Abstract
Cercospora sojina Hara, the causal agent of frogeye leaf spot of soybean (Glycine max (L.) Merr.), causes yield reductions worldwide. Although the phenotypic diversity (physiological races) of this pathogen has been assessed through its ability to affect soybean lines with different resistant genes (Rcs genes), little is known about the pathogen's genetic diversity. In order to better understand the genetic diversity that exists with C. sojina, a historical collection of 62 C. sojina isolates from Brazil (10 isolates), China (7 isolates), Nigeria (1 isolate), and United States (44 isolates) was used for genetic diversity analysis with amplified fragment length polymorphism (AFLP) markers. The average genetic similarity of the isolates was 0.56 on a scale between 0 and 1, indicating a high degree of genetic diversity within the species. Cluster analysis resulted in two major clusters and seven sub-clusters. Two isolates collected from Georgia were clustered together, and two isolates from China were clustered together. Besides these four isolates, no clear separation of isolates based on origin was found. Our results provide evidence that substantial genetic diversity exists within the species of C. sojina and that selection for broad-spectrum host-resistance should be targeted in soybean breeding programmes.
Résumé
Cercospora sojina Hara, l'agent causal de l’œil de grenouille chez le soja (Glycine max [L.] Merr), cause des pertes de rendement partout dans le monde. Bien que la diversité phénotypique (races physiologiques) de cet agent pathogène ait été évaluée relativement à sa capacité d'infecter les lignées de soja possédant différents gènes de résistance (gènes Rcs), nous en savons peu sur sa diversité génétique. Afin de mieux comprendre cette dernière, une collection historique de 62 isolats de C. sojina, dont 10 du Brésil, 7 de la Chine, 1 du Nigéria et 44 des États-Unis, a été utilisée pour analyser la diversité génétique à l'aide des marqueurs du polymorphisme de longueur de fragments amplifiés (AFPL). La similarité génétique moyenne chez les isolats était de 0.56, sur une échelle de 0 à 1, indiquant un degré élevé de diversité génétique au sein de l'espèce. L'analyse par grappes a donné deux grappes majeures et sept sous-grappes. Deux isolats collectés en Géorgie étaient groupés, ainsi que deux collectés en Chine. Mis à part ces deux groupes, aucune séparation nette des isolats, basée sur l'origine, n'a été décelée. Nos résultats prouvent qu'il existe une réelle diversité génétique chez les espèces de C. sojina et que, sur le plan de l'amélioration du soja, la résistance à un grand éventail d'hôtes devrait être ciblée.
Keywords:
Mots clés:
Introduction
Cercospora sojina Hara, the causal agent of frogeye leaf spot of soybean (Glycine max (L.) Merr.), causes yield reductions worldwide. Wrather et al. (Citation2010) estimated that frogeye leaf spot reduced soybean yields by 427 600 metric tons across the top eight soybean-producing countries in 2006; of this, 300 and 345 100 metric ton reductions were estimated for Canada and the United States, respectively. In the United States, estimated soybean yield reductions caused by frogeye leaf spot ranged between 183 868 and 345 148 metric tons from 2006 to 2009 (Koenning & Wrather, Citation2010). The use of host resistance has been an economical way to manage frogeye leaf spot, but the development of new virulent races that overcome single genes for resistance (Rcs genes) has been a hindrance to developing cultivars with durable resistance to C. sojina (Athow et al., Citation1962; Ross, Citation1968; Phillips & Boerma, Citation1981; Mian et al., Citation2008). Application of foliar fungicides has been another method of managing frogeye leaf spot; however, C. sojina isolates with resistance to quinone outside inhibitor (QoI) fungicides have been reported recently (Zhang et al., Citation2012a ).
Based on the results of assays that measured virulence on soybean differential lines and sensitivity to QoI fungicides, the variability within C. sojina appeared high. Mian et al. (Citation2008) evaluated 98 C. sojina isolates from different countries on 12 differential soybean lines, and found that the majority of these isolates could be classified within 11 different race groups. Evaluating 50 C. sojina isolates from Ohio on 12 differential soybean lines, Cruz & Dorrance (Citation2009) reported a total of 20 different pathotype groups. Zhang et al. (Citation2012b ) evaluated the in vitro sensitivities of 163 individual C. sojina isolates to the QoI fungicides azoxystrobin, pyraclostrobin, and trifloxystrobin, and found that the range of effective concentrations that inhibited 50% of conidial germination (EC50) varied from 19-fold to 52-fold among these isolates, depending on the fungicide. Although the diversity of C. sojina has been measured in the aforementioned ways, little is known about the actual genetic diversity within C. sojina. The objective of this study was to determine the genetic diversity of a worldwide collection of C. sojina isolates with amplified fragment length polymorphism (AFLP) markers.
Table 1. Collection of 62 Cercospora sojina isolates used in the genetic diversity study
Materials and methods
A set of C. sojina isolates was used to determine the genetic diversity within the species. These isolates were collected from soybean in a number of locations within the United States (44 isolates) and from Brazil (10 isolates), China (7 isolates), and Nigeria (1 isolate) (). The isolates were a subset of a collection that had been collected over a span of approximately 30 years by Dr Daniel Phillips (approximately mid-1970s to mid-2000s). These purified isolates were subcultured on soybean stem lima bean agar (SSLBA) (Phillips & Boerma, Citation1981) and incubated for about 1 week at room temperature (25 ± 2 °C) under ambient light until the agar surface was covered with mycelia and spores. For DNA extraction from these isolates, plugs (0.3 cm2) of each isolate were aseptically transferred to 50 mL aliquots of potato dextrose broth (PDB; HiMedia Laboratories, Mumbai, India) and vortexed for 30 s. The fungal suspensions were incubated on a shaker (140 rpm) for 5 days at room temperature under ambient light, and mycelial mats were harvested and placed into 1.5 mL microcentrifuge tubes and centrifuged at 11 500 rpm for 5 min (centrifuge model Marathon 16KM, Fisher Scientific, Hampton, NH) and the supernatants were discarded. The tubes and contents were re-centrifuged and the supernatants were discarded. The pellet was transferred to FastDNA kit tubes (Qbiogene, Inc., Carlsbad, CA) and DNA was extracted following the manufacturer's instructions. In addition, PCR inhibitors were removed following the methods published by Malvick & Grunden (Citation2005). DNA concentrations were determined by electrophoresis in agarose gel with Lambda DNA (New England BioLabs, Inc., Ipswich, MA) diluted to 5, 10, 50, 100, 150, 200, 250 and 300 ng μg−1. If the DNA concentration was less than 30 ng μg−1 for a C. sojina isolate, DNA was extracted again from that isolate until a DNA concentration of at least 30 ng μg−1 was achieved.
A preliminary screen for polymorphisms was carried out on 10 C. sojina isolates. The protocol outlined by Vos et al. (Citation1995) was used for digestion of genomic DNA with the following adjustment in the digestion conditions: 250 ng of DNA was digested with 5 U of EcoR I and 5 U of Mse I at 37 °C for 3 h. The reaction conditions for adapter ligation and pre-amplification described by Vos et al. (Citation1995) were used with the exception that 1 U of Taq polymerase was used. IRDye™ 700 and Dye™ 800 labelled EcoR I primers were purchased from Li-Cor (Lincoln, NE) and Mse I primers were obtained from Bioneer (Alameda, CA). The reaction conditions described in the AFLP Protocols for Use on the Li-Cor System (Lincoln, NE) were followed.
An equal volume of loading dye (95% formamide, 20 mM EDTA, 0.08% bromophenol blue) was added to each sample, which was then denatured at 95 °C for 3 min and placed on ice for 2 min before loading. The sample volume was set to 1.0 μL and loaded with a Hamilton multi-channel pipette onto 25 cm gels prepared with 6.5% KB Plus Polyacrylamide Gel Matrix. Electrophoresis and detection of the AFLP fragments were performed on a Li-Cor IR2 Automated DNA Sequencer (Li-Cor, Lincoln, NE). The electrophoresis parameters were set to: 1500 V, 40.0 mA, 40.0 W, 50 °C and scan speed of 4. The run time was set to 2.0 h and gel images were saved as TIF files for analysis.
The gel images were scored using a binary scoring system, where the presence and absence of bands were scored as 1 and 0, respectively. The data were exported into a spreadsheet and formatted for the NTSYSpc (v. 2.1) cluster analysis software (Exeter Software Co., New York). The AFLP marker data were used to compute pair-wise simple matching similarity coefficients. Cluster analysis was performed on the similarity matrix using the “unweighted pair group method using arithmetic means” (UPGMA) algorithm provided in the software package NTSYSpc. The cophenetic correlation coefficient was calculated to test the goodness of fit between the similarity and the cophenetic matrices.
Results and discussion
The polymorphism rate varied among AFLP primer combinations. A survey of AFLP primer combinations using 10 C. sojina isolates led to the selection of eight sets of EcoR I/Mse I primers with three nucleotide extensions for fingerprinting the entire collection. The number of polymorphic markers generated by each primer set ranged from one to eight. A total of 40 polymorphic markers were scored and analysed ().
Table 2. Amplified fragment length polymorphism (AFLP) primers used to assess the genetic diversity among Cercospora sojina isolates
The average genetic similarity of the 62 C. sojina isolates was 0.56 on a scale between 0 and 1, indicating a high degree of genetic diversity within the species. Other Cercospora species have been reported to have a substantial amount of genetic diversity, such as C. beticola Sacc. (Moretti et al., Citation2004, Citation2006; Groenewald et al., Citation2008), C. canescens Ellis & Martin (Joshi et al., Citation2006), C. coffeicola Berk. & Cooke (Martins et al., Citation2008), and C. kikuchii (Matsumoto & Tomoy.) Gardner (Almeida et al., Citation2005; Cai & Schneider, Citation2005; Lura et al., Citation2011). Conversely, C. zeae-maydis Tehon & Daniels and C. zeina Crous & Braun (originally reported as C. zeae-maydis Group I and Group II, respectively) (Wang et al., Citation1998; Dunkle & Levy, Citation2000; Okori et al., Citation2003; Crous et al., Citation2006) and C. sorghi Ellis & Everh. (Okori et al., Citation2004) have little reported genetic diversity within a species.
From our research, cluster analysis resulted in two major clusters and seven sub-clusters (). Two isolates collected from Georgia (S8 and S14) were the most closely related, sharing a similarity of 0.97. Two isolates from China (S20 and S26) also were clustered together with a similarity of 0.94. Besides these four isolates, no clear separation of isolates based on origin was found. Cercospora sojina isolates collected from the midwestern United States (Illinois, Iowa and Wisconsin) were in sub-clusters 1, 4 and 5; isolates from the southern United States (Alabama, Arkansas, Georgia, Louisiana, Mississippi and South Carolina) were represented in all seven sub-clusters; and isolates from Brazil were represented in sub-clusters 1, 2, 4, 5 and 6. Isolates from China were represented in sub-clusters 2, 3, 4 and 7, and the isolate from Nigeria was in sub-cluster 7 (). Cluster analysis of 93 C. sojina isolates, which included 59 of the same isolates we tested, based on the reaction of 10 soybean differential cultivars, resulted in 13 clusters (Mian et al., Citation2008). In that study, Mian et al. (Citation2008) reported that isolates from the midwestern United States were represented in 2 clusters, isolates from the southern United States were represented in all 13 clusters, isolates from Brazil were represented in 8 clusters, and isolates from China were represented in 7 clusters. Our results confirm those reported by Mian et al. (Citation2008) that a high level of diversity exists among these C. sojina isolates.
Fig. 1. Phenogram based on simple matching similarity coefficients among 62 Cercospora sojina isolates collected from four countries. The seven clusters are indicated by Roman numerals. The last letter of the isolate name represents the country where the isolate was collected; U: United States; B: Brazil, C: China; N: Nigeria. The cophenetic correlation coefficient r = 0.80.
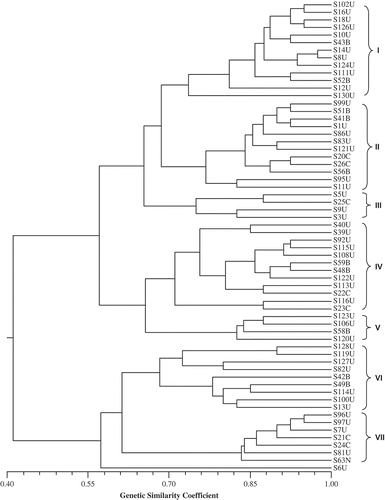
Table 3. Geographic distribution of the Cercospora sojina isolates among the seven sub-clusters based on amplified fragment length polymorphism (AFLP) markers
We reported a high level of diversity among C. sojina isolates herein, but no sexual stage for C. sojina is known. Although sexual recombination is not the only factor that determines genetic diversity, fungi with sexual fungal populations tend to be more genetically diverse than asexual populations (Milgroom, Citation1996). Groenewald et al. (Citation2006) evaluated several Cercospora species for mating type distributions, and found evidence that suggests sexual cycles may be active in C. beticola, C. zeae-maydis and C. zeina populations. Additional research by Groenewald et al. (Citation2008) and Bolton et al. (Citation2012) reaffirmed the idea that some populations of C. beticola may be reproducing sexually. Cai & Schneider (Citation2008) also suggested that a cryptically functioning sexual stage may be active in some C. kikuchii populations. In light of the evidence that suggests potential active sexual stages in other Cercospora species, along with the high level of genetic diversity we found in C. sojina, additional research should be conducted to determine the frequency of mating type genes in C. sojina populations. Asexual processes such as vegetative compatibility, parasexual recombination, and mutation also should be considered as possible reasons for the high level of genetic diversity in C. sojina, since no sexual stage has been identified for this fungus. Vegetative compatibility has been demonstrated in other species of Cercospora, such as C. kikuchii and C. coffeicola (Cai & Schneider, Citation2005; Martins et al., Citation2008), and additional research should be conducted to investigate vegetative compatibility in C. sojina.
Our results provide evidence that substantial genetic diversity exists within the species of C. sojina and that selection for broad-spectrum host resistance should be targeted in soybean breeding programmes. Three single genes are recognized as providing resistance to C. sojina, which are Rcs1, Rcs2 and Rcs3 (Athow & Probst, Citation1952; Athow et al., Citation1962; Phillips & Boerma, Citation1982; Boerma & Phillips, Citation1983). Virulent races of C. sojina that can affect cultivars with the Rcs1 and Rcs2 genes have been reported (Athow et al., Citation1962; Ross, Citation1968; Phillips & Boerma, Citation1981), but the Rcs3 gene has been reported to confer resistance to all observed C. sojina populations in the United States (Phillips & Boerma, Citation1982; Mian et al., Citation1998; Phillips, Citation1999; Cruz & Dorrance, Citation2009). In light of the high level of diversity present among C. sojina isolates reported in this article and by Mian et al. (Citation2008), the selection of virulent races that can overcome Rcs3 is a risk. Recently, potentially new sources of resistance to frogeye leaf spot were identified (Mian et al., Citation2009; Mengistu et al., Citation2011). These potential new sources of resistance should continue to be characterized, and additional sources of broad resistance to multiple populations of C. sojina should continue to be identified.
Recently, C. sojina isolates resistant to QoI fungicides were reported in North America (Zhang et al., Citation2012a ). The high level of diversity among C. sojina isolates likely was a factor in the selection of QoI fungicide-resistant isolates. The selection of fungicide-resistant strains of other phytopathogenic Cercospora species has been somewhat common. Strains of C. arachidicola Hori, C. beticola and C. kikuchii with resistance to methyl benzimidazole carbamate (MBC) fungicides have been reported (Georgopoulos & Dovas, Citation1973; Clark et al., Citation1974; Littrell, Citation1974; Rupel & Scott, Citation1974; Smith & Littrell, Citation1980; Bugbee, Citation1996; Campbell et al., Citation1998; Weiland & Halloin, Citation2001; Imazaki et al., Citation2006). In addition, populations of C. beticola with reduced sensitivity to demethylation inhibitor (DMI) fungicides (Karaoglanidis et al., Citation2000, Citation2002; Secor et al., Citation2010), QoI fungicides (Malandrakis et al., Citation2006; Secor et al., Citation2010), and triphenyltin hydroxide fungicides (Giannopolitis, Citation1978; Bugbee, Citation1995, 1996; Campbell et al., Citation1998) have been reported. Considering the high level of genetic diversity within C. sojina, and the fact that strains of C. sojina and other Cercospora species have been selected with resistance to fungicides, the risk of selecting strains of C. sojina with resistance to additional fungicide classes may be elevated.
References
- Almeida , A.M.R. , Piuga , F.F. , Marin , S.R.R. , Binneck , E. , Sartori , F. , Costamilan , L.M. , Teixeira , M.R.O. and Lopes , M. 2005 . Pathogenicity, molecular characterization, and cercosporin content of Brazilian isolates of Cercospora kikuchii. Fitopat. Brasil . 30 : 594 – 602 .
- Athow , K.L. and Probst , A.H. 1952 . The inheritance of resistance to frogeye leaf spot of soybeans . Phytopathology , 42 : 660 – 662 .
- Athow , K.L , Probst , A.H. , Kartzman , C.P. and Laviolette , F.A. 1962 . A newly identified physiological race of Cercospora sojina on soybean . Phytopathology , 52 : 712 – 714 .
- Boerma , H.R. and Phillips , D.V. 1983 . Genetic implications of the susceptibility of Kent soybean to . Cercospora sojina. Phytopathology , 74 : 1666 – 1668 .
- Bolton , M.D. , Secor , G.A. , Rivera , V. , Weiland , J.J. , Rudolph , K. , Birla , K. , Rengifo , J. and Campbell , L.G. 2012 . Evaluation of the potential for sexual reproduction in field populations of Cercospora beticola from USA . Fungal Biol. , 116 : 511 – 521 .
- Bugbee , W.M. 1995 . Cercospora beticola tolerant to triphenyltin hydroxide . J. Sugarbeet Res. , 32 : 167 – 174 .
- Bugbee , W.M. 1996 . Cercospora beticola strains from sugar beet tolerant to triphenyltin hydroxide and resistant to thiophanate methyl . Plant Dis. , 80 : 103
- Cai , G. and Schneider , R.W. 2005 . Vegetative compatibility groups on Cercospora kikuchii, the causal agent of Cercospora leaf blight and purple seed stain in soybean . Phytopathology , 95 : 257 – 261 .
- Cai , G. and Schneider , R.W. 2008 . Population structure of Cercospora kikuchii, the causal agent of cercospora leaf blight and purple seed stain in soybean . Phytopathology , 98 : 823 – 829 .
- Campbell , L.G. , Smith , G.A. , Lamey , H.A. and Cattanach , A.W. 1998 . Cercospora beticola tolerant to triphenyltin hydroxide and resistant to thiophanate methyl in North Dakota and Minnesota . J. Sugarbeet Res. , 35 : 29 – 41 .
- Clark , E.M. , Backman , P.A. and Rodriguez-Kabana , R. 1974 . Cercospora and Cercosporidium tolerance to benomyl and related fungicides in Alabama peanut fields . Phytopathology , 64 : 1476 – 1477 .
- Crous , P.W. , Groenewald , J.Z. , Groenewald , M. , Caldwell , P. , Braun , U. and Harrington , T.C. 2006 . Species of Cercospora associated with grey leaf spot of maize . Studies Mycol. , 55 : 189 – 197 .
- Cruz , C.D. and Dorrance , A.E. 2009 . Characterization and survival of Cercospora sojina in Ohio. Online . Plant Health Progress , doi: 10.1094/PHP-2009-0512-03-RS
- Dunkle , L.D. and Levy , M. 2000 . Genetic relatedness of African and United States populations of Cercospora zeae-maydis . Phytopathology , 90 : 486 – 490 .
- Georgopoulos , S.G. and Dovas , C. 1973 . Occurrence of Cercospora beticola strains resistant to benzimidazole fungicides in northern Greece . Plant Dis. Rep. , 62 : 321 – 324 .
- Giannopolitis , C.N. 1978 . Occurrence of strains of Cercospora beticola resistant to triphenyltin fungicides in Greece . Plant Dis. Rep. , 62 : 205 – 208 .
- Groenewald , M. , Groenewald , J.Z. , Harrington , T.C. , Abeln , E.C.A. and Crous , P.W. 2006 . Mating type gene analysis in apparently asexual Cercospora species is suggestive of cryptic sex . Fungal Genet. Biol. , 43 : 813 – 825 .
- Groenewald , M. , Linde , C.C. , Groenewald , J.Z. and Crous , P.W. 2008 . Indirect evidence for sexual reproduction in Cercospora beticola populations from sugar beet . Plant Pathol. , 57 : 25 – 32 .
- Imazaki , I. , Ishikawa , K. , Yasuda , N. , Miyasaka , A. , Kawasaki , S. and Koizumi , S. 2006 . Incidence of thiophanate-methyl resistance in Cercospora kikuchii within a single lineage based on amplified fragment length polymorphisms in Japan . J. Gen. Plant Pathol. , 72 : 77 – 84 .
- Joshi , A. , Souframanien , J. , Chand , R. and Pawar , S.E. 2006 . Genetic diversity study of Cercospora canescens (Ellis & Martin) isolates, the pathogen of cercospora leaf spot in legumes . Curr. Sci. , 90 : 564 – 568 .
- Karaoglanidis , G.S. , Ioannidis , P.M. and Thanassoulopoulos , C.C. 2000 . Reduced sensitivity of Cercospora beticola isolates to sterol-demethylation-inhibiting fungicides . Plant Pathol. , 49 : 567 – 572 .
- Karaoglanidis , G.S. , Ioannidis , P.M. and Thanassoulopoulos , C.C. 2002 . Changes in sensitivity of Cercospora beticola populations to sterol-demethylation-inhibiting fungicides during a 4-year period in northern Greece . Plant Pathol. , 51 : 55 – 62 .
- Koenning , S.R. and Wrather , J.A. 2010 . Suppression of soybean yield potential in the continental United States by plant diseases from 2006 to 2009. Online . Plant Health Progress , doi: 10.1094/PHP-2010-1122-01-RS
- Littrell , R.H. 1974 . Tolerance in Cercospora arachidicola to benomyl and related fungicides . Phytopathology , 64 : 1377 – 1378 .
- Lura , M.C. , Rapela , M.G.L. , Vaccari , M.C. , Maumary , R. , Soldano , A. , Mattio , M. and Gonzalez , A.M. 2011 . Genetic diversity of Cercospora kikuchii isolates from soybean cultured in Argentina as revealed by molecular markers and cercosporin production . Mycopathologia , 171 : 361 – 371 .
- Malandrakis , A.A. , Markoglou , A.N. , Nikou , D.C. , Vontas , J.G. and Ziogas , B.N. 2006 . Biological and molecular characterization of laboratory mutants of Cercospora beticola resistant to Qo inhibitors . Eur. J. Plant Pathol. , 116 : 155 – 166 .
- Malvick , D.K. and Grunden , E. 2005 . Isolation of fungal DNA from plant tissues and removal of DNA amplification inhibitors . Mol. Ecol. Notes , 5 : 958 – 960 .
- Martins , R.B. , Maffia , L.A. and Mizubuti , E.S.G. 2008 . Genetic variability of Cercospora coffeicola from organic and conventional coffee plantings, characterized by vegetative compatibility . Phytopathology , 98 : 1205 – 1211 .
- Mengistu , A. , Bond , J. , Mian , R. , Nelson , R. , Shannon , G. and Wrather , A. 2011 . Identification of soybean accessions resistant to Cercospora sojina by field screening, molecular markers, and phenotyping . Crop Sci. , 51 : 1101 – 1109 .
- Mian , M.A.R. , Boerma , H.R. , Phillips , D.V. , Kenty , M.M. , Shannon , G. , Shipe , E.R. , Soffes Blount , A.R. and Weaver , D.B. 1998 . Performance of frogeye leaf spot resistant and susceptible near isolines of soybean . Plant Dis. , 82 : 1017 – 1021 .
- Mian , M.A.R. , Missaoui , A.M. , Walker , D.R. , Phillips , D.V. and Boerma , H.R. 2008 . Frogeye leaf spot of soybean: a review and proposed race designations for isolates of Cercospora sojina Hara . Crop Sci. , 48 : 14 – 24 .
- Mian , R. , Bond , J. , Joobeur , R. , Mengistu , A. , Wiebold , W. , Shannon , G. and Wrather , A. 2009 . Identification of soybean genotypes resistant to Cercospora sojina by field screening and molecular markers . Plant Dis. , 93 : 408 – 411 .
- Milgroom , M.G. 1996 . Recombination and the multilocus structure of fungal populations . Annu. Rev. Phytopathol. , 34 : 457 – 477 .
- Moretti , M. , Saracchi , M. and Farina , G. 2004 . Morphological, physiological and genetic diversity within a small population of Cercospora beticola Sacc . Annals Microbiol. , 54 : 129 – 150 .
- Moretti , M. , Karaoglanidis , G. , Saracchi , M. , Fontana , A. and Farina , G. 2006 . Analysis of genotypic diversity in Cercospora beticola Sacc. field isolates . Annals Microbiol. , 56 : 215 – 221 .
- Okori , P. , Fahleson , J. , Rubaihayo , P.R. , Adipala , E. and Dixelius , C. 2003 . Assessment of genetic variation among east African . Cercospora zeae-maydis. Afric. Crop Sci. J. , 11 : 75 – 85 .
- Okori , P. , Rubaihayo , P.R. , Ekwamu , A. , Fahleson , J. and Dixelius , C. 2004 . Genetic characterization of Cercospora sorghi from cultivated and wild sorghum and its relationship to other Cercospora fungi . Phytopathology , 94 : 743 – 750 .
- Phillips , D.V. 1999 . “ Frogeye leaf spot ” . In Compendium of Soybean Diseases , Fourth , Edited by: Hartman , G.L. , Sinclair , J.B. and Rupe , J.C. 20 – 21 . St. Paul , MN : American Phytopathological Society Press .
- Phillips , D.V. and Boerma , H.R. 1981 . Cercospora sojina race 5: a threat to soybean in the southeastern United States . Phytopathology , 71 : 334 – 336 .
- Phillips , D.V. and Boerma , H.R. 1982 . Two genes for resistance to race 5 of Cercospora sojina in soybeans . Phytopathology , 72 : 764 – 766 .
- Ross , J.T. 1968 . Additional physiological races of Cercospora sojina on soybean in North Carolina . Phytopathology , 58 : 708 – 709 .
- Rupel , E.G. and Scott , P.R. 1974 . Strains of Cercospora resistant to benomyl in the U.S.A . Plant Dis. Rep. , 58 : 434 – 436 .
- Secor , G.A. , Rivera , V.V. , Khan , M.F.R. and Gudmestad , N.C. 2010 . Monitoring fungicide sensitivity of Cercospora beticola of sugar beet for disease management decisions . Plant Dis. , 94 : 1272 – 1282 .
- Smith , D.H. and Littrell , R.H. 1980 . Management of peanut foliar diseases with fungicides . Plant Dis. , 64 : 356 – 360 .
- Vos , P. , Hogers , R. , Bleeker , M. , Reijans , M. , van de Lee , T. , Hornes , M. , Frijters , A. , Pot , J. , Peleman , J. , Kuiper , M. and Zabeau , M. 1995 . AFLP: a new technique for DNA fingerprinting . Nucleic Acids Res. , 23 : 4407 – 4414 .
- Wang , J. , Levy , M. and Dunkle , L.D. 1998 . Sibling species of Cercospora associated with gray leaf spot of maize . Phytopathology , 88 : 1269 – 1275 .
- Weiland , J.J. and Halloin , J.M. 2001 . Benzimidazole resistance in Cercospora beticola sampled from sugarbeet fields in Michigan, U.S.A . Can. J. Plant Pathol. , 23 : 78 – 82 .
- Wrather , A. , Shannon , G. , Balardin , R. , Carregal , L. , Esobar , R. , Gupta , G.K. , Ma , Z. , Morel , W. , Ploper , D. and Tenuta , A. 2010 . Effect of diseases on soybean yield in the top eight producing countries in 2006. Online . Plant Health Progress , doi: 10.1094/PHP-2010-0125-01-RS
- Zhang , G. , Pedersen , D.K. , Phillips , D.V. and Bradley , C.A. 2012b . Sensitivity of Cercospora sojina isolates to quinone outside inhibitor fungicides . Crop Prot. , 40 : 63 – 68 .
- Zhang , G.R. , Newman , M.A. and Bradley , C.A. 2012a . First report of the soybean frogeye leaf spot fungus (Cercospora sojina) resistant to quinone outside inhibitor fungicides in North America . Plant Dis. , 96 : 767