ( k), and the most pathogenic isolate to strawberry (P. cactorum Ph407) was only slightly pathogenic to rhododendron ( k and e).
The frequencies of serious symptoms (lesions covering over half of the stem perimeter) caused by the different Phytophthora species on various plant species are given in . There were significant differences between the pathogenicity of the four Phytophthora species in almost all host plants tested ().
Table 3. The abilities of the Phytophthora species to cause serious symptoms on various plant species tested in this study (i.e. the frequencies of test plants with a lesion covering over half of the stem perimeter), and their 95% confidence intervals
Discussion
EVIRA annually inspected c. 120 Finnish nurseries during the study period, and one of them was found to harbour P. ramorum. Since the first observation, P. ramorum-specific PCR has given a positive result each year. We were also able to isolate P. ramorum together with other Phytophthora species from lesions on rhododendron plants in 2004–2007, 2009 and 2010. Phytophthora ramorum is subject to emergency European Commission (EC) phytosanitary measures, which means that all rhododendron seedlings in addition to other plants and growth media are destroyed each year in the infected site. However, consecutive isolations indicate that the pathogen remained in the nursery in spite of an annual sanitation programme. Persistence of P. ramorum in specific sites despite hygiene and eradication measures has also been reported in Belgium (Vercauteren et al., Citation2010). These findings show the failure of the EU commission sanitation protocols (2002/757/EY) to prevent the spread and establishment of P. ramorum.
Recent technology has revealed many morphospecies of Phytophthora to be composed of complexes (Hansen et al., Citation2009; Jung & Burgess, Citation2009; Scott et al., Citation2009; Hong et al., Citation2011). Molecular and isozyme studies have been used to investigate and parse diversity in the P. citricola complex (Oudemans et al., Citation1994; Kong et al., Citation2003; Gallegly & Hong, Citation2008; Jung & Burgess, Citation2009; Scott et al., Citation2009; Bezuidenhout et al., Citation2010). It has since been divided into seven subclades, among which new species (P. plurivora, P. multivora P.M. Scott & T. Jung and P. capensis C.M. Bezuidenhout, Denman, A. McLeod & S.A. Kirk) have been described. Our P. plurivora isolates were originally misidentified as P. inflata (Lilja et al., Citation2007), but have since been shown to be P. plurivora, which was separated from the morphospecies P. citricola and described in 2009 (Jung & Burgess, Citation2009). P. citricola subgroup I was recently found to be identical to P. pini, a species described by Leonian (Citation1925), then merged with P. citricola by Waterhouse in Citation1963 and finally resurrected to species level by Hong et al. (Citation2011). Here only one isolate proved to be P. pini.
Phytophthora plurivora is abundant in forests, semi-natural ecosystems and nurseries of Western, Central, Eastern and Southern Europe and is also found in the eastern USA and Canada, where it causes bark necroses, fine root losses and dieback on at least 45 woody host species, including Pedunculate oak (Jung & Blaschke, Citation1996; Jung et al., Citation2003; Jung & Burgess, Citation2009) and Common alder (Jung & Blaschke, Citation2004; Jung & Nechwatal, Citation2008). Phytophthora plurivora has also recently been found to be present in Denmark and Norway where it causes dieback and mortality in several deciduous tree species (Thinggaard, Citation2009; Talgø et al., Citation2009). Phytophthora pini is widespread in the eastern USA where it damages and kills the introduced species European beech (Fagus sylvatica L.) and Common lilac (Syringa vulgaris L.) (Jung et al., Citation2005; Weiland et al., Citation2010; Hong et al., Citation2011), but in Europe it has only been found in nurseries, indicating a very recent introduction to the continent (Jung & Burgess, Citation2009). It is a new species to Finland and other Nordic countries. Both of the above-mentioned species have also been found in forest streams of Oregon (Reeser et al., Citation2011). However, baiting tests of samples taken from nearby water bodies in our study resulted only in isolates of Pythium spp. which are common in natural water bodies (Hamm et al., Citation1988). Nonetheless, the baiting method chosen can also affect the species that are recovered. For example, in the study by Reeser et al. (Citation2011), a slightly different assortment of Phytophthora species was recovered by pear fruit baits than by using foliar baits or a filtration method. It is possible that Pythium species can also outcompete some slow-growing Phytophthora species after plating; thus, using a selection of different baiting methods would increase the success of recovering Phytophthora. Another species isolated from Rhododendron plants in this study was P. cactorum, which has been present in Finland since 1990 and caused losses in both agriculture and forestry and was found to be present in a natural pond (Hantula et al., Citation1997, Citation2000; Rytkönen et al., Citation2008).
Also Pestalotiopsis spp. were commonly isolated from our Rhododendron samples. The taxonomy of this genus and its relation to Pestalotia are not clear according to current knowledge (Maharachchikumbura et al., Citation2011), but representatives of these genera are known as weak parasites and endophytes in various ornamentals and other cultivated plants including Rhododendron (e.g. Guba et al., Citation1932; Xu et al., Citation2010; Aly et al., Citation2011; Maharachchikumbura et al., Citation2011). We report Pestalotiopsis as the most common fungal genus isolated from Finnish domestic production of rhododendrons.
In this study, one aim was to assign P. ramorum isolates to EU or NA lineages, since micropropagated plants have been imported to the nursery both from Europe and the USA. Recent studies on the mating type and population distribution of the three distinct lineages of P. ramorum suggest that separate introductions may have occurred to Europe and the USA and thus it should be possible to identify the origin of isolates (Brasier, Citation2003; Rizzo et al., Citation2005; Goss et al., Citation2009; Prospero et al., Citation2009). First we attempted to identify lineages with RAMS fingerprinting, a method which we have successfully used earlier with P. cactorum and other pathogens (Hantula et al., Citation1997, Citation2000; Rytkönen et al., Citation2008, Citation2011). However, RAMS fingerprinting could not detect any intraspecific variation among our isolates and the European and North American reference isolates of P. ramorum, perhaps due to the relative uniformity of the introduced pathogen. Because RAMS was deemed to be rather inefficient, the microsatellite multilocus genotype of each isolate was determined with seven markers, resulting in five of six P. ramorum isolates being identified as the EU1MG1 genotype. Vercauteren and colleagues (Citation2010) showed that the Belgian population of P. ramorum was dominated (68%) by the same genotype (EU1MG1). This was suggested to be the original genotype introduced to Belgium whereas other less common genotypes were probably derived from it (Vercauteren et al. Citation2010). Strain EU1MG1 might be considered the most widespread genotype in Europe, and probably representative of the original founder population.
Risk analyses assume that the consequences of pest introduction are positively correlated with the host range of the pest (Cave et al., Citation2007). A good example of this assumption is P. ramorum, which has several hosts in many different plant families (Denman et al., Citation2005; Hansen et al., Citation2005). Our P. ramorum isolates caused stem lesions in over 80% of inoculated silver birch and at least one third of the inoculated common alder seedlings. In inoculation trials conducted by Denman et al. (Citation2005) with detached foliage, P. ramorum was also pathogenic to birch and alder. In our tests, more serious symptoms were observed on blueberries, almost all of which died after inoculation. Also, 20% of bog bilberries produced necrotic tissue at inoculation points. Some variation in aggression between different isolates of P. ramorum was observed on wayfaring trees, but still at least 80% of inoculated plants developed necrotic lesions. All these plant species are native and abundant in Finnish nature. As such, P. ramorum would find suitable hosts if it escapes a nursery.
Detached needle assays have suggested that Norway spruce might be susceptible to P. ramorum, and Scots pine is most likely resistant (Denman et al., Citation2005). However, wound inoculations have resulted in small-size lesions on pine logs (Moralejo et al., Citation2009b ). The more effective defence of living seedlings could account for the resistance of both conifers observed in this study, as well as the different susceptibility of needle tissue compared with the suberized stems of seedlings. Thus, Scots pine, which is the dominant tree species in Finland, is not likely to be infected by P. ramorum. In the case of Norway spruce, although the pathogen might be able to colonize individual needles (Denman et al., Citation2005), the formation of stem cankers is unlikely. However, when considering the consequences of the possible introduction of P. ramorum to Finnish natural ecosystems, it should be taken into account that the climate matching model (CLIMEX) predicts that the risk is low for establishment and epidemic spread in Northern Europe under current climatic conditions (RAPRA, Citation2009).
Among other diseases, P. cactorum causes crown rot in Finnish strawberries (Hantula et al., Citation1997, Citation2000). Micropropagated strawberries are cultivated in the infected nursery. Thus, it was not surprising to isolate P. cactorum from rhododendrons which are known to attract Phytophthora (Erwin & Ribeiro, Citation1996). However, the pathogenicity of different P. cactorum isolates varied on strawberry plants ( k). Isolates Ph407 and Ph410 (isolated in 2005) caused more severe symptoms to strawberry than Ph440 and Ph445 (isolated in 2007). According to previous investigations, Finnish P. cactorum isolates from strawberry and birch are morphologically distinguishable in terms of the relative size of oogonia, oospores and sporangia. Also, DNA fingerprinting placed isolates from birch and European strawberry into separate clusters (Hantula et al., Citation1997, Citation2000; Lilja et al., Citation1998). Furthermore, isolates from birch are not pathogenic to strawberry (Hantula et al., Citation1997, Citation2000; Lilja et al., Citation1998). Here, all P. cactorum isolates were pathogenic to rhododendron, but as previously told, differed in their pathogenicity towards strawberry. More isolates and tests would reveal the underlying patterns of host specificity. Our isolates likely represent at least two separate introductions of different P. cactorum genotypes, although this was not tested by molecular means. According to a Swedish study (Molin et al., Citation1960), P. cactorum was found to cause damping off in young Scots pine seedlings. In our trials, Scots pine was resistant. Seedling age, genotype and pathogen strain might affect the success of infection.
In our pathogenicity trials, P. plurivora was able to infect most plants tested. It also had more hosts than P. ramorum (). Weiland et al. (Citation2010) compared the pathogenicity of P. cactorum, P. plurivora and P. pini to European beech and common lilac. Isolates of P. cactorum were the least aggressive and caused less necrosis than isolates of P. pini and P. plurivora (Weiland et al., Citation2010). Also, our results indicate that P. pini and P. plurivora cause quicker-advancing infections and more severe symptoms than P. cactorum on silver birch, Norway spruce, blueberry, highbush blueberry, strawberry and rhododendron. It was also evident that P. pini and P. plurivora were the most aggressive species on Norway spruce. Our results in addition to those of Luedemann et al. (Citation2005) and Nechwatal & Oßwald (Citation2001) suggest that at least juvenile trees and seedlings are vulnerable to these pathogens. Phytophthora plurivora (under the previous name P. citricola) has also been isolated in a German nursery from P. abies plants showing natural infection with symptoms such as chlorosis, dieback and mortality (Jung & Blaschke, Citation2004). Phytophthora plurivora was the most aggressive species compared with P. cactorum and P. ramorum towards all of the tested species except wayfaring tree and bog bilberry (). P. pini had similar infection patterns to P. plurivora, except that it was not able to infect strawberry.
In our pathogenicity trials, none of the four Phytophthora species were able to infect Q. robur. The results of Jung & Blaschke (Citation1996) as well as Brasier & Jung (Citation2003) showed that P. plurivora (under its previous name P. citricola) was aggressive to the bark of 5-year-old Q. robur seedlings and mature Q. robur stems, causing extensive bark lesions within 3 months and 5 weeks, respectively. The duration of the assessments in the pathogenity test here was 4 weeks, which might have not been enough time to induce symptoms on the 6-year-old Q. robur seedlings; this could also be the case with P. sylvestris. Scots pine was also resistant to all isolates of Phytophthora tested. However, varieties of Scots pine can exhibit considerable variation in resistance towards, for example, Sphaeropsis sapinea (Fr.) Dyco et Sutton (Gerhold et al., Citation1994), so it would be prudent to include more seedlings from different provenances or clones and strains of the various of Phytophthora species in further pathogenicity trials.
Generally, it has to be noted that the susceptibility of fine roots and suberized bark tissue can differ significantly, and several devastating tree declines in natural or semi-natural ecosystems are primarily driven by Phytophthora-caused fine root losses. Therefore, soil infestation trials for testing the susceptibility of fine root systems of Nordic tree species to P. cactorum, P. plurivora, P. pini and P. ramorum will be an important subject for future research.
The results presented here indicate that species of Phytophthora isolated from rhododendron pose a disease threat to woody species in commercial and natural situations. Phytophthora species can be considered a threat to forest trees and seedlings due to their aggressive infection of one or more hosts (Rizzo et al., Citation2002; Brasier et al., Citation2004a , Citation2005; Jung & Blaschke, Citation2004; Jung & Burgess, Citation2009; Robin et al., Citation2010). The ability of P. ramorum to remain in the nursery in spite of an annual sanitation programme and the escape of P. plurivora from a nursery into a seminatural ecosystem in Norway (Talgø et al., Citation2009) underline the seriousness of the threat posed by these pathogens. Furthermore, Phytophthora spp. currently enjoy human-mediated dispersal to new locations with non-adapted susceptible vegetation (Brasier, Citation2008; Goss et al., Citation2011) where climate change will most likely favour their survival and their ability to spread, infect and cause damage (Sturrock et al., Citation2011). However, for a complete picture of the impact of these pathogens, the significance of possible soil-borne inoculum and root susceptibility should be further investigated. It is highly likely that species of Phytophthora will establish themselves in Finland and other Nordic countries unless efficient phytosanitary control in the international plant trade is implemented.
Acknowledgements
We acknowledge the help given to us by Mrs Marjaana Virtanen and Ritva Vanhanen. We would also like to thank Dr Michael Hardman who revised the English and improved the text. The study was partly supported by Suomen Kulttuurirahasto. We also acknowledge the European Union COST FP0801 project, which has given us the possibility to get to know recent results in Phytophthora research and to cooperate with Dr Annelies Vercauteren. We thank also Jussi Nuutinen and Suonenjoki nursery for providing seedlings.
References
- Altschul , S.A. , Madden , T.L. , Schäffer , A.A. , Zhang , J. , Zhang , Z. , Miller , W. and Lipman , D.J. 1997 . Gapped BLAST and PSI-BLAST: a new generation of protein database search programs . Nucleic Acids Res , 25 : 3389 – 3402 .
- Aly , A.H. , Debbab , A. and Proksch , P. 2011 . Fungal endophytes: unique plant inhabitants with great promises . Appl. Microbiol. Biotechnol , 90 : 1829 – 1845 .
- Anonymous . 2004a . Sudden Oak death (Phytophthora ramorum) discovered on trees in Europe . Mycol. Res , 108 : 1108 – 1109 .
- Anonymous . 2004b . Phytophthora ramorum in Amerikaanse eik . Gewasbescherming , 35 : 126
- Bezuidenhout , C.M. , Denman , S. , Kirk , S.A. , Botha , W.J. , Mostert , L. and McLeod , A. 2010 . Phytophthora taxa associated with cultivated Agathosma, with emphasis on the P. citricola complex and P. capensis sp. nov . Persoonia , 25 : 32 – 49 .
- Bilodeau , G.J. , Levesque , C.A. , de Cock , A.W.A.M. , Duchaine , C. , Briere , S. Uribe , P. 2007 . Molecular detection of Phytophthora ramorum by real-time polymerase chain reaction using TaqMan, SYBR Green, and molecular beacons . Phytopathology , 97 : 632 – 642 .
- Brasier , C. and Webber , J. 2010 . Plant pathology: Sudden larch death . Nature , 466 : 824 – 825 .
- Brasier , C.M. 1996 . Phytophthora cinnamomi and oak decline in southern Europe. Environmental constraints including climate change . Ann. Sci. Forest , 53 : 347 – 358 .
- Brasier , C.M. 2003 . Sudden oak death exhibits transatlantic differences . Mycol. Res , 107 : 258 – 259 .
- Brasier , C.M. 2008 . The biosecurity threat to the UK and global environment from international trade in plants . Plant Pathol , 57 : 792 – 808 .
- Brasier , C.M. and Jung , T. 2003 . “ Progress in understanding Phytophthora diseases of trees in Europe ” . In Phytophthora in Forests and Natural Ecosystems , Edited by: McComb , J. , Hardy , G. and Tommerup , I. 4 – 18 . Perth , WA : Murdoch University Press .
- Brasier , C.M. , Kirk , S.A. , Delcan , J. , Cooke , D.E.L. , Jung , T. and Man in't Veld , W.A. 2004a . Phytophthora alni sp. nov. and its variants: designation of emerging heteroploid hybrid pathogens spreading on Alnus trees . Mycol. Res , 108 : 1172 – 1184 .
- Brasier , C.M. , Denman , S. , Rose , J. , Kirk , S.A. , Hughes , K.J.D. Griffin , R.L. 2004b . First report of ramorum bleeding canker on Quercus falcata caused by Phytophthora ramorum . Plant Pathol , 53 : 804
- Brasier , C.M. , Beales , P.A. , Kirk , S.A. , Denman , S. and Rose , J. 2005 . Phytophthora kernoviae sp. nov. an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis on ornamentals in the UK . Mycol. Res , 109 : 853 – 859 .
- Cave , G.L. , Randall-Schadel , B. and Redlin , S.R. 2007 . Risk analysis for Phytophthora ramorum Werres, de Cock & In't Veld, causal agent of Sudden Oak Death, Ramorum leaf blight, and Ramorum dieback , 87 USDA-APHIS-PPQ .
- Cooke , D.E.L. , Drenth , A. , Duncan , J.M. , Wagels , G. and Brasier , C.M. 2000 . A molecular phylogeny of Phytophthora and related oomycetes . Fungal Genet. Biol , 30 : 17 – 32 .
- Davidson , J.M. , Wickland , A.C. , Patterson , H.A. , Falk , K.R. and Rizzo , D.M. 2005 . Transmission of Phytophthora ramorum in mixed-evergreen forest in California . Phytopathology , 95 : 587 – 596 .
- Denman , S. , Kirk , S.A. , Brasier , C.M. and Webber , J.F. 2005 . In vitro leaf inoculation studies as an indication of tree foliage susceptibility to Phytophthora ramorum in the UK . Plant Pathol , 54 : 512 – 521 .
- EPPO (2010). Data sheets on quarantine pests Phytophthora ramorum http://www.eppo.org/QUARANTINE/Alert_List/fungi/PHYTRA.htm (http://www.eppo.org/QUARANTINE/Alert_List/fungi/PHYTRA.htm)
- Erwin , D.C. and Ribeiro , O.K. 1996 . “ Phytophthora ” . In Diseases Worldwide , 562 St. Paul , MN : The American Phytopathological Society (APS) Press .
- Forestry Commission (2010). Phytophthora ramorum http://www.forestry.gov.uk/pramorum (http://www.forestry.gov.uk/pramorum)
- Gallegly , M.E. and Hong , C. 2008 . “ Phytophthora ” . In Identifying species by morphology and DNA fingerprints , 158 St. Paul , MN : The American Phytopathological Society (APS) Press .
- Gerhold , H.D. , Rhodes , H.L.H. and Wenner , N.G. 1994 . Screening Pinus sylvestris for resistance to Sphaeropsis sapinea . Silvae Genet , 43 : 333 – 337 .
- Goss , E.M. , Larsen , M. , Chastagner , G.A. , Givens , D.R. and Grünwald , N.J. 2009 . Population genetic analysis infers migration pathways of Phytophthora ramorum in US nurseries . Plos Pathog , 5 : e1000583
- Goss , E.M. , Larsen , M. , Vercauteren , A. , Werres , S. , Heungens , K. and Gruenwald , N.J. 2011 . Phytophthora ramorum in Canada: evidence for migration within North America and from Europe . Phytopathology , 101 : 166 – 171 .
- Grünwald , N.J. , Goss , E.M. , Ivors , K. , Garbelotto , M. , Martin , F.N. , Prospero , S. and Hansen , E. 2009 . Standardizing the nomenclature for clonal lineages of the Sudden Oak Death pathogen, Phytophthora ramorum . Phytopathology , 99 : 792 – 795 .
- Guba , E.F. 1932 . Monograph of the genus Pestalotia: Part II . Mycologia , 24 : 355 – 397 .
- Hamm , P.B. , Hansen , E.M. , Hennon , P.E. and Shaw , C.G. 1988 . Pythium species from forests and muskeg areas of Southeast Alaska . Trans. Br. Mycol. Soc , 91 : 385 – 388 .
- Hansen , E.M. , Parke , J.L. and Sutton , W. 2005 . Susceptibility of Oregon forest trees and shrubs to Phytophthora ramorum: a comparison of artificial inoculation and natural infection . Plant Dis , 89 : 63 – 70 .
- Hansen , E.M. , Wilcox , W.F. and Reeser , P.W. 2009 . Phytophthora rosacearum and P. sansomeana, new species segregated from the Phytophthora megasperma “complex” . Mycologia , 101 : 129 – 135 .
- Hantula , J. , Dusabenyagasani , M. and Hamelin , R.C. 1996 . Random amplified microsatellites (RAMS) – a novel method for characterizing genetic variation within fungi . Eur. J. Forest Pathol , 26 : 159 – 166 .
- Hantula , J. , Lilja , A. and Parikka , P. 1997 . Genetic variation and host specificity of Phytophthora cactorum in Europe . Mycol. Res , 101 : 565 – 572 .
- Hantula , J. , Lilja , A. , Nuorteva , H. , Parikka , P. and Werres , S. 2000 . Pathogenicity, morphology and genetic variation of Phytophthora cactorum from strawberry, apple, rhododendron and silver birch . Mycol. Res , 104 : 1062 – 1068 .
- Hong , C. , Gallegly , M.E. , Richardson , P.A. and Kong , P. 2011 . Phytophthora pini Leonian resurrected to distinct species status . Mycologia , 103 : 351 – 360 .
- Hulvey , J. , Gobena , D. , Finley , L. and Lamour , K. 2010 . Co-occurrence and genotypic distribution of Phytophthora species recovered from watersheds and plant nurseries of eastern Tennessee . Mycologia , 102 : 1127 – 1133 .
- Ivors , K.I. , Hayden , K.J. , Bonants , P.J.M. , Rizzo , D.M. and Garbelotto , M. 2004 . AFLP and phylogenetic analyses of North American and European populations of Phytophthora ramorum . Mycol. Res , 108 : 378 – 392 .
- Ivors , K.I. , Garbelotto , M. , Vries , I.D.E. , Ruyter-Spira , C. , Hekkert , B.T. , Rosenzweig , N. and Bonants , P. 2006 . Microsatellite markers identify three lineages of Phytophthora ramorum in US nurseries, yet single lineages in US forest and European nursery populations . Mol. Ecol , 15 : 1493 – 1505 .
- Jung , T. and Blaschke , M. 1996 . Phytophthora root rot in declining forest trees . Phyton , 36 : 95 – 102 .
- Jung , T. and Blaschke , M. 2004 . Phytophthora root and collar rot of alders in Bavaria: distribution, modes of spread and possible management strategies . Plant Pathol , 53 : 197 – 208 .
- Jung , T. and Burgess , T.I. 2009 . Re-evaluation of Phytophthora citricola isolates from multiple woody hosts in Europe and North America reveals a new species, Phytophthora plurivora sp. nov . Persoonia , 22 : 95 – 110 .
- Jung , T. and Nechwatal , J. 2008 . Phytophthora gallica sp. nov. a new species from rhizosphere soil of declining oak and reed stands in France and Germany . Mycol. Res , 112 : 1195 – 1205 .
- Jung , T. , Nechwatal , J. , Cooke , D.E.L. , Hartmann , G. , Blaschke , M. Osswald , W.F. 2003 . Phytophthora pseudosyringae sp. nov. a new species causing root and collar rot of deciduous tree species in Europe . Mycol. Res , 107 : 772 – 789 .
- Jung , T. , Hudler , G.W. , Jensen-Tracy , S.L. , Griffiths , H.M. and Obwald , W. 2005 . Involvement of Phytophthora species in the decline of European beech in Europe and the USA . Mycologist , 19 : 159 – 166 .
- Kliejunas , J.T. 2010 . Sudden Oak Death and Phytophthora ramorum: A summary on the Literature , 2010 , 181 Albany , CA : Department of Agriculture, Forest Service, Pacific Southwest Research Station . Gen. Tech. Rep. PSW-GTR-234
- Kong , P. , Hong , C. , Richardson , P.A. and Gallegly , M.E. 2003 . Single-strand-conformation polymorphism of ribosomal DNA for rapid species differentiation in genus Phytophthora . Fungal Genet. Biol , 39 : 238 – 249 .
- Leonian , L.H. 1925 . Physiological studies on the genus Phytophthora . Am. J. Bot , 12 : 444 – 498 .
- Lilja , A. , Karjalainen , R. , Parikka , P. , Kammiovirta , K. and Nuorteva , H. 1998 . Pathogenicity and genetic variation of Phytophthora cactorum from silver birch and strawberry . Eur. J. Plant Pathol , 104 : 529 – 535 .
- Lilja , A. , Rytkönen , A. , Kokkola , M. , Parikka , P. and Hantula , J. 2007 . First report of Phytophthora ramorum and P. inflata in ornamental rhododendrons in Finland . Plant Dis , 91 : 1055
- Luedemann , G. , Matyssek , R. , Fleischmann , F. and Grams , T.E.E. 2005 . Acclimation to ozone affects host/pathogen interaction and competitiveness for nitrogen on juvenile Fagus sylvatica and Picea abies trees infected with Phytophthora citricola . Plant Biol , 7 : 640 – 649 .
- Maharachchikumbura , S.S.N. , Guo , L.D. , Chukeatirote , E. , Bahkali , A.H. and Hyde , K.D. 2011 . Pestalotiopsis-morphology, phylogeny, biochemistry and diversity . Fungal divers , 50 : 167 – 187 .
- Molin , N. , Persson , M. and Persson , S. 1960 . Root parasites on forest tree seedlings . Meddelanden från Statens Skogsforskningsinstitut , 49 : 1 – 17 .
- Moralejo , E. , Perez-Sierra , A.M. , Alvarez , L.A. , Belbahri , L. , Lefort , F. and Descals , E. 2009a . Multiple alien Phytophthora taxa discovered on diseased ornamental plants in Spain . Plant Pathol , 58 : 100 – 110 .
- Moralejo , E. , Garcia-Muñoz , J.A. and Descals , E. 2009b . Susceptibility of Iberian trees to Phytophthora ramorum and P. cinnamomi . Plant Pathol , 58 : 271 – 283 .
- Nechwatal , J. and Oßwald , W. 2001 . Comparative studies on the fine root status of healthy and declining spruce and beech trees in the Bavarian Alps and occurrence of Phytophthora and Pythium species . Forest Pathol , 31 : 257 – 273 .
- Oudemans , P. , Föster , H. and Coffey , M.D. 1994 . Evidence for distinct isozyme subgroups within Phytophthora citricola and close relationships with P. capsici and P. citrophora . Mycol. Res , 98 : 189 – 199 .
- Prospero , S. , Hansen , E.M. , Grunwald , N.J. and Winton , L.M. 2007 . Population dynamics of the sudden oak death pathogen Phytophthora ramorum in Oregon from 2001 to 2004 . Mol. Ecol , 16 : 2958 – 2973 .
- Prospero , S. , Gruenwald , N.J. , Winton , L.M. and Hansen , E.M. 2009 . Migration patterns of the emerging plant pathogen Phytophthora ramorum on the west coast of the United States of America . Phytopathology , 99 : 739 – 749 .
- Reeser , P.W. , Sutton , W. , Hansen , E.M. , Remigi , P. and Adams , G.C. 2011 . Phytophthora species in forest streams in Oregon and Alaska . Mycologia , 103 : 22 – 35 .
- RAPRA. Risk analysis of Phytophthora ramorum, a newly recognised pathogen threat to Europe and the cause of Sudden Oak Death in the USA (2009). EU Sixth Framework Project, deliverable report D28. http://rapra.csl.gov.uk/RAPRA-PRA_26feb09.pdf (http://rapra.csl.gov.uk/RAPRA-PRA_26feb09.pdf)
- Rizzo , D.M. , Garbelotto , M. , Davidson , J.M. and Slaugter , G.W. 2002 . Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California . Plant Dis , 86 : 205 – 214 .
- Rizzo , D.M. , Garbelotto , M. and Hansen , E.A. 2005 . Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests . Annu. Rev. Phytopathol , 43 : 309 – 335 .
- Robin , C. , Prou , D. , Feau , N. , Douzon , G. , Schenck , N. and Hansen , E.M. 2010 . Root and aerial infections of Chamaecyparis lawsoniana by Phytophthora lateralis: a new threat for European countries . Forest Pathol , 41 : 417 – 424 .
- Rytkönen , A. , Lilja , A. , Petäistö , R.-L. and Hantula , J. 2008 . Irrigation water and Phytophthora cactorum in a forest nursery . Scand. J. Forest Res , 23 : 404 – 411 .
- Rytkönen , A. , Lilja , A. , Drenkhan , R. , Gaitnieks , T. and Hantula , J. 2011 . First record of Chalara fraxinea in Finland and genetic variation among isolates sampled from Åland, mainland Finland, Estonia and Latvia . Forest Pathol , 41 : 169 – 174 .
- Scott , P.M. , Burgess , T.I. , Barber , P.A. , Shearer , B.L. , Stukely , M.J.C. , Hardy , G.E. , St , J. and Jung , T. 2009 . Phytophthora multivora sp. nov., a new species recovered from declining Eucalyptus, Banksia, Agonis and other plant species in Western Australia . Persoonia , 22 : 1 – 13 .
- Sturrock , R.N. , Frankel , S.J. , Brown , A.V. , Hennon , P.E. , Kliejunas , J.T. Lewis , K.J. 2011 . Climate change and forest diseases . Plant Pathol , 60 : 133 – 149 .
- Talgø , V. , Herrero , M.-L. , Toppe , B. , Brurberg , M.B. , Thurston , R. and Stensvand , A. 2009 . Phytophthora plurivora, ny skadegjerar på tre i Noreg . Bioforsk FOKUS , 5 : 238 – 239 . In Norwegian
- Themann , K. , Werres , S. , Lüttmann , R. and Diener , H.-A. 2002 . Observations of Phytophthora spp. in water recirculation systems in commercial hardy ornamental nursery stock . Eur. J. Plant Pathol , 108 : 337 – 343 .
- Thinggaard , K. 2009 . Phytophthora – en ny og alvorlig trussel mod de danske skove . Skoven , 2009/11 : 478 – 481 . in Danish
- TIKE Information Centre of Ministry of Agriculture and Forestry . 2011 . Horticultural Statistics 2010 , 126 Helsinki : Edita Oy . ISSN 1799-084X. [In Finnish].
- Vainio , E.J. and Hantula , J. 1999 . Variation of RAMS markers within the intersterility groups of Heterobasidion annosum in Europe . Eur. J. Forest Pathol , 29 : 231 – 246 .
- Vainio , E.J. , Korhonen , K. and Hantula , J. 1998 . Genetic variation in Phlebiopsis gigantea as detected with random amplified microsatellite (RAMS) markers . Mycol. Res , 102 : 187 – 192 .
- Venette , R.C. and Cohen , S.D. 2006 . Potential climatic suitability for establishment of Phytophthora ramorum within the contiguous United States . Forest Ecol. Manag , 231 : 18 – 26 .
- Vercauteren , A. , De Dobbelaere , I. , Grünwald , N.J. , Bonants , P. , van Bockstaele , E. , Maes , M. and Heungens , K. 2010 . Clonal expansion of the Belgian Phytophthora ramorum populations based on new microsatellite markers . Mol. Ecol , 19 : 92 – 107 .
- Waterhouse , G.M. 1963 . “ Key to the species of Phytophthora de Bary ” . In Mycology Paper No , Vol. 92 , 22 UK : CMI Kew .
- Werres , S. and De Merlier , D. 2003 . First detection of Phytophthora ramorum mating type A2 in Europe . Plant Dis , 87 : 1266
- Werres , S. , Marwitz , R. , Man In't Veld , W.A. , De Cock , W.A.M. , Bonants , P.J.M. De Weert Themann , K. 2001 . Phytophthora ramorum sp. nov. a new pathogen on Rhododendron and Viburnum . Mycol. Res , 105 : 115 – 165 .
- Weiland , J.E. , Nelson , A.H. and Hudler , G.W. 2010 . Aggressiveness of Phytophthora cactorum, P. citricola I, and P. plurivora from European beech . Plant Dis , 94 : 1009 – 1014 .
- Yakabe , L.E. , Thomas , S.L. and MacDonald , J.D. 2009 . Identification and frequency of Phytophthora species associated with foliar diseases in California ornamental nurseries . Plant Dis , 93 : 883 – 890 .
- Xu , J. , Ebada , S.S. and Proksch , P. 2010 . Pestalotiopsis a highly creative genus: chemistry and bioactivity of secondary metabolites . Fungal Diver , 44 : 15 – 31 .