Abstract
Pseudozyma flocculosa (syn: Sporothrix flocculosa) was first discovered and described in 1987 as an epiphytic yeast on powdery mildew-infected clover leaves. It was subsequently found to be a powerful antagonist of powdery mildews which prompted its study and development as a biocontrol agent (BCA). Most BCAs exert their activity through the manifestation of one or more of the following modes of action: competition, parasitism, antibiosis and induced resistance. In the case of P. flocculosa, in vitro bioassays, electron microscopy studies, and chemical analyses all pointed to a single mode of action: antibiosis. This conclusion was reinforced by the characterization and purification of an active molecule, flocculosin, and by the demonstration of its powerful antimicrobial activity. This was further supported by the discovery of a complex gene cluster regulating the synthesis of flocculosin, a molecule nearly identical to ustilagic acid – a compound produced by U. maydis under the control of a similar gene cluster. Despite this strong evidence, there is new evidence to indicate that flocculosin plays a secondary, if any, role in the antagonistic activity of P. flocculosa. The biocontrol process instead appears to be mediated by an intricate interaction involving nutrients produced by the plant, harvested by the phytopathogen and exploited by P. flocculosa. With the imminent completion of sequencing of the P. flocculosa genome, the recent publication of the barley powdery mildew genome (Blumeria graminis f. sp. hordei), and the availability of many plant genomes, the latest developments in DNA sequencing and transcriptomic analyses will allow unparalleled insight into the complex and delicate balance defining this unique tripartite interaction.
Résumé
Pseudozyma flocculosa (syn.: Sporothrix flocculosa) a été découvert et décrit pour la première fois en 1987 comme une levure épiphyte se développant sur les feuilles de trèfle infectées par le blanc. Par la suite, il a été démontré qu'il s'agissait d'un puissant antagoniste des blancs, ce qui a suscité son étude et son développement à titre d'agent de lutte biologique (ALB). La plupart des ALB agissent selon un ou plusieurs des modes suivants: compétition, parasitisme, antibiose et résistance induite. Dans le cas de P. flocculosa, des épreuves biologiques in vitro, des études au microscope électronique et des analyses chimiques ont toutes indiqué un seul mode d'action: l'antibiose. Cette conclusion a été renforcée par la caractérisation et la purification d'une molécule active, la flocculosine, ainsi que par la démonstration de sa puissante activité antimicrobienne. Ceci a de plus été appuyé par la découverte d'un groupe de gènes complexe qui régule la synthèse de la flocculosine, une molécule presque identique à l'acide ustilagique – un composé produit par Ustilago maydis, déterminé également par un groupe de gènes semblable. Malgré cette solide preuve, il y a de nouveaux indices laissant croire que la flocculosine ne joue qu'un rôle secondaire, voire aucun, dans l'activité antagoniste de P. flocculosa. Le processus de lutte biologique semble plutôt relié à une interaction complexe impliquant des nutriments produits par la plante, récoltés par l'agent phytopathogène et utilisés par P. flocculosa. Avec la sortie imminente du séquençage du génome de P. flocculosa, la publication récente du génome du blanc de l'orge (Blumeria graminis f. sp. hordei) et la disponibilité du génome de nombreuses plantes, les dernières avancées dans le domaine du traitement des données et des analyses transcriptomiques nous offriront un aperçu sans précédent de l'équilibre complexe et fragile caractérisant cette unique interaction tripartite.
Introduction
The development and study of biocontrol agents (BCAs) against plant pathogens has been and continues to remain the focal point of research in many laboratories. While practical implementation of biological control has failed to meet early expectations, many scientific, legal and commercial reasons can explain this situation (Paulitz & Bélanger, Citation2001; Fravel, Citation2005). In many instances, our inability to properly exploit the intrinsic properties of a BCA accounts for this limited success.
Our laboratory has been working with the yeast-like epiphyte Pseudozyma flocculosa (Traquair, L. A. Shaw & Jarvis) Boekhout & Traquair (syn: Sporothrix flocculosa) for more than 20 years. From its initial discovery on powdery mildew-infected clover leaves in 1988 (Traquair et al., Citation1988), its efficacy and ability at controlling members of the Erysiphales have been the driving force for past and current initiatives to develop a biocontrol product with P. flocculosa as the main active ingredient. However, from a research perspective, the bulk of our efforts have been devoted to trying to decipher its mode of action in order to improve the expression of its biocontrol properties. From what appeared to be a straightforward case of antibiosis, this endeavour has proven to be significantly more challenging than initially anticipated.
We review in this paper the many milestones that have both enlightened and influenced the study of the mode of action of P. flocculosa over the last 20 years. At the same time, we will describe how the path has been filled with fortuitous discoveries, and unexpected phenomena that have led the way to some remarkable and unique research opportunities that could not have been anticipated 20 or even 10 years ago.
The mode of action of BCAs
Most BCAs identified to date have been categorized as exerting their activity through the manifestation of one or more of four modes of action: competition, parasitism, antibiosis and induced resistance (Whipps, Citation2001; Bélanger & Avis, Citation2002). Understanding precisely how BCAs kill their target has always been perceived as the key to maximize the efficacy of BCAs in the field. From a theoretical point of view, if the mode of action can be reduced to a single trait, this greatly facilitates manipulation and over-expression of the trait in key situations for a BCA to suppress a plant pathogen. As a matter of fact, this strategy has been tried with a few systems with mitigated results. For instance, in cases where parasitism appeared to be the predominant mode of action, several attempts have been made to increase production of lytic enzymes such as chitinases and glucanases (Kubicek et al., Citation2001; Lorito et al., Citation2001). The approach targeted either the selection of BCA strains with superior ability to produce such enzymes or the direct cloning and over-expression of relevant genes conferring greater degrading properties. These initiatives, while conceptually sound, failed to deliver BCAs with notable increased activity. In retrospect, it was generally agreed that parasitism (or at least the enzymes associated with this mode of action) is not, as initially proposed (Elad et al., Citation1982), the key factor in the biocontrol activity of some fungi, notably in the case of Trichoderma spp. (Woo et al., Citation2006).
The mode of action became paramount to the study of every BCA in the early 1990s. At the time, there seemed to have been a necessity to associate a BCA with a specific mode of action. This trend appeared to take its origin in the efforts to register a BCA as a biocontrol product. A rumour quickly circulated that if a BCA acted by antibiosis, i.e. produced antibiotics, regulation agencies around the world would deny or delay registration of the BCA. The source of this misinformation is unknown, but it had a profound impact on the scientific community working in biological control. Scientists started screening bacteria and fungi through bioassays, and systematically rejected organisms that displayed antibiosis in vitro. At the same time, some researchers went to great lengths to demonstrate that their BCAs did not act by antibiosis (Castoria et al., Citation1997; Elad et al., Citation1998). While the notion that antibiotic-producing organisms may be more difficult to register still persists, regulation agencies will not automatically reject them. In fact, one of the first biocontrol agents approved for use in Canada, Mycostop (Streptomyces griseoviridis) acts primarily through antibiosis. A thorough description of the properties of the active molecules and their fate in the environment will satisfy most requirements.
Antibiosis: the case of Pseudozyma flocculosa
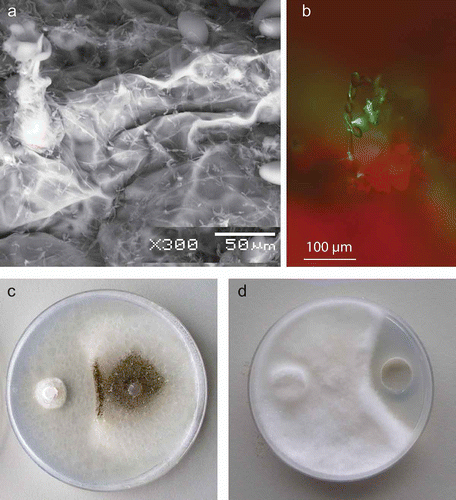
It became apparent early on that P. flocculosa had all the characteristics of acting by antibiosis, as much as we would have liked to describe it as a non-antibiotic producer. Electron microscopic studies showed that the antagonist did not penetrate powdery mildew cells but induced a rapid cell collapse ( a; Hajlaoui & Bélanger, Citation1993). In addition, we observed by using gold-labelling of chitin residues that the cell walls of powdery mildew hyphae were not degraded in the presence of P. flocculosa. These results eliminated the possibility that the fungus acted through parasitism and production of cell wall-degrading enzymes. The biological fact that powdery mildew fungi are biotrophs de facto ruled out competition for substrate. No evidence of induced resistance was ever observed, so antibiosis appeared to be the sole and logical mode of action by which P. flocculosa antagonized fungi from the Erysiphales. This conclusion was reinforced by in vitro bioassays showing clear zones of lysis when P. flocculosa was confronted to other fungi ().
Fig. 1. Pseudozyma flocculosa, 24 h post-inoculation, on powdery mildew infected leaves (a) under scanning electron microscopy; b, under fluorescent microscopy (when using a P. flocculosa strain expressing GFP; c, P. flocculosa against Phomopsis sp. on potato dextrose agar (PDA) medium after 2 days; d, flocculosin against Pythium ultimum grown for 2 days on PDA.
The search for active molecules
We began working on the premise that P. flocculosa produced molecules with antifungal properties, so we sought to isolate and purify these active compounds. Our initial approach was based on the understanding that secondary metabolites were produced in the later stage of culture. Our work identified three fatty acids displaying strong antimicrobial properties (Choudhury et al., Citation1994, Citation1995). Purification and synthesis of the most active molecule, 6-methyl-9-heptadocenoic acid, provided the opportunity to carry out bioassays aimed at identifying the molecular target of the compound.
The fatty acid induced a rapid plasmolysis of sensitive cells with the plasma membrane being seemingly affected in the presence of the molecule. By testing it against a number of fungi, it appeared that the fatty acid was more deleterious to certain fungi than others. Analysis of the plasma membrane revealed that fungi with a high sterol content were much less sensitive than fungi containing none (e.g. Pythiaceae) or limited sterols (Benyagoub et al., Citation1996; Avis & Bélanger, Citation2001). This explained, at least in part, why P. flocculosa, with a high sterol content, was not affected by its own secondary metabolites. However, given the fairly large spectrum of fungi affected by the fatty acid, it still raised the question as to why P. flocculosa was nearly exclusively antagonistic towards members of the Erysiphales.
Toward molecular approaches
A seemingly trivial change occurred at the time we were trying to decipher the mode of action of P. flocculosa. Based on new molecular tools to classify fungi, Boekhout (Citation1995) suggested that ascomycetous fungi known hitherto as Sporothrix anamorphs, including Sporothrix flocculosa, were in fact more closely related to the basidiomycetes and should be reclassified as such under the genus Pseudozyma. Further analyses confirmed this finding and Begerow et al. (Citation2000) officially renamed Sporothrix flocculosa as Pseudozyma flocculosa (). We paid little attention to this new classification until we ventured into molecular approaches.
Fig. 2. Phylogenetic relationship among different members of Ustilaginales. Note the close relationship between Pseudozyma flocculosa and Ustilago maydis in the upper clade. (Adapted from Begerow et al., Citation2000.)
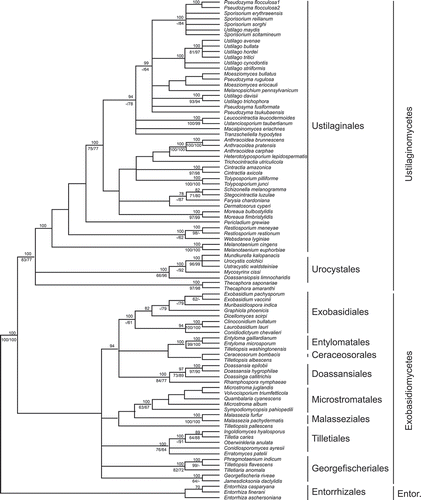
Our initial attempts to transform P. flocculosa in the early 2000s were unsuccessful. Based on the premise that the fungus was a yeast-like organism, we tried all the tools available that had been developed with the widely studied yeast Saccharomyces cerevisiae, but to no avail (Cheng et al., Citation2001). Those failures served as incentives to rethink our approach and this was when we considered for the first time the implications of the new classification. What if P. flocculosa responded better to tools developed for genetic manipulation of basidiomycete fungi? Upon further analysis, we realized that P. flocculosa belonged to the Ustilaginales and was actually closely related to the model fungus Ustilago maydis (). This prompted us to try a plasmid commonly used in U. maydis transformation. Within a fortnight, we obtained our first P. flocculosa transformants (Cheng et al., Citation2001). Through random insertion of the plasmid, some transformants had seemingly lost their ability to produce antibiotics. By analysing and comparing the metabolites present in the culture media of both the wild-type and the antibiotic-minus transformants, we discovered a compound that was abundant and unique to the wild-type. Although the compound, later named flocculosin, contained fatty acid chains, it appeared to be quite different from the fatty acids identified some 10 years earlier. As a matter of fact, it was so unusual and so complex that the structure we first described turned out to be incorrect (Cheng et al., Citation2003).
Flocculosin and ustilagic acid
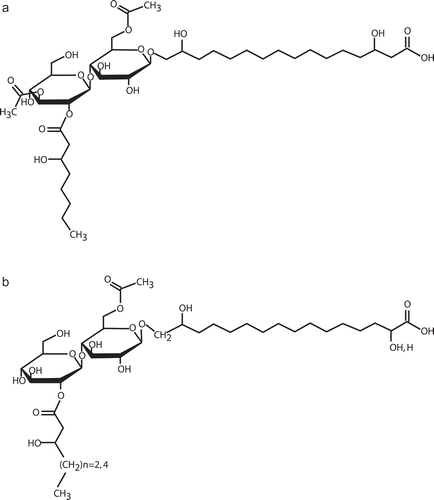
The fortuitous discovery of a paper dating back to the early 1950s and describing a glycolipid, ustilagic acid, was arguably the most altering event in our work with P. flocculosa. For one, ustilagic acid was nearly similar to flocculosin in its chemical composition and it allowed us to define the proper structure of the latter molecule (; Mimee et al., Citation2005). It consists of cellobiose, O-glycosidically linked to 3,15,16-trihydroxypalmitic acid. The sugar moiety is acylated with 2-hydroxyoctanoic acid and acetylated at two positions. Secondly and most importantly, ustilagic acid was produced by U. maydis, a coincidence reinforcing the phylogenetic link between P. flocculosa and U. maydis. Furthermore, both molecules were reported to display strong antibiotic properties (Haskins & Thorn, Citation1951; Mimee et al., Citation2005, Citation2009). While this discovery opened up the field of potential research endeavours, it raised the question about the role of ustilagic acid. If flocculosin conferred a biocontrol activity to P. flocculosa, what was the function of ustilagic acid for U. maydis considering that the fungus was strictly known as a plant pathogen?
Fig. 3. Chemical structure of (A) flocculosin and (B) ustilagic acid produced by Pseudozyma flocculosa and Ustilago maydis, respectively.
On the basis of the similarity between the two molecules, M. Bölker's group in Germany took advantage of the annotated genome of U. maydis and the particular structure of the long chain fatty acid on the glycolipid to hypothesize that a gene coding for a cytochrome P450 monooxygenase had to be involved in the synthesis of ustilagic acid. Hewald et al. (Citation2005) were thus able to identify the gene cyp1 responsible for the terminal hydroxylation of the long chain fatty acid conferring the link between the fatty acid and the sugar moiety. This discovery was the cornerstone of scientific inferences leading to the description of a unique cluster of 12 genes involved in the complete synthesis of the glycolipid (Teichmann et al., Citation2007).
We followed in the footsteps of that discovery and the phylogenetic link between P. flocculosa and U. maydis to hypothesize that P. flocculosa must contain a homolog of cyp1 in order to synthesize flocculosin. Using the U. maydis cyp1 cDNA as a probe against all known species of Pseudozyma, we were indeed able to show that it hybridized specifically with P. flocculosa (Marchand et al., Citation2009) which was the only other strain producing flocculosin. This indicated that cyp1 had to be involved in flocculosin production. The presence of cyp1 in P. flocculosa raised the obvious possibility of the existence of a cluster similar to the one found in U. maydis regulating the production of flocculosin.
Genomic sequencing of P. flocculosa
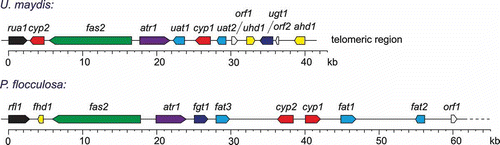
It soon became evident that genome sequencing and assembly of P. flocculosa were a necessity if we were to make significant headway into the understanding of the implication of flocculosin in the biocontrol activity of P. flocculosa. Using Roche 454 Titanium technology, we generated 525 Mb of shotgun data and 167 Mb of 2.6 and 4.5 kb paired-end sequences for a c. 30× coverage of the genome. The latest assembly contains 40 scaffolds and has a N50 value of nearly 1 Mb for an estimated genome size of 22 Mb, similar to that of U. maydis. On the basis of sequence homology with genes found in U. maydis, we were able to find a gene cluster comprising 10 genes that were necessary for the biosynthesis of flocculosin (Teichmann et al., Citation2010) (). In contrast to the cluster of U. maydis, the flocculosin biosynthesis cluster contains an additional gene encoding an acetyl-transferase and is lacking a gene homologous to the α-hydroxylase Ahd1 necessary for UA hydroxylation. The functions of three acyl/acetyl-transferase genes (Fat1, Fat2 and Fat3) including the additional acetyl-transferase were studied by complementing the corresponding U. maydis mutants (Teichmann et al., Citation2011). This showed that the additional acetyl-transferase is necessary for acetylation of the glucose moiety, explaining the differences between the two molecules.
Is flocculosin involved in the biocontrol activity of P. flocculosa?
The fortuitous discovery that two seemingly unrelated fungi with opposite lifestyles would be found to be very similar genetically has created a very unique opportunity to investigate the subtle genetic and molecular phenomena that differentiate the two types of pathogens; a BCA and a plant pathogen. At the same time, it has raised important questions about the role of the glycolipids in the respective ecology of the two fungi.
On the basis of the new phylogeny of Ustilaginales, it is quite clear that Pseudozyma spp. are either anamorph vestiges or descendants of Ustilago spp. which have lost the ability (or the necessity) for sexual reproduction. However, as the diploid state is essential to initiate the infection process in smut fungi (Bakkeren et al., Citation2008), Pseudozyma spp. have not maintained the pathogenic state of their diploid counterparts. In the same vein, it is quite revealing that U. maydis can produce ustilagic acids only in the haploid state (Hewald et al., Citation2005). Therefore, is the glycolipid-production trait in U. maydis really linked to pheromone recognition as suggested by Hewald et al. (Citation2005), considering that P. flocculosa, the only other known producer of similar glycolipids, has lost its mating capabilities? Is this trait essential for P. flocculosa survival or more a measure of its fitness? If it is the latter case, does it have the same ecological role during the anamorph state of U. maydis which could explain the conservation of the trait among related species?
We had always assumed that antibiosis was the main mode of action of P. flocculosa based on the overwhelming evidence supporting this conclusion. Because it appeared to be dictated by the ability to produce flocculosin, it would thus stand to reason that any organism capable of synthesizing a similar glycolipid would have biocontrol properties. Bölker's group (Teichmann et al., Citation2007) had suggested that U. maydis had biocontrol potential against Botrytis cinerea. However, this suggestion came from the limited observation that ustilagic acid was fungitoxic to the spores of B. cinerea.
In an effort to correlate, or refute, the production of flocculosin (or ustilagic acid) with biocontrol properties, we tested and compared P. flocculosa, U. maydis and other species of Pseudozyma which did or did not produce glycolipids. In all our bioassays, only P. flocculosa was capable of antagonizing powdery mildews. Using GFP-technology, we observed that none of the other tested fungi could grow over the colonies, while P. flocculosa developed abundantly in the presence of the powdery mildew host. Interestingly, this result was confirmed by q-PCR analyses whereby the population of P. flocculosa increased dramatically within the first 72 h while that of the other compared fungi declined (; Clément-Mathieu et al., Citation2008).
Fig. 5. a, Microscope observations of leaf colonization by four GFP-transformed Pseudozyma spp. on Podosphaera xanthii-infected leaf disks from Cucumis sativus L. ‘Corona’, 24 h after treatment. b, Quantification by RT (real time)-PCR of population development over time of four Pseudozyma spp. following inundative application of conidial suspensions on P. xanthii-infected leaf disks from Cucumis sativus L. ‘Corona’. Ratios represent the number of cells at a specific time ([t]) divided by the initial number of sprayed cells ([t0]). Error bars represent the standard error for each treatment.
![Fig. 5. a, Microscope observations of leaf colonization by four GFP-transformed Pseudozyma spp. on Podosphaera xanthii-infected leaf disks from Cucumis sativus L. ‘Corona’, 24 h after treatment. b, Quantification by RT (real time)-PCR of population development over time of four Pseudozyma spp. following inundative application of conidial suspensions on P. xanthii-infected leaf disks from Cucumis sativus L. ‘Corona’. Ratios represent the number of cells at a specific time ([t]) divided by the initial number of sprayed cells ([t0]). Error bars represent the standard error for each treatment.](/cms/asset/e7a0c761-b578-4052-8738-fd4e7c12030e/tcjp_a_726649_o_f0005g.jpg)
We concluded from these latter results that the biocontrol specificity of P. flocculosa could not be attributed to its ability to produce glycolipids alone, given that other similar organisms producing the same glycolipids were incapable of biocontrol activity. The results rather suggested that the particular properties of P. flocculosa, if modulated by flocculosin, were highly dependent on other factors stimulating the growth and development of the yeast-like fungus in presence of powdery mildew colonies.
The search for an alternate mode of action
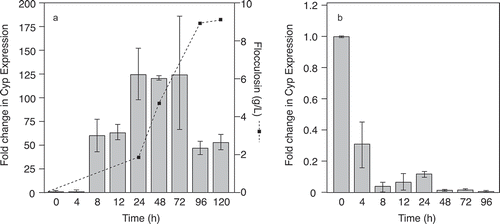
We had to contemplate a new hypothesis after nearly 20 years of working on the assumption that antibiosis was the mode of action of P. flocculosa. With access to new genetic tools, we set out to determine if subtle factors came into play to provide P. flocculosa with special mechanisms to release flocculosin when in contact with powdery mildews. For this purpose, we followed the expression of cyp1, a key gene involved in the synthesis of flocculosin. Initially, in vitro experiments confirmed that the gene was highly over-expressed when the fungus was grown under conditions conducive to flocculosin release ( a; Marchand et al., Citation2009). This result confirmed that the gene was a reliable marker to follow flocculosin release in situ. We proceeded to follow the expression of the gene over time following the confrontation between P. flocculosa and powdery mildew colonies. We surmised that if flocculosin was important in the process, the fungus would release it in the early stages of antagonism given that it usually overtook colonies within 12 h. This notion was proven incorrect as we could not find any significant increase in cyp1 expression at any time during the antagonistic process ( b). It thus appears that in spite of all the evidence and the conservation of a complex trait aimed at producing an antifungal compound, the latter bears no direct role in the biocontrol activity of P. flocculosa. The ultimate demonstration would obviously come from engineering a flocculosin-minus mutant and further assessment of the phenotype. However, despite repeated attempts and application of different approaches, it has been impossible so far to obtain P. flocculosa mutants via homologous recombination. On the other hand, Bölker's group was able to obtain U. maydis mutants altered in their ability to produce ustilagic acid. Such mutants were still able to mate and infect, which prompted Hewald et al. (Citation2005) to suggest that ustilagic acid acted possibly as a pheromone facilitating recognition between two compatible mating strains. If it is indeed the case, this would mean that the trait serves a different purpose for P. flocculosa, given that it is the only other known producer of similar glycolipids, and it has lost its mating capabilities.
Fig. 6. Reverse transcription RT-PCR analysis of Pseudozyma flocculosa actin-normalized cyp1 expression with (a) flocculosin quantification within a growth medium conducive to flocculosin production; and (b) after inundative applications of conidia on powdery mildew-infected cucumber leaf discs. Error bars represent the standard error for each treatment.
It is now clear that antibiosis, if involved at all, represents a minor component in the overall antagonistic process of P. flocculosa against powdery mildews. Considering that BCAs are reported to act by one (or more) of four modes of action – competition, antibiosis, parasitism or resistance inducer – we have seemingly run out of alternatives. Parasitism and induced resistance can be ruled out on the basis of overwhelming evidence against either mode of action (Marchand et al., Citation2007). This leaves competition, a mechanism highly improbable in the context of the specificity of P. flocculosa against biotrophic powdery mildews. Indeed, considering the latter having no saprophytic phase, it is hard to conceive how competition for substrate or ecological niche would take place. Besides, the activity of P. flocculosa is manifest when the powdery mildews are anchored into the epidermal cells.
A possible explanation came from the observation of a peculiar phenomenon. Pseudozyma flocculosa will display distinct morphologies in culture depending on the nutrients in the media. Incidentally, the morphology resembling the most typical of P. flocculosa growth on powdery mildew colonies can be easily replicated by adjusting micro-elements. By systematically adding or subtracting individual micro-elements in the medium, we observed that the complex of Zn/Mn played a key role in the interaction. On the other hand, U. maydis did not appear to have the same nutritional requirements and hence lacked the ability to colonize powdery mildews. Strangely enough, a simple solution of Zn/Mn sprayed on a leaf stimulated the development of P. flocculosa in absence of the pathogen (Hammami et al., Citation2011). The latter interactions indicate that some form of competition or exchange of nutrients takes place between P. flocculosa and powdery mildews. The BCA relies on micronutrients for its development and would draw them from the pathogen, which draws them from the plant. It would thus constitute an intricate tripartite interaction. Evidence supporting this sophisticated system of antagonism is threefold : (1) the development of P. flocculosa is halted as soon as the powdery mildew colonies have collapsed thus interrupting the flow of nutrients from the plants; (2) P. flocculosa will not antagonize powdery mildew spores separated from their host; and (3) this phenomenon can only be observed with the biotrophic powdery mildews that maintain an intimate association with the plant and not with necrotrophs such as Botrytis cinerea (Hammami et al., Citation2011). Interestingly, the only instance under which we have observed the upregulation of genes involved in flocculosin synthesis is when P. flocculosa was sprayed on leaves infected with B. cinerea. We surmise that sugars released by macerated plant tissues explain this phenomenon since flocculosin will invariably be released in vitro in media rich in sugars and poor in nutrients (Hammami et al., Citation2008).
Transcriptomic analyses
What remains unclear are the factors that would elicit such an unusual interaction? How does P. flocculosa recognize powdery mildews as a host? What are the genes involved in this process? What is present (or activated) in P. flocculosa and not in U. maydis to explain their diametrically opposed lifestyles in spite of their close genetic relatedness? We believe that the latest developments in high-throughput DNA sequencing offers unparalleled opportunities to decipher the minute details of this complex BCA–plant pathogen–plant system. Indeed, the powdery mildew genome of Blumeria graminis f. sp. hordei (powdery mildew of barley) has recently been sequenced (Spanu et al., Citation2010) and is now publicly available. We are in the process of completing the annotation of P. flocculosa genome, a task that is highly facilitated by relying on the reference fungi U. maydis, U. hordei and Sporisorium reilianum. This means that it is now possible to contemplate the in-depth analysis of the differential transcriptomic response over time of both P. flocculosa and powdery mildew when the former antagonizes the latter. This future project should reveal the salient genes involved in the antagonistic activity of P. flocculosa. This can be further supported by using U. maydis as a negative control and looking into the expression, or lack thereof, of virulence genes. This should provide invaluable information into the evolutionary separation between plant pathogens and BCAs. At some point, it will also be interesting to incorporate the host plant and conduct a simultaneous analysis of the transcriptome of all actors in this tripartite interaction. A difficult task, but one that may be well worth the effort.
Conclusion
Pseudozyma flocculosa is an effective antagonist of powdery mildews but its specific activity toward this particular group of plant pathogens appears to be a lot more intricate and complex than what was hypothesized for nearly 20 years. In light of all the accumulated evidence, it does seem counter-intuitive, however, that a BCA would conserve an elaborate trait such as a potent and unusual antifungal molecule and yet evolve a different mechanism to antagonize other fungi. Recent advancements in DNA sequencing offer powerful tools to investigate these subtle genetic determinants of BCA–pathogen–plant interactions. Exploiting these advancements will reveal fascinating phenomena and useful information to optimize biological control of plant pathogens.
References
- Avis , T.J. and Bélanger , R.R. 2001 . Specificity and mode of action of the antifungal fatty acid cis-9-heptadecenoic acid produced by Pseudozyma flocculosa . Appl. Environ. Microbiol. , 67 : 956 – 960 .
- Bakkeren , G. , Kämper , J. and Schirawski , J. 2008 . Sex in smut fungi: structure, function and evolution of mating-type complexes . Fungal Genet. Biol. , 45 : S15 – S21 .
- Begerow , D. , Bauer , R. and Boekhout , T. 2000 . Phylogenetic placements of ustilaginomycetous anamorphs as deduced from nuclear LSU rDNA sequences . Mycol. Res. , 104 : 53 – 60 .
- Bélanger , R.R. and Avis , T.J. 2002 . “ Ecological processes and interactions occurring in leaf surface fungi ” . In Phyllosphere microbiology , Edited by: Lindow , S.E. , Hecht-Poinar , E.I. and Elliott , V.J. 193 – 207 . St. Paul , MN : APS Press .
- Benyagoub , M. , Willemot , C. and Bélanger , R.R. 1996 . Influence of a subinhibitory dose of antifungal fatty acids from Sporothrix flocculosa on cellular lipid composition in fungi . Lipids , 31 : 1077 – 1082 .
- Boekhout , T. 1995 . Pseudozyma Bandoni emend. Boekhout, a genus for yeast-like anamorphs of ustilaginales . J. Gen. App. Microbiol. , 41 : 359 – 366 .
- Castoria , R. , De Curtis , F. , Lima , G. and De Cicco , V. 1997 . ß-1,3-glucanase activity of two saprophytic yeasts and possible mode of action as biocontrol agents against postharvest diseases . Postharvest Biol. Technol. , 12 : 293 – 300 .
- Cheng , Y. , Belzile , F. , Tanguay , P. , Bernier , L. and Bélanger , R.R. 2001 . Establishment of a gene transfer system for Pseudozyma flocculosa, an antagonistic fungus of powdery mildew fungi . Mol. Genet. Genomics , 266 : 96 – 102 .
- Cheng , Y. , Mcnally , D.J. , Labbé , C. , Voyer , N. , Belzile , F. and Bélanger , R.R. 2003 . Insertional mutagenesis of a fungal biocontrol agent led to discovery of a rare cellobiose lipid with antifungal activity . Appl. Environ. Microbiol. , 69 : 2595 – 2602 .
- Choudhury , S.R. , Traquair , J.A. and Jarvis , W.R. 1994 . 4-methyl-7,11-heptadecadienal and 4-methyl-7,11-heptadecadienoic acid: new antibiotics from Sporothrix flocculosa and Sporothrix rugulosa . J. Nat. Prod. , 57 : 700 – 704 .
- Choudhury , S.R. , Traquair , J.A. and Jarvis , W.R. 1995 . New extracellular fatty acids in culture filtrates of Sporothrix flocculosa and S. rugulosa . Can. J. Chem. , 73 : 84 – 87 .
- Clément-Mathieu , G. , Chain , F. , Marchand , G. and Bélanger , R.R. 2008 . Leaf and powdery mildew colonization by glycolipid-producing Pseudozyma species . Fungal Ecol. , 1 : 69 – 77 .
- Elad , Y. , Chet , I. and Henis , Y. 1982 . Degradation of plant pathogenic fungi by Trichoderma harzianum. Can. J . Microbiol. , 28 : 719 – 725 .
- Elad , Y. , Kirshner , B. and Yehuda , N. 1998 . Management of powdery mildew and gray mold of cucumber by Trichoderma harzianum T39 and Ampelomyces quisqualis AQ10 . Biocontrol , 43 : 241 – 251 .
- Fravel , D.R. 2005 . Commercialization and implementation of biocontrol . Annu. Rev. Phytopathol. , 43 : 337 – 359 .
- Hajlaoui , M.R. and Bélanger , R.R. 1993 . Antagonism of the yeast-like phylloplane fungus Sporothrix flocculosa against Erysiphe graminis var tritici . Biocontrol Sci. Technol. , 3 : 427 – 434 .
- Hammami , W. , Labbé , C. , Chain , F. , Mimee , B. and Bélanger , R.R. 2008 . Nutritional regulation and kinetics of flocculosin synthesis by Pseudozyma flocculosa . Appl. Microbiol. Biotechnol. , 80 : 307 – 315 .
- Hammami , W. , Quiroga Castro , C. , Rémus-Borel , W. , Labbé , C. and Bélanger , R.R. 2011 . Ecological basis of the interaction between Pseudozyma flocculosa and powdery mildew fungi . Appl. Environ. Microbiol. , 77 : 926 – 933 .
- Haskins , R.H. and Thorn , J.A. 1951 . Biochemistry of the ustilaginales: VII. Antibiotic activity of ustilagic acids . Can. J. Bot. , 29 : 585 – 592 .
- Hewald , S. , Josephs , K. and Bölker , M. 2005 . Genetic analysis of biosurfactant production in Ustilago maydis . Appl. Environ. Microbiol. , 71 : 3033 – 3040 .
- Kubicek , C.P. , Mach , R.L. , Peterbauer , C.K. and Lorito , M. 2001 . Trichoderma: from genes to biocontrol . J. Plant Pathol. , 83 : 11 – 23 .
- Lorito , M. , Scala , F. , Zoina , A. and Woo , S.L. 2001 . “ Enhancing biocontrol of fungal pests by exploiting the Trichoderma genome ” . In Enhancing Biocontrol Agents and Handling Risks , Edited by: Gressel , J. and Vurro , M. 248 – 259 . Amsterdam : IOS Press .
- Marchand , G. , Clément-Mathieu , G. , Neveu , B. and Bélanger , R.R. 2007 . “ Enhancing biological control efficacy of yeasts to control fungal diseases through biotechnology ” . In Biotechnology and Plant Disease Management , Edited by: Punja , Z.K. , Deboer , S. and Sansfacon , H. 518 – 531 . Wallingford , , UK : CAB International .
- Marchand , G. , Rémus-Borel , W. , Chain , F. , Hammami , W. , Belzile , F. and Bélanger , R.R. 2009 . Identification of genes potentially involved in the biocontrol activity of Pseudozyma flocculosa . Phytopathology , 99 : 1142 – 1149 .
- Mimee , B. , Labbé , C. , Pelletier , R. and Bélanger , R.R. 2005 . Antifungal activity of flocculosin, a novel glycolipid isolated from Pseudozyma flocculosa . Antimicrob. Agents Chemother. , 49 : 1597 – 1599 .
- Mimee , B. , Pelletier , R. and Bélanger , R.R. 2009 . In vitro antibacterial activity and antifungal mode of action of flocculosin, a membrane-active cellobiose lipid . J. Appl. Microbiol. , 107 : 989 – 996 .
- Paulitz , T.C. and Bélanger , R.R. 2001 . Biological control in greenhouse systems . Annu. Rev. Phytopathol. , 39 : 103 – 133 .
- Spanu , P.D. , Abbott , J.C. , Amselem , J. , Burgis , T.A. , Soanes , D.M. Stüber , K. 2010 . Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism . Science , 330 : 1543 – 1546 .
- Teichmann , B. , Linne , U. , Hewald , S. , Marahiel , M.A. and Bölker , M. 2007 . A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis . Mol. Microbiol. , 66 : 525 – 533 .
- Teichmann , B. , Labbé , C. , Lefebvre , F. , Bölker , M. , Linne , U. and Bélanger , R.R. 2010 . Identification of a biosynthesis gene cluster for flocculosin, a cellobiose lipid produced by the biocontrol agent Pseudozyma flocculosa . Mol. Microbiol. , 79 : 1483 – 1495 .
- Teichmann , B. , Lefebvre , F. , Labbé , C. , Bölker , M. , Linne , U. and Bélanger , R.R. 2011 . Beta hydroxylation of glycolipids from Ustilago maydis and Pseudozyma flocculosa by an NADPH-dependent B-hydroxylase . Appl. Environ. Microbiol. , 77 : 7823 – 7829 .
- Traquair , J.A. , Shaw , L.A. and Jarvis , W.R. 1988 . New species of Stephanoascus with Sporothrix anamorphs . Can. J. Bot. , 66 : 926 – 933 .
- Whipps , J.M. 2001 . Microbial interactions and biocontrol in the rhizosphere . J. Exp. Bot. , 52 ( Suppl. 1 ) : 487 – 511 .
- Woo , S.L. , Scala , F. , Ruocco , M. and Lorito , M. 2006 . The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants . Phytopathology , 96 : 181 – 185 .