Abstract
Vegetative compatibility groups (VCGs) of 12 potato and nine sunflower isolates of Verticillium dahliae were determined using both nitrate non-utilizing (nit) mutants and multiplex-nested-PCR approaches. Based on analysis of nit mutants, most potato isolates showed strong compatibility with VCG 4A and weak compatibility with VCG 4B; one potato isolate was also weakly compatible with VCGs 2A, 2B and 3. Sunflower isolates were more variable and most of them showed weak reactions with VCG 4A and 4B. One sunflower isolate, Vs06-14, was compatible only with VCG3 testers, whereas another isolate, Vs06-20, was compatible with all VCG groups except VCG2A. Vegetative compatibility of four selected isolates, two from potato and two from sunflower, was also investigated after passing them through potato or sunflower for four generations. The VCG of one potato isolate did not change, whereas the other one changed from VCG4B to 4A. One sunflower isolate, initially compatible with both VCG4A and 4B, became incompatible with both. The other sunflower isolate was not compatible with any VCG and remained so. In a second part of this work, multiplex-nested-PCR was carried out to characterize VCGs of the same isolates of V. dahliae. Vegetative compatibility grouping using this approach assigned the V. dahliae isolates to VCG1, 2A, 3 or 4A. Six of the 12 potato isolates and four of the nine sunflower isolates of V. dahliae could not be identified or placed in any VCG group based on PCR results. In the present study, the multiplex-nested-PCR results were confirmed using nit mutants. As such, PCR-based methods will require more specific primers before they can replace the approach based on nit mutants. Footnote
Current address of A.F. El-Bebany: Department of Plant Pathology, Faculty of Agriculture, Alexandria University, El-Shatby, 21545, Alexandria, Egypt.
Résumé
Les groupes de compatibilité végétative (GCV) de 21isolats de Verticillium dahliae dont 12 issus de pomme de terre et 9 de tournesols ont été déterminés à l'aide des méthodes faisant appel aux mutants n'utilisant pas les nitrates (nit) et à la PCR multiplexe. Selon les analyses des mutants nit, la majorité des isolats de pomme de terre affichait une forte compatibilité avec le GCV 4A et une faible compatibilité avec le GCV 4B; un isolat de pomme de terre était également faiblement compatible avec les GCV 2A, 2B et 3. Les isolats de tournesol présentaient plus de variabilité et la plupart affichaient de faibles réactions avec les testeurs des GCV 4A et 4B. Un isolat de tournesol, Vs06-14, était compatible avec les souches contrôles de GCV3, tandis qu'un autre isolat, Vs06-20, était compatible avec tous les GCV, sauf GCV 2A. La compatibilité végétative de quatre isolats choisis, deux de la pomme de terre et deux du tournesol, a été étudiée après passage sur pomme de terre ou tournesol pour quatre générations. Le GCV d'un isolat de pomme de terre n'a pas changé tandis que l'autre est passé du GCV 4B au GCV 4A. Un isolat de tournesol, initialement compatible avec GCV 4A et 4B, est devenu incompatible avec les deux. L'autre isolat de tournesol n’était compatible avec aucun des GCV et n'a pas changé. Dans la deuxième partie de cette étude, une PCR multiplexe et par amorces incluses a été réalisée pour caractériser les GCV des mêmes isolats de V. dahliae. Le groupage de la compatibilité végétative basé sur cette approche a attribué les isolats de V. dahliae aux GCV1, 2A, 3 ou 4A. Six des douze isolats de pomme de terre et quatre des neuf isolats de tournesol de V. dahliae n'ont pu être identifiés ou classés dans aucun GCV sur la base des résultats obtenus par PCR. Dans la présente étude, les résultats de la PCR multiplexe et par amorces incluses ont été confirmés à l'aide des mutants nit. En tant que telles, les méthodes basées sur la PCR requerront des amorces plus spécifiques avant qu'elles puissent remplacer l'approche fondée sur les mutants nit.
Introduction
Verticillium dahliae (Kleb.) is a soilborne fungus that causes vascular wilt in hundreds of economically important plants worldwide (Pegg & Brady, Citation2002). In association with the nematode Pratylenchus penetrans and other soilborne pathogens, V. dahliae causes potato early dying (PED) syndrome (Rowe & Powelson, Citation2002; Mahran et al., Citation2008), which affects both the yield and quality of infected potatoes. Such associations also happen in other crops (Johnson & Santo, Citation2001). Management of verticillium wilt represents a real challenge due to the persistence of microsclerotia, the resting structure of the pathogen in the soil (Wilhelm, Citation1955). In order to reduce losses due to this disease, potato growers usually rely on cultural practices optimized for their region and budget, and may include such practices as rotations, soil fumigation or solarization (Johnson & Dung, Citation2010). More recently, several biological control approaches have been proposed (Mahran et al., Citation2008; Uppal et al., 2008). However, the wide host range of V. dahliae represents another challenge for management of this disease.
For many years, Verticillium wilt has been an impediment to optimal production of both potato and sunflower in southern Manitoba and other growing regions of Canada, as well as in other countries (Uppal et al., Citation2007). Cross-pathogenicity of isolates of V. dahliae between potato and sunflower has been confirmed and is potentially damaging to both crops, especially to sunflower when the original host is potato (Alkher et al., Citation2009 a, Citation2009b ). However, it is now clear that V. dahliae isolates possess a high level of pathogenic diversity under different environmental conditions and when they originate from different hosts or locations. Therefore, characterizing the diversity of this pathogen and predicting the virulence of isolates from a given field, location or region, to other crops may be helpful in making recommendations to growers i.e. which crops may not be suited for their fields and rotations. Pathogenicity of V. dahliae isolates and the host plant of origin were correlated with vegetative compatibility groups (VCGs) (Daayf et al., Citation1995). Vegetative compatibility refers to the ability of two individual isolates to form heterokaryons through anastomosis (Puhalla, Citation1979; Leslie, Citation1993). It is considered to be a source of genetic diversity in V. dahliae and may contribute to pathogenicity and other features in the pathogen (Collado-Romero et al., Citation2008). For example, Collado-Romero et al. (Citation2010) conducted phylogenetic analyses of DNA sequences of some conserved genes in V. dahliae isolates representing several geographic regions, VCGs and hosts. Their results suggested that hybridization events between isolates of V. dahliae VCG1 and/or VCG4A may have led to the appearance of V. dahliae var. longisporum.
For many years, VCGs of V. dahliae have been determined using nitrate non-utilizing (nit) mutants (Puhalla & Hummel, Citation1983; Joaquim & Rowe, Citation1990). Recently, Collado-Romero et al. (Citation2009) developed a new multiplex-nested-PCR-based procedure to detect and identify V. dahliae VCGs in planta using specific PCR primers that produce differential PCR marker bands of 334, 688 and 964 bp in size. Characterization of VCGs using this approach was conducted according to the differential pattern of three multiplex-nested-PCR-amplified marker bands. However, VCGs of some V. dahliae isolates could not be differentiated.
In North America, the majority of V. dahliae potato isolates were suggested to belong to either VCG4A or VCG4B subgroups (Omer et al., Citation1997; Dobinson et al., Citation2000). The isolates of V. dahliae in subgroup VCG4A were described as highly virulent on potato when compared with those from VCG4B and VCG2 (Joaquim & Rowe, Citation1991). Verticillium dahliae VCGs were investigated extensively using isolates from many other hosts, such as cotton (Daayf et al., Citation1995), artichoke and other vegetables (Berbegal et al., Citation2010), chrysanthemum (Göre, Citation2009) and olive trees (Bellahcene et al., Citation2005), and in different regions of the world (Strausbaugh, Citation1993; Hiemstra & Rataj-Guranowska, Citation2003).
Pathogenicity of V. dahliae isolates from potato and sunflower fields in Manitoba was previously assessed both on their original and alternative hosts (Uppal et al., Citation2007; Alkher et al., Citation2009a ). Their aggressiveness after passing them through their original or alternative host for several generations was also studied to investigate any possible variation as a result of multiple exposure to a given host plant (Alkher et al., Citation2009a , Citation2009b ). The objectives of the current study were to identify the VCGs of these isolates and compare the use of nitrate non-utilizing (nit) mutants and multiplex-nested-PCR in determining their VCGs and genetic diversity. It was previously shown that Verticillium sp. isolates initially non-pathogenic on tomato became pathogenic on this host after one to five successive inoculations, while losing pathogenicity on their original host, peppermint (Fordyce & Green, Citation1963). However, it is not known whether such a change in pathogenicity would be accompanied by a change in vegetative compatibility status. Therefore, the third objective of this study was to test the effect of passing selected isolates of V. dahliae through the original and an alternative host for four generations on the stability of the isolate's VC grouping.
Materials and methods
Verticillium dahliae isolates and testers
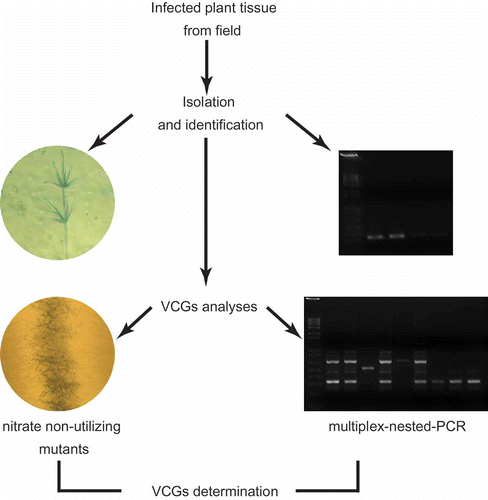
A total of 21 isolates of V. dahliae were used in the current investigation, including 10 from potato and nine from sunflower. These isolates were previously characterized based on their morphology, microsclerotia production and specific primers for molecular identification (Alkher et al., Citation2009a ). The two remaining isolates are used in our laboratory as standard highly aggressive isolates (Vd1396-9 and Vd1398-21) (Uppal et al., Citation2007). Pathogenicity of all isolates was assessed and variation in their aggressiveness levels was tested by passing them for four generations through potato or sunflower plants (Uppal et al., Citation2007; Alkher et al., Citation2009a , Citation2009b ). The V. dahliae nit1 and nitM tester isolates used in the present study were kindly provided by Dr Dennis A. Johnson, Department of Plant Pathology, Washington State University. Our approach to identify the VCGs of V. dahliae isolates using nit mutants and PCR is summarized in
Characterization of VCGs of V. dahliae using nitrate non-utilizing (nit) mutants
Production of nit mutants. All 21 isolates of V. dahliae were grown on chlorate minimal medium (CMM: minimal medium (MM) amended with 30 g L−1 potassium chlorate) according to Puhalla & Spieth (Citation1983). Some of the sunflower isolates would only grow on a modified CMM containing 15 g L−1 potassium chlorate (CMM15). Sections were transferred from the chlorate resistant colonies to MM to verify nit mutation then transferred back to potato dextrose agar medium (PDA, Difco) to produce conidia for single spore cultures of the nit mutants.
The nit mutants were tested on MM with four different nitrogen sources as follows: Minimal medium minus the 2 g L−1 sodium nitrate was prepared with the addition of either 0.2 g L−1 sodium nitrate, 0.4 g L−1 sodium nitrite, 0.5 g L−1 hypoxanthine or 0.8 g L−1 ammonium tartrate buffered with 0.5 g L−1 calcium carbonate. Isolates which were unable to utilize nitrate but could utilize nitrite, ammonium or hypoxanthine were selected as nit1 mutants. Isolates that were incapable of utilizing nitrate or hypoxanthine but all other sources were labelled nitM mutants. No nit3 mutants were produced from the tested isolates.
Assessment of nit mutants of V. dahliae isolates with tester nit mutants. Nit mutants were paired with tester isolates to determine VCG1, VCG1A, VCG2A, VCG2B, VCG3, VCG4A or VCG4B groups. The pairings with complementary nit mutant tester isolates that produced dense aerial mycelium were rated (++) or (+++). Those that formed microsclerotia or thin mycelium were rated (+), whereas pairings which resulted in no reaction were rated (0). Pairings with only a few patches of aerial mycelium were rated (+/−).
Characterization of VCGs of V. dahliae using multiplex-nested-PCR
Genomic DNA extraction. DNA was extracted according to Wally et al. (Citation2008) from 4-week-old isolates of V. dahliae grown on PDA at room temperature. Mycelium (50–100 mg) from each isolate was harvested, placed into a sterile 1.5-mL Eppendorf tube and ground using a micro-pestle with 500 μL extraction buffer (2% CTAB, 100 mM Tris-HCl, pH 8.0, 20 mM EDTA, 5% PVPP, 1.4 M NaCl) at 60 °C. Samples were vortexed for 15 s and incubated at 60 °C for 30–90 min. Five hundred μL of cold chloroform : isoamyl alcohol (24 : 1) was added to the sample. The Eppendorf tube containing the sample was inverted 6–10 times and centrifuged at 9000 rpm for 15 min. The supernatant was transferred into a new 1.5-mL Eppendorf tube, an additional 500 μL of chloroform : isoamyl alcohol was added, mixed again by inversion and centrifuged for an additional 15 min at 9000 rpm. The supernatant was again transferred into a new Eppendorf tube followed by the addition of 250 μL of cold isopropanol, mixed by inversion and incubated at −20 °C for 30 min. Following incubation, the sample was centrifuged at 14,000 rpm for 15 min. The supernatant was carefully decanted and the pellet was washed with 500 μL of 70% ethanol, then incubated at room temperature for 15 min and centrifuged at 14,000 rpm for 15 min. After centrifugation, all traces of ethanol were removed and the pellet air-dried. The pellet was then dissolved in 200 μL of TE (10 mM Tris-HCL, 1mM EDTA, pH 8.0) and stored at −20 °C until used in the PCR reactions.
Multiplex-nested-PCR. A multiplex-PCR reaction using 1 μL of a 1 : 50 dilution, in nanopure water, of V. dahliae genomic DNA extracted from each isolate was carried out. The primers used in the multiplex-PCR were: DB19 and DB22 (400 nM) and NDf and NDr (200 nM) as described by Collado-Romero et al. (Citation2009). The final volume used for the multiplex-PCR reaction was 25 μL. The PCR mixture contained 200 μM of each dNTP, 2.5 mM MgCl2, 0.5 U Taq polymerase and 2.5 μL of 10× PCR reaction buffer. PCR amplification was conducted in a Bio-Rad thermocycler (MyCyclerTM thermal cycler, Bio-Rad Laboratories, Inc.) with cycling conditions of 94 °C for 4 min, 35 cycles of 94 °C for 1 min., 54 °C for 45 s and 72 °C for 1 min followed by final extension at 72 °C for 5 min.
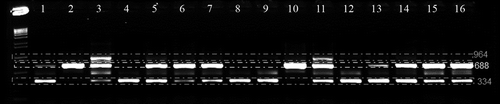
Nested-PCR was run using 1 μL of the PCR amplification product from the initial multiplex-PCR and the following primers: DB19 and espdef01 (200 nM) and INTD2f, INTD3r and M2CR2B (100 nM) as described by Collado-Romero et al. (Citation2009). The PCR mixture components had the same concentrations as mentioned above in a final volume of 25 μL. The PCR cycling protocol was 94 °C for 4 min, 35 cycles of 94 °C for 1 min, 60 °C for 45 S and 72 °C for 1 min, followed by a final step at 72 °C for 5 min. Ten μL of the multiplex-nested-PCR amplification product were mixed with 3 μL of loading buffer and run on a 1.5% agarose gel containing 0.5 μg mL−1 ethidium bromide at 100 volts for 30 min. Five μL of the 1 Kb Plus DNA ladder (10 μg mL−1) (Invitrogen) was run in parallel with the multiplex-nested-PCR product. The gels were visualized using an AlphaImager HP gel documentation system (Alpha Innotech Corporation, San Leandro, CA, USA) equipped with a P93D thermoprinter (Mitsubishi Electric Co., Tokyo, Japan). The assessment was based on three bands at 334, 688 and 964 bp ().
Fig. 2. Example of an agarose gel showing multiplex-nested-PCR products for VCG determination of Verticillium dahliae tester isolates (1: T9-NM, 2: PH-NM, 3: 115-NM, 4: 70-21-NM, 5: BB-NM, 6: S39-NM, 7: MT-NM, 8: V-44-N1, 9: T9-N1, 10: PH-N1, 11: 115-N1, 12: PCW-N1, 13: 70-21-N1, 14: BB-N1, 15: S39-N1, and 16: MT-N1). The primers used in the multiplex-nested-PCR were DB19, DB22, NDf, NDr, espdef01, INTD2f, INTD3r and M2CR2B (Collado-Romero et al., Citation2009). VCG characterization was based on the differential pattern of the three amplified multiplex-nested-PCR product (964, 688 and 334 bp) markers.
Results
Vegetative compatibility groups of V. dahliae using nit mutants
Results of the pairings between nit mutants of the tested isolates of V. dahliae and universal testers are shown in . All isolates from potato were scored as VCG4A, except one that was VCG4B. However, all VCG4A isolates also had minor compatibility with VCG3 and VCG4B. One potato isolate, Vs04-41, that had a strong reaction with both VCG4A and VCG4B, also produced a weak reaction with VCGs 2A, 2B and 3. There was a much higher diversity among sunflower isolates, with five that ranged in both VCG4A and VCG4B, one in both VCG3 and VCG4A, one in VCG3, one in all VCGs except VCG2A, and one that was not compatible with any of the testers used. In general, heterokaryon formation with testers was much weaker in sunflower isolates compared with those from potato.
Table 1. Results of vegetative compatibility pairings of mutants produced from V. dahliae isolates with tester isolates
None of the tested isolates had any reaction with VCG2A or VCG2B, except Vs04-41 from potato and Vs06-20 from sunflower. Both potato and sunflower isolates had very weak reactions with VCG1 and VCG1A. Because many isolates had weak reactions with testers from more than one VCG, compatibility pairings between nit1s and nitMs from all available testers was conducted. Besides the strong reaction of testers from the same VCG, interestingly, many testers had a reaction with testers from more than one VCG ().
Table 2. Results of vegetative compatibility pairings between nit1 and nitM mutants of V. dahliae VCG tester isolates
Vegetative compatibility groups of V. dahliae using multiplex-nested-PCR
Most potato isolates identified using nested PCR were in VCG4A, and five isolates from sunflower were in VCG1, 2 or 3. Eight isolates (six from potato and two from sunflower) had the same VCG results using either nit mutants or PCR-based approach; these isolates are Vs04-21, Vs04-28, Vs04-35, Vs04-38, Vs04-41, Vs04-47, Vs06-03 and Vs06-11. Based on multiplex-nested-PCR, the potato isolates belonged to one VCG (), whereas their nit mutants were compatible with testers from more than one VCG ().
Table 3. Results of vegetative compatibility groups (VCGs) obtained using multiplex-nested-PCR approach of all the tested V. dahliae isolates
Vegetative compatibility groups of V. dahliae after serial passages on the alternative host
Vegetative compatibility of four selected isolates, two from potato and two from sunflower, was investigated after passing them through an alternative host (sunflower or potato) for four generations. The VCG of one potato isolate (Vs04-47) did not change, which was confirmed using the multiplex-nested-PCR approach (). The other potato isolate (Vs04-09) changed from VCG4B to VCG4A, but this was not confirmed because the multiplex-nested-PCR approach did not allow differentiation among all VCGs. One sunflower isolate, initially compatible with both VCG4A and 4B, became incompatible with both. The other sunflower isolate was not compatible with any VCG and remained so (). Based on PCR, these sunflower isolates would belong to VCGs 1, 2 or 3.
Table 4. Vegetative compatibility groups (VCGs) of V. dahliae isolates after four serial passages on potato (P4) or sunflower (S4)
Discussion
The high genetic diversity of V. dahliae and its wide host range are considered as obstacles to successful management of verticillium wilt (Powelson & Rowe, Citation1993; Klosterman et al., Citation2009). Identification of VCGs of V. dahliae is important when isolates have the ability to infect several crops in the same region. We previously assessed the pathogenicity of local isolates from Manitoba on potato (Uppal et al., Citation2007), then their cross-pathogenicity both on potato and sunflower (Alkher et al., Citation2009a , Citation2009b ). Those results included the isolates tested in the current study and indicated that isolates originally collected from potato are highly aggressive on both host plants compared with those from sunflower. Isolates from a specific VCG with more aggressiveness on a specific crop than other VCGs have been described previously (Göre, Citation2009).
The V. dahliae isolates tested in this study belonged to more than one VCG, but the general trend was that potato isolates belonged to VCG4A whereas sunflower isolates belonged to VCG4A, VCG4B or VCG3. Interestingly, according to the multiplex PCR tests, the sunflower isolates would belong in VCG1, VCG2 or VCG3. Placement of a single isolate into more than one VCG may suggest the existence of several variants within that isolate. However, all of these isolates were initially derived from single spore cultures. In addition, some level of compatibility was also confirmed using the different VCG testers (). Compatibility with multiple groups may also be due to a transitional status of these isolates between given VCGs (Daayf et al., Citation1995), especially in regions where different crops are grown in rotations.
Proteomic and transcriptomic differences (El-Bebany et al., Citation2010a , Citation2011) between two of the V. dahliae isolates tested here, Vs06-14 (VCG3) and Vd1396-9 (VCG4A), show the extent of potential relationships between VCGs and other characteristics. Signalling of defence reactions, through reactive oxygen species, salicylic and jasmonic acid biosynthesis pathways, were also differentially expressed in potato and sunflower after inoculation with these two isolates (Derksen et al., Citation2011; Yao et al., Citation2011). Furthermore, secondary metabolites, e.g. rutin, also accumulated differentially in potato in response to inoculation with these and other isolates (El-Bebany et al., Citation2010b ; El Hadrami et al., Citation2011). Such variations in host plant defence responses to the two isolates parallel their different pathogenicity levels and VCG status. To date, no studies have explored the mechanisms of such links between VCGs and the behaviour of pathogens in planta.
The effect of passing the tested isolates of V. dahliae for four generations through the original or the alternative host (either potato or sunflower) on the stability of their pathogenicity was previously examined by Alkher et al. (Citation2009b ). Two of these isolates from potato (Vs04-09 and Vs04-47) and two from sunflower (Vs06-07 and Vs0609) were tested for their VCG status (). Alkher et al. (Citation2009b ) reported that V. dahliae isolates may gain pathogenicity when repeatedly passed through a given variety, but according to the current study, this does not necessarily affect the VCG status of the isolates, or at least not after four generations. Pathogenicity of the isolates tested in this study is being investigated on other crops with the aim of detecting the relationship between their VCGs and potential host adaptation.
Several molecular-based techniques have been used to detect genetic variation among isolates of V. dahliae. These included random amplified polymorphic DNA (RAPD), restriction fragment length polymorphism (RFLP) and amplified fragment length polymorphism (AFLP) (Ramsay et al., Citation1996; Dobinson et al., Citation2000; Bhat et al., Citation2003; Collado-Romero et al., Citation2006; El-Bebany et al., Citation2011). In the current study, vegetative compatibility grouping of V. dahliae based on the multiplex-nested-PCR protocol developed by Collado-Romero et al. (Citation2009) proved to be less specific than using paired complementary nit mutants. This level of specificity relates to the fact that the multiplex-nested-PCR procedure identifies VCGs indirectly and does not consider the VCG alleles themselves. Also, it does not distinguish all the known VCGs. However, using molecular approaches helps determine the VCGs quickly and can be used for VCG determination in planta (Collado-Romero et al., Citation2009) and to confirm results obtained using nit mutants. In any event, molecular approaches currently available cannot replace the VCG testing that actually shows the presence or absence of compatibility. Vegetative compatibility of isolates of V. dahliae from many geographical regions and countries such as the USA (Vallad et al., Citation2006), Europe (Rataj-Guranowska, Citation2006), and Japan (Wakatabe et al., Citation1997) was investigated to explain genetic variation in this important worldwide pathogen.
All the tested isolates of V. dahliae, except one, were compatible with both VCGs 4A and 4B. It has been reported that VCGs 4A and 4B were dominant in isolates of V. dahliae from potato plants and fields in North America (Omer et al., Citation1997; Dobinson et al., Citation2000). Identification of VCGs of V. dahliae from Manitoba will not only provide information about genetic diversity of this pathogen in this region, but will permit comparisons of the distribution of VCGs of V. dahliae worldwide. The present VCG characterization of V. dahliae isolates may help predict the disease severity and incidence in specific parts and direct options to avoid yield losses. Integrating such data into the pool of information about the fungus and its environment should assist in establishing an efficient management programme for verticillium wilt in Canada and abroad.
Acknowledgements
We thank Dr D.A. Johnson (Department of Plant Pathology, Washington State University) for providing the V. dahliae VCG tester isolates. We acknowledge research funding from MAAS-ARDI to F. Daayf, and financial support from the Libyan government to H. Alkher.
Notes
Current address of A.F. El-Bebany: Department of Plant Pathology, Faculty of Agriculture, Alexandria University, El-Shatby, 21545, Alexandria, Egypt.
References
- Alkher , H. , El Hadrami , A. , Rashid , K.Y. , Adam , L.R. and Daayf , F. 2009a . Verticillium dahliae cross-pathogenicity between potato and sunflower . Eur. J. Plant Pathol. , 124 : 505 – 519 .
- Alkher , H. , El Hadrami , A. , Rashid , K.Y. , Adam , L.R. and Daayf , F. 2009b . Pathogenic variation of Verticillium dahliae after serial passages through potato and sunflower . Can. J. Plant Pathol. , 31 : 427 – 438 .
- Bellahcene , M. , Fortas , Z. , Fernandez , D. and Nicole , M. 2005 . Vegetative compatibility of Verticillium dahliae isolated from olive trees (Olea europea L.) in Algeria . Afr. J. Biotechnol. , 4 : 963 – 967 .
- Berbegal , M. , Ortega , A. , Jiménez-Gasco , M.M. , Olivares-García , C. , Jiménez-Díaz , R.M. and Armengol , J. 2010 . Genetic diversity and host range of Verticillium dahliae isolates from artichoke and other vegetable crops in Spain . Plant Dis. , 94 : 396 – 404 .
- Bhat , R.G. , Smith , R.F. , Koike , S.T. , Wu , B.M. and Subbarao , K.V. 2003 . Characterization of Verticillium dahliae isolates and wilt epidemics of pepper . Plant Dis. , 87 : 789 – 797 .
- Collado-Romero , M. , Mercado-Blanco , J. , Olivares-García , C. , Valverde-Corredor , A. and Jiménez-Díaz , R.M. 2006 . Molecular variability within and among Verticillium dahliae vegetative compatibility groups determined by fluorescent amplified fragment length polymorphism and polymerase chain reaction markers . Phytopathology , 96 : 485 – 495 .
- Collado-Romero , M. , Mercado-Blanco , J. , Olivares-García , C. and Jiménez-Díaz , R.M. 2008 . Phylogenetic analysis of Verticillium dahliae vegetative compatibility groups . Phytopathology , 98 : 1019 – 1028 .
- Collado-Romero , M. , Berbegal , M. , Jiménez-Díaz , R.M. , Armengol , J. and Mercado-Blanco , J. 2009 . A PCR-based ‘molecular tool box’ for in planta differential detection of Verticillium dahliae vegetative compatibility groups infecting artichoke . Plant Pathol. , 58 : 515 – 526 .
- Collado-Romero , M. , Jiménez-Díaz , R.M. and Mercado-Blanco , J. 2010 . DNA sequence analysis of conserved genes reveals hybridization events that increase genetic diversity in Verticillium dahliae . Fungal Biol. , 114 : 209 – 218 .
- Daayf , F. , Nicole , M. and Geiger , J.-P. 1995 . Differentiation of Verticillium dahliae populations on the basis of vegetative compatibility and pathogenicity on cotton . Eur. J. Plant Pathol. , 101 : 69 – 79 .
- Derksen , H. , Badawi , M. , Henriquez , M.A. and Daayf , F. 2011 . Raging hormones, the role of hormone defence signalling in the potato–Verticillium dahliae interaction . Can. J. Plant Pathol. , 33 : 274 Abstr
- Dobinson , K.F. , Harrington , M.A. , Omer , M. and Rowe , R.C. 2000 . Molecular characterization of vegetative compatibility group 4A or 4B isolates of Verticillium dahliae associated with potato early dying . Plant Dis , 84 : 1241 – 1245 .
- El-Bebany , A.F. , Rampitsch , C. and Daayf , F. 2010a . Proteomic analysis of the phytopathogenic soilborne fungus Verticillium dahliae reveals differential protein expression in isolates that differ in aggressiveness . Proteomics , 10 : 289 – 303 .
- El-Bebany , A.F. , Adam , L.R. and Daayf , F. 2010b . “ Phenolic profiling in potato after inoculation with highly versus weakly aggressive V. dahliae isolates ” . In Polyphenols Communications 2010 , Edited by: Ageorges , A. , Cheynier , V. , Lefer , P. and Sarni-Manchado , P. Vol. 1 , 277 – 278 . Montpellier, France : Publisher: Groupe Polyphenols .
- El-Bebany , A.F. , Henriquez , M.A. , Badawi , M. , Adam , L.A. , El Hadrami , A. and Daayf , F. 2011 . Induction of pathogenicity-related candidate genes in Verticillium dahliae in response to elicitation with potato root extracts . Environ. Exp. Bot. , 72 : 251 – 257 .
- El Hadrami , A. , Adam , L.R. and Daayf , F. 2011 . Biocontrol treatments confer protection against Verticillium dahliae infection of potato by inducing anti-microbial metabolites . Mol. Plant–Microbe Interact. , 24 : 328 – 335 .
- Fordyce , C. and Green , R.J. 1963 . Alteration of pathogenicity of Verticillium albo-atrum var. menthae . Phytopathology , 53 : 701 – 704 .
- Göre , M.E. 2009 . Vegetative compatibility and pathogenicity of Verticillium dahliae isolates from chrysanthemum in Turkey . Phytoparasitica , 37 : 87 – 94 .
- Hiemstra , J.A. and Rataj-Guranowska , M. 2003 . Vegetative compatibility groups in Verticillium dahliae isolates from the Netherlands as compared to VCG diversity in Europe and in the USA . Eur. J. Plant Pathol. , 109 : 827 – 839 .
- Joaquim , T.R. and Rowe , R.C. 1990 . Reassessment of vegetative compatibility relationships among strains of Verticillium dahliae using nitrate-nonutilizing mutants . Phytopathology , 80 : 1160 – 1166 .
- Joaquim , T.R. and Rowe , R.C. 1991 . Vegetative compatibility and virulence of strains of Verticillium dahliae from soil and potato plants . Phytopathology , 81 : 552 – 558 .
- Johnson , D.A. and Dung , J.K.S. 2010 . Verticillium wilt of potato – the pathogen, disease and management . Can. J. Plant Pathol. , 32 : 58 – 67 .
- Johnson , D. A. and Santo , G. S. 2001 . Development of wilt in mint in response to infection by two pathotypes of Verticillium dahliae and co-infection by Pratylenchus penetrans . Plant Dis. , 85 : 1189 – 1192 .
- Klosterman , S.J. , Atallah , Z.K. , Vallad , G.E. and Subbarao , K.V. 2009 . Diversity, pathogenicity, and management of Verticillium species . Annu. Rev. Phytopathol. , 47 : 39 – 62 .
- Leslie , J.F. 1993 . Fungal vegetative compatibility . Annu. Rev. Phytopathol. , 31 : 127 – 150 .
- Omer , M. , Beery , W. , Johnson , D. and Rowe , R.C. 1997 . Vegetative compatibility among isolates of Verticillium dahliae from potato seed tubers and stems from western USA . Phytopathology , 87 : S72
- Mahran , A. , Tenuta , M. , Hanson , M.L. and Daayf , F. 2008 . Mortality of Pratylenchus penetrans by volatile fatty acids from liquid hog manure . J. Nematol. , 40 : 119 – 126 .
- Pegg , G.F. and Brady , B.L. 2002 . Verticillium wilts , Wallingford : CAB International. 552 pages .
- Powelson , M.L. and Rowe , R.C. 1993 . Biology and management of early dying of potatoes . Annu. Rev. Phytopathol. , 31 : 111 – 126 .
- Puhalla , J.E. 1979 . Classification of isolates of Verticillium dahliae based on heterokaryon incompatibility . Phytopathology , 69 : 1186 – 1189 .
- Puhalla , J.E. and Hummel , M. 1983 . Vegetative compatibility groups within Verticillium dahliae . Phytopathology , 73 : 1305 – 1308 .
- Puhalla , J.E. and Spieth , P.T. 1983 . Heterokaryosis in Fusarium moniliforme . Exp. Mycol. , 7 : 328 – 335 .
- Ramsay , J.R. , Multani , D.S. and Lyon , B.R. 1996 . RAPD-PCR identification of Verticillium dahliae isolates with differential pathogenicity on cotton . Austral. J. Agric. Res. , 47 : 681 – 693 .
- Rataj-Guranowska , M. 2006 . Vegetative compatibility in Verticillium dahliae from several European countries . Phytopathol. Pol. , 42 : 5 – 12 .
- Rowe , R.C. and Powelson , M.L. 2002 . Potato early dying: management challenges in a changing production environment . Plant Dis. , 86 : 1184 – 1193 .
- Strausbaugh , C.A. 1993 . Assessment of vegetative compatibility and virulence of Verticillium dahliae isolates from Idaho potatoes and tester strains . Phytopathology , 83 : 1253 – 1258 .
- Uppal , A.K. , El Hadrami , A. , Adam , L.R. , Tenuta , M. and Daayf , F. 2007 . Pathogenic variability of Verticillium dahliae isolates from potato fields in Manitoba and screening of bacteria for their biocontrol . Can. J. Plant Pathol. , 29 : 141 – 152 .
- Uppal , A.K. , El Hadrami , A. , Adam , L.R. , Tenuta , M. and Daayf , F. 2008 . Biological control of potato Verticillium wilt under controlled and field conditions using selected bacterial antagonists and plant extracts . Biol. Control , 44 : 90 – 100 .
- Vallad , G.E. , Qin , Q.-M. , Grube , R. , Hayes , R.J. and Subbarao , K.V. 2006 . Characterization of race-specific interactions among isolates of Verticillium dahliae pathogenic on lettuce . Phytopathology , 96 : 1380 – 1387 .
- Wakatabe , D. , Nagao , H. , Arai , H. , Shiraishi , T. , Koike , M. and Iijima , T. 1997 . Vegetative compatibility groups of Japanese isolates of Verticillium dahliae . Mycoscience , 38 : 17 – 23 .
- Wally , O. , El Hadrami , A. , Khadhair , A. , Adam , L.R. , Shinners-Carnelley , T. , Elliott , B. and Daayf , F. 2008 . Sequencing reveals false positives during the detection in leafhoppers of carrot aster yellows phytoplasmas . Sci. Hort. , 116 : 130 – 137 .
- Wilhelm , S. 1955 . Longevity of Verticillium wilt fungus in the laboratory and field . Phytopathology , 45 : 180 – 181 .
- Yao , Z. , Rashid , K.Y. , Adam , L.R. and Daayf , F. 2011 . Verticillium dahliae’s VdNEP acts both as a plant defense elicitor and a pathogenicity factor in the interaction with Helianthus annuus . Can. J. Plant Pathol. , 33 : 375 – 388 .