Abstract
Phytophthora capsici is an oomycete pathogen of vegetable crops worldwide, causing crop losses exceeding 50% and possibly resulting in crop failure. Extensive research has been conducted on various facets of this pathogen since it was first described in 1922. Information from past research efforts has enhanced the understanding of the biology and management of P. capsici. However, this pathogen remains a continuous challenge to vegetable production. This mini-review succinctly provides (i) an appraisal of the current state of management approaches and outlines a framework for designing an integrated management system for P. capsici; and (ii) identifies knowledge gaps and delineates new research perspectives for control of this pathogen.
Résumé
Phytophthora capsici est un oomycète pathogène qui s'attaque aux cultures de légumes partout dans le monde, causant des pertes de plus de 50 % et occasionnant parfois de mauvaises récoltes. Des recherches approfondies ont été menées sur différentes facettes de cet agent pathogène depuis sa description initiale en 1922. L'information émanant des efforts en matière de recherche a amélioré la compréhension de la biologie et de la gestion de P. capsici. Malgré tout, l'agent représente un défi permanent quant à la production légumière. Ce bref examen: (i) fournit une évaluation de l'état actuel des approches de gestion et expose les grandes lignes d'un cadre de travail visant à concevoir un système de lutte intégrée pour gérer P. capsici; et (ii) révèle les lacunes sur le plan des connaissances et présente de nouvelles hypothèses de recherche quant à la lutte contre cet agent pathogène.
Introduction
Phytophthora blight, caused by Phytophthora capsici, is an important soilborne disease that affects a wide array of crops across many global agro-ecosystems (Erwin & Ribeiro, Citation1996). In the USA, P. capsici is rated as a high research priority in a number of Pest Management Strategic Plans published in the National Information System of the Regional IPM centres (http://www.ipmcenters.org/pmps). The importance of managing P. capsici has led to discussions at three international conferences held in 2007, 2009 and 2011 in Florida and to a global discussion session during the 19th International Pepper Conference in September 2008 in New Jersey.
Phytophthora capsici can inflict crop losses exceeding 50% in diverse agro-ecosystems (Xie et al., Citation1999; Babadoost, Citation2000). Similar to other species of Phytophthora, P. capsici is characterized by biological and ecological complexities that contribute to its success as a plant pathogen (Ploetz et al., Citation2002; Sanogo, Citation2006; Sanogo & Clary, Citation2006). There are at least four significant factors that greatly enhance the success of this plant pathogen in its ability to cause disease: (i) the wide host range which enables the pathogen to thrive in diverse agro-ecosystems; (ii) the production of several types of propagules that are specialized for the purposes of survival, reproduction and dispersal; (iii) the ability of the pathogen to infect all plant parts including roots, stems, leaves and fruit; and (iv) the potential for sexual recombination via the presence of two mating types leading to new, more aggressive genotypes.
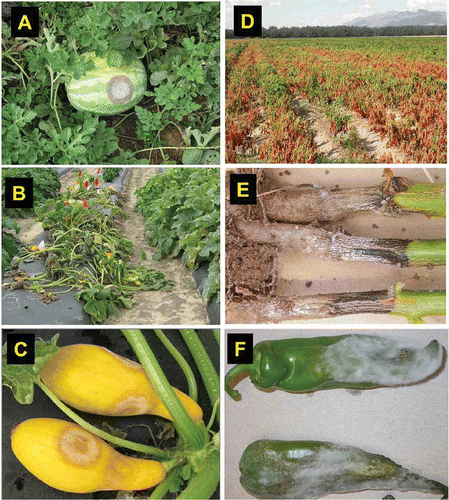
Although other plant hosts may be infected by this pathogen, this mini-review is limited to solanaceous and cucurbitaceous vegetable crops that are affected by P. capsici in the USA and many countries worldwide (Erwin & Ribeiro, Citation1996; Ristaino & Johnston, Citation1999; Hausbeck & Lamour, Citation2004; Sholberg et al., Citation2007) () . However, some work, even though not conducted on these two groups of crops, was included for relevancy to discussion on unexplored aspects of the biology of P. capsici. Previous reviews on P. capsici focused on different aspects of this pathogen (Erwin & Ribeiro, Citation1996; Ristaino & Johnston, Citation1999; Hausbeck & Lamour, Citation2004). This mini-review outlines a conceptual framework for integrated control of P. capsici, follows a unique approach aimed at identifying gaps in knowledge on P. capsici with respect to well-defined categories of production environment variables, and delineates research needs for integrated control of P. capsici.
Integrated management framework: old and new perspectives
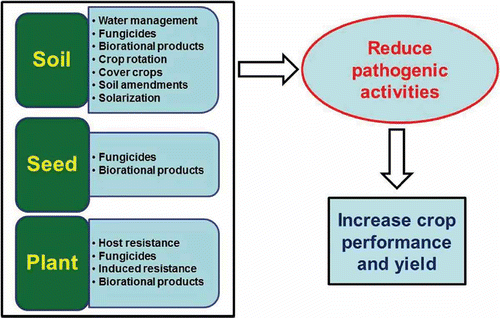
There is a need to develop an integrated disease management system for Phytophthora blight (Erwin & Ribeiro, Citation1996; Ristaino & Johnston, Citation1999; Hausbeck & Lamour, Citation2004). provides a conceptual framework for developing such a system with emphasis on three tactics, namely soil, seed and plant treatment. The goal is to combine various tactics to reduce pathogen activities, promote and maintain stand establishment, and reduce yield losses.
Fig. 2. Conceptual framework for developing an effective and economical management system for Phytophthora capsici that integrates soil, seed and plant treatment to reduce pathogen activities and increase crop performance and yield.
Seed treatment
Stand establishment is determined by good seed germination and seedling emergence. Crop yield may be significantly reduced by reduction in seed germination and seedling emergence. Seedling damping-off caused by P. capsici has resulted in significant plant losses in many vegetable crops (Lee et al., Citation2001; Islam & Babadoost, Citation2002). Chemical fungicides and biofungicides (bacteria-based and fungi-based formulations) may be used to protect vegetable crop seeds from infection by P. capsici. In the absence of seed treatment, seedling stands of pumpkin in soil infested with P. capsici was approximately 45% at 35 days after planting; however, when seeds were treated with mefenoxam and metalaxyl prior to planting, seedling stands increased to 77 and 75%, respectively (Babadoost & Islam, Citation2003). The effectiveness of a liquid formulation of phosphonate, containing potassium salt of phosphorous acid and copper sulphate with citrate as a chelating agent, was evaluated for suppression of damping-off and root rot of cucumber caused by P. capsici under growth room conditions (Abbasi et al., Citation2011). Cucumber seeds were soaked in phosphonate solution for 10 min before seeding in a peat-based mix inoculated with three levels of pathogen density. Seed treatment provided significant damping-off protection at all three inoculum levels and increased the percentage of healthy cucumber seedlings by more than two-fold at low inoculum level, eight-fold at medium inoculum level and 14-fold at high inoculum level (Abbasi et al., Citation2011).
Seed treatment with biological fungicides may enhance germination and reduce damping-off. In pepper, seed germination at 20 days after planting was approximately 94% in the absence of P. capsici, and was reduced to 62% in the presence of P. capsici; however, seed germination was 87% in the presence of P. capsici when seeds were treated with some strains of Bacillus thuringiensis (Mojica-Marín et al., Citation2009). A volatile-producing bacterium, Flavobacterium johnsoniae strain GSE09, demonstrated biocontrol activity against P. capsici in pepper (Sang & Kim, Citation2012). GSE09 strain forms biofilms and produces indolic compounds, biosurfactant, and a volatile compound 2,4-di-tert-butylphenol. Germinated pepper seeds treated with 2,4-di-tert-butylphenol or strain GSE09 significantly reduced radicle infection by P. capsici without radicle growth inhibition (Sang & Kim, Citation2012). The effect of seed treatment with Trichoderma harzianum on necrosis in pepper stems caused by P. capsici was studied under growth chamber conditions (Ahmed et al., Citation2000). Seed treatment with spores of T. harzianum significantly reduced stem necrosis, by as much as 50% compared with the non-treated control. Seed treatment with the biocontrol agent appeared to increase the concentration of capsidiol in pepper stems that might be related to enhanced plant resistance against the disease (Ahmed et al., Citation2000). The benefit of seed treatment with chemical and biological fungicides may be augmented with novel approaches such as treatment of seedlings with red light before transplanting (Islam & Babadoost, Citation2002). Seedling damping-off was reduced by up to nearly 80% when seedlings of pepper, pumpkin and tomato were exposed to continuous irradiation with red light (600–700 nm).
Soil and plant treatment
Soil represents an important reservoir of P. capsici that should be targeted to reduce the inoculum potential of this pathogen. Several tactics, some described briefly below, may be used to reduce inoculum potential of P. capsici in soil. These tactics include soil water management, chemical fungicides, biorational products, crop rotation, cover crops, solarization and soil amendments. Benefits of soil treatment can be augmented with tactics targeting the plant such as host resistance, induced resistance through the use of chemical and microbial activators and microorganisms, grafting, and application of other biorational products.
Water management
Soil water is a critical factor in the management of P. capsici. Water from rain or surface and subsurface irrigation is the primary vehicle for inoculum dispersal in fields through splashing and flowing water. Additionally, water from rivers or ponds used for irrigation may be a source of inoculum of P. capsici (Roberts et al., Citation2005; Gevens et al., Citation2007; Wang et al., Citation2009). Moisture and its relation to the biology of P. capsici have been well studied. Moisture must be reduced to effectively manage P. capsici. The use of irrigation and cultural methods, such as drip irrigation, raised beds and plastic mulching, that minimize high soil moisture conditions and soil splash is essential in restricting inoculum production and movement, which can reduce disease severity (Erwin & Ribeiro, Citation1996; Ristaino & Johnston, Citation1999; Hausbeck & Lamour, Citation2004). Studies have shown that while less frequent irrigation is effective in the control of Phytophthora blight on susceptible vegetable plants, it could negatively affect crop performance depending on other environmental factors such as temperature (Café-Filho & Duniway, Citation1995). The challenge for producers is to use less water and yet secure profitable crop yield and quality while minimizing any negative impact from P. capsici. Certain potential approaches may assist producers in facing this challenge. For instance, drought-tolerant genotypes could provide an innovative approach for P. capsici management while maintaining crop performance. Drought-tolerant genotypes may be ideal in irrigated fields in semi-arid environments. However, they may not be tenable options in areas with high rainfall.
Reducing inoculum level of P. capsici in irrigation water sources such as rivers and ponds must also be considered in the management of irrigation water. Algaecides have been shown to inhibit zoospore motility and cause zoospore mortality (Granke & Hausbeck, Citation2010), and therefore could be used for treating irrigation water infested with P. capsici. Treating irrigation water sources may be essential and effective for production systems within enclosed environments such as greenhouses, in which irrigation water is recirculated. However, it may be difficult or impractical to implement such water treatment approaches in open production fields.
Fungicides and soil fumigants
Chemical fungicides have been and remain an important component for managing both the aerial and soil phases of Phytophthora blight (Erwin & Ribeiro, Citation1996). The existing pool of these fungicides has been enriched with new chemistries with curative activity, such as mandipropamid and cyazofamid, foliar penetrants, and fluopicolide, a penetrant and systemic material. Mandipropamid and cyazofamid belong to the mandelamide and cyanoimidazole classes, respectively. Mandipropamid inhibits lipids and membrane synthesis and cyazofamid causes respiratory inhibition at Complex III in the mitochondria (Mitani et al., Citation2001; Kuck & Gisi, Citation2008; Blum et al., Citation2010). Fluopicolide belongs to the benzamide class and the pyridine class and appears to have a new mode of action of delocalizing a spectrin-like protein that is cytoskeleton-associated (Toquin et al., Citation2008). These newer fungicides have been demonstrated to be effective in reduction of diseases caused by oomycete pathogens including Phytophthora blight on vegetables (Matheron & Porchas, Citation2007; Jackson et al., Citation2010; Ji et al., Citation2011).
Combined use of soil and foliar fungicides has been explored in several studies (Ji et al., Citation2008, Citation2011). While more commercially available fungicides are for foliar applications, fungicides that can be used for soil treatment to suppress Phytophthora blight are limited. This situation is exacerbated due to the development of resistance in P. capsici populations to mefenoxam, a fungicide that was commonly used for crown and soil treatment. More research should be directed at identifying new fungicides with promise to protect against infections from soil inoculum and be used as soil treatments to effectively suppress P. capsici in soils and reduce Phytophthora blight on vegetables.
Soil fumigants have been demonstrated to be integral components of Phytophthora blight management. Metam sodium in combination with alternate-row irrigation was shown to decrease disease incidence and increase yield of green chile pepper (Biles et al., Citation1992). In soil fumigated with methyl bromide and chloropicrin, P. capsici was reduced to undetectable levels (French-Monar et al., Citation2007). With the restrictions placed on the use of methyl bromide, other alternatives such as chloropicrin-based fumigants, biofumigation and mycofumigation are increasingly being explored or used to alleviate many soilborne diseases including Phytophthora blight.
Continuous use of the same fungicides may be associated with the development of resistant strains of P. capsici. Fungicides containing the active ingredient mefenoxam have been used as the chemical standard for control of P. capsici; however, mefenoxam-resistant strains of P. capsici have developed in several states in the USA (Parra & Ristaino, Citation2001; Ploetz et al., Citation2002; Gevens et al., Citation2007; Keinath, Citation2007; Jackson et al., Citation2010). Studies indicated that prior application of mefenoxam-containing fungicides was a good predictor of the level of sensitivity to mefenoxam in field isolates (Keinath, Citation2007). There was a higher recovery of resistant isolates from fields where mefenoxam had been applied alone than in combination with other fungicides (Parra & Ristaino, Citation2001; Keinath, Citation2007). Resistance of P. capsici to some newer fungicides, such as mandipropamid and fluopicolide, has not been reported. However, a great proportion of P. capsici isolates from vegetable crops in Georgia were resistant to another newer fungicide, cyazofamid, based on mycelial growth or sporangial production, although the isolates were sensitive to this compound based on zoospore germination (Jackson et al., Citation2012). Effective management of this oomycete should include strategies for resistance management. Combined or alternated use of fungicides with different mode of actions, and integrated use of chemical fungicides with other biological, physical, cultural and genetic strategies, may enhance disease control efficacy and reduce selection pressure for the pathogen to develop resistance.
Biorational products
The prospect of using microbial-based formulations and botanical extracts has been and remains the focus of research on finding alternatives to chemical fungicides and fumigants. Biofungicides have been used infrequently for control of P. capsici in spite of the fundamental research that has been conducted to identify microorganisms with activity against this oomycete pathogen (Nemec et al., Citation1996; Mao et al., Citation1998; Mercier & Manker, Citation2005). A diverse group of bacterial and fungal microorganisms has been the focus of research on biocontrol of P. capsici, and includes, for example, species of Bacillus, Streptomyces, Trichoderma and Penicillium (Ma et al., Citation2008).
Recently, arbuscular mycorrhizal fungi in the genus Glomus have been explored for their ability to protect plants against P. capsici (Ozgonen & Erkilic, Citation2007; Cimen et al., Citation2009). Disease severity in pepper inoculated with P. capsici was reduced by G. mosseae by approximately 92, 43 and 57% under pot, greenhouse and field conditions, respectively (Ozgonen & Erkilic, Citation2007). In pepper plants inoculated with G. intraradices, plant mortality due to P. capsici was reduced by approximately 15% at the end of the season, and total yield was increased by approximately 40% (Cimen et al., Citation2009). These results suggest that arbuscular mycorrhizal fungi have the potential to reduce the impact of Phytophthora blight.
Other fungal microorganisms such as species of Muscodor are characterized by the production of volatile organic compounds, which have been shown to suppress several soilborne pathogens including P. capsici. Muscodor albus was reported to provide complete control of root rot of bell pepper (Mercier & Manker, Citation2005). Although mycofumigation may not eliminate P. capsici in soil, it may reduce disease severity in tolerant solanaceous and cucurbitaceous crops (Camp et al., Citation2008).
There is an increasing interest in the use of entomopathogenic bacteria such as Xenorhabdus bovienii strain YL002 and Bacillus thuringiensis. Cell-free culture filtrate from X. bovienii completely inhibited mycelium growth of P. capsici, and treatment of pepper plants by foliar sprays with the filtrate from X. bovienii provided both protective and curative effects of 65 and 68%, respectively, against P. capsici (Fang et al., Citation2011). In a study conducted on 64 strains of B. thuringiensis, Mojica-Marin et al. (Citation2009) identified 19 strains that significantly reduced mycelial growth of P. capsici. Thus, entomopathogenic bacteria may provide new tools in controlling P. capsici.
Commercial biofungicides contain as active ingredients predominantly species of Streptomyces (e.g. S. lydicus), Trichoderma (e.g. T. harzianum, T. asperellum and T. gamsii) and Bacillus (e.g. B. subtilis). Results from tests conducted on the efficacy of biofungicides against P. capsici have been variable. Biofungicides capable of providing disease control similar to recommended chemical fungicides have not yet been identified; however, these products may be useful in an integrated disease management context to enhance and sustain disease suppression capacity of other tactics such as chemical fungicides.
In addition to microbial-based products or formulations, essential oils and extracts from several plant species have been researched for their potential to inhibit P. capsici and reduce Phytophthora blight (Muller-Riebau et al., Citation1995; Shafi et al., Citation2004; Bajpai et al., Citation2009; Bajpai & Kang, Citation2010; Bi et al., Citation2012). There is increasing interest in botanical oils and extracts because of their benefits not only in disease control but they offer environmentally sound alternatives to chemical fungicides. Much work remains to be done before essential oils and extracts become commercially adopted as a viable component of the management of Phytophthora blight.
Host plant resistance
Host resistance against P. capsici has been identified in certain vegetable crops, particularly pepper (Bosland & Lindsey, Citation1991). Although a number of commercial pepper cultivars are known with various levels of resistance to Phytophthora blight, the pool of these cultivars is still narrow and they are only moderately resistant to the disease. The pepper line CM334 is a primary source of resistance currently used in pepper breeding programmes against root and crown rot caused by P. capsici (Oelke et al., Citation2003; Glosier et al., Citation2008). There are a few commercial pepper cultivars that are regarded as moderately resistant to Phytophthora root and crown rot, such as ‘Aristotle’, ‘Revolution’, ‘Excursion’ and ‘Paladin’. In a study with 49 P. capsici isolates collected from vegetable crops in Georgia, ‘Paladin’, ‘Excursion’, ‘Aristotle’, and a known susceptible cultivar ‘Camelot’ were susceptible to 20, 65, 88 and 94% of the isolates, respectively, while CM334 was resistant to all the isolates (Yin et al., Citation2012). Average disease severity on ‘Paladin’, ‘Excursion’, ‘Aristotle’ and ‘Camelot’ was 1.3, 2.3, 3.3 and 3.9, respectively, indicating ‘Paladin’ was the most resistant among the commercial cultivars evaluated. However, ‘Paladin’ is not resistant to all the isolates and it has not been widely adopted by growers due to unsatisfactory horticultural characteristics such as smaller fruit size. Continued effort is being made in attempts to increase the pool of resistant genetic sources of peppers (Candole et al., Citation2010).
On cucurbits, studies have been conducted in recent years to evaluate and identify Cucurbita pepo (Padley et al., Citation2008) and C. moschata (Chavez et al., Citation2011) accessions with resistance to Phytophthora crown rot. However, limited success has been achieved so far in identifying commercial cultivars of cucurbit crops with resistance to P. capsici. In addition, resistance to crown and root rot caused by P. capsici was evaluated in tomato and its wild relatives (Quesada-Ocampo & Hausbeck, Citation2010). However, further breeding will be required before tomato cultivars with high resistance become commercially available. A challenge to breeding for host resistance and using host resistance for management of P. capsici is the existence of races within P. capsici (Oelke et al., Citation2003; Glosier et al., Citation2008; Sy et al., Citation2008; Monroy-Barbosa & Bosland, Citation2011).
In many studies, the increase in plant resistance with age, known as ontogenic resistance or age-related resistance, has been recognized as an important mechanism through which the effect of P. capsici may be significantly reduced in solanaceous crops such as pepper (Kim et al., Citation1989; Biles et al., Citation1993) and cucurbitaceous crops such as cucumber (Cucumis sativus L.), melon (Cucumis melo), butternut squash (Cucurbita moschata), watermelon (Citrullus lanatus), courgette, yellow summer squash, acorn squash and pumpkin (Cucurbita pepo) (Ando et al., Citation2009). Age-related resistance is heritable and may be governed by at least two genes in pepper (Reifschneider et al., Citation1992). However, ontogenic resistance has not been practically exploited in production systems.
Another avenue for using host resistance is grafting, whereby scions from a desirable line or cultivar are grafted onto rootstocks from another desirable line or cultivar. Grafting is an established horticultural technique that is emerging as a tool to reduce the incidence of many soilborne diseases including Phytophthora blight (Ros et al., Citation2005; King et al., Citation2008; Gisbert et al., Citation2010). Much detailed information on the use of grafting for disease resistance has been provided by King et al. (Citation2008) and Louws et al. (Citation2010). The application of the technique for vegetable crops has received much scepticism with respect to its cost and practicality. However, the technique has been employed extensively in Southeast Asia, and is used in southern Europe and Mediterranean regions. Its rapid adoption in these regions has generated increased confidence in the process.
Induced resistance
There is a growing interest in induced resistance, whether as induced systemic resistance (ISR) or systemic acquired resistance (SAR), which has the potential to reduce Phytophthora blight on vegetables (Khan et al., Citation2004; Baysal et al., Citation2005; Sang et al., Citation2010). A systemic acquired resistance inducer, acibenzolar-S-methyl (ASM), has been studied for control of several diseases. ASM acts as a functional analogue of salicylic acid in the SAR signalling pathway, and was shown to reduce Phytophthora blight on pepper and squash under greenhouse conditions (Matheron & Porchas, Citation2002; Koné et al., Citation2009) and in the field (Kousik & Subramanya, Citation2001; Ji et al., Citation2011). According to Baysal et al. (Citation2005), plant treatment with ASM may result in the induction of defence-related enzymes and accumulation of phenolics and PR proteins, and thereby enhance plant resistance to P. capsici. Due to the reported potential negative effects of ASM on plant growth (Romero et al., Citation2001; Matheron & Porchas, Citation2002), application rates and timing can be critical for successful disease suppression and yield increase. Other chemical compounds with the potential for inducing plant resistance deserve evaluation. For example, in a recent study, application of silicon reduced Phytophthora blight on bell pepper under greenhouse conditions (French-Monar et al., Citation2010).
In addition to using plant activators such as acibenzolar-S-methyl, induced resistance may be achieved through application of beneficial microorganisms. In greenhouse studies, Trichoderma hamatum significantly reduced the severity of Phytophthora root and crown rot or leaf blight on cucumber (Khan et al., Citation2004). This effect did not differ significantly from that provided by acibenzolar-S-methyl or mefenoxam. Trichoderma hamatum remained spatially separated from the pathogen in the bioassays, suggesting that induced resistance was involved and the effects were systemic in nature (Khan et al., Citation2004). In more recent studies, some endophytic Trichoderma spp. induced defence-related expressed sequence tags (EST) in hot pepper and delayed development of Phytophthora blight (Bae et al., Citation2011). Diaz et al. (Citation2005) showed that pre-inoculation of pepper with the tomato wilt pathogen, Fusarium oxysporum f. sp. lycopersici (FOL) decreased the incidence of root rot caused by P. capsici by nearly 40%, and the area under the disease progress curve was reduced by 50% compared with control plants that were not pre-inoculated with FOL. Induced resistance by microorganisms appears to be promising for management of Phytophthora blight, but their effectiveness and stability in disease suppression need to be evaluated under various field conditions.
Host plant attributes
There are several attributes of host plants that may affect the impact of Phytophthora blight. For example, in pickling cucumber (Cucumis sativus L.), Ando & Grumet (Citation2006) found that incidence of fruit infection by P. capsici was lower in cucumber lines with compact growth that held fruit off the ground than in other lines that did not have such attributes. Other plant characteristics such as heat (spiciness) level and fruit cuticle thickness are also important attributes that merit further examination in terms of their relationship to level of Phytophthora blight. Biles et al. (Citation1993) reported that cuticle thickness contributes to resistance of pepper fruit to P. capsici, and suggested that cuticle thickness should be considered in breeding programmes. Tahboub et al. (Citation2008) showed that there was no relationship between Phytophthora blight and heat level in pepper, which is due to the production of capsaicinoids in the placenta of fruit. Further studies conducted under field conditions are needed in this area.
Cover crops and crop rotation
Numerous studies have examined the role of cover crops and crop rotation on Phytophthora blight (McGrath, Citation1997, Citation2009; Lamour & Hausbeck, Citation2003; Liu et al., Citation2008). Despite the benefits of these tactics, they are not systematically implemented owing to economic impracticalities in many growing areas. Phytophthora capsici produces oospores that can remain viable for long periods of time in the absence of host plants, and this may represent a major challenge to the use of crop rotation. The efficacy of crop rotation and cover crops has been attributed to several factors, including reduction in initial soil inoculum, increased host nutrition, and increased antagonistic microbial populations. Using cover crops may also reduce inoculum of P. capsici through reduction in weed populations since some common weeds on vegetable farms could serve as a host for this pathogen (Ploetz et al., Citation2002; Tian & Babadoost, Citation2004; French-Monar et al., Citation2006). Little research has been done on the effect of cover crops on dispersal of P. capsici. Dispersal of inoculum of P. capsici and development of Phytophthora blight were significantly suppressed when bell pepper was planted in stubble of a no-till wheat cover crop, which was sown in the autumn, sprayed with paraquat in the spring, and mowed before planting pepper (Ristaino et al., Citation1997).
Another area that has been little explored with respect to Phytophthora blight is the use of bioactive crop residues and by-products from cruciferous crops (Ji et al., Citation2012). One of the characteristics of bioactive cruciferous crops is the presence of glucosinolate compounds (Mazzola et al., Citation2007), which mediate the suppression of several soil pests and pathogens. There is some evidence that glucosinolate-containing amendments could reduce the impact of P. capsici on pepper and other vegetables. Soil amendments with mustard or canola as green manures significantly reduced Phytophthora blight on squash under greenhouse and field conditions (Ji et al., Citation2012). It may be beneficial to develop disease management programmes integrating Brassica crop amendments and effective fungicides and other established practices.
Soil amendments
Soil amendment with various organic and synthetic materials has long been considered in research aimed at reducing Phytophthora blight on vegetables (Kim et al., Citation1997), and there is a renewed interest in this area (Liu et al., Citation2008). Effective amendments should enhance soil physical, chemical and microbial characteristics that restrict pathogen movement and growth, and reduce conditions favourable for infection and disease onset. Development of Phytophthora blight on pepper transplants was less in soil amended with rye-vetch green manure, composted poultry manure and synthetic fertilizers than in soil amended with cotton gin trash (Liu et al., Citation2008). Two types of compost, yard-waste and brewery-waste composts, were evaluated for their efficacy against Phytophthora blight of pumpkin (McGrath & Rangarajan, Citation2002; McGrath, Citation2004). Although these studies showed that compost amendments had no significant effect on Phytophthora blight, they provide new avenues for research in this area.
Solarization
Solarization has been previously described in several other studies, with its basic tenants being the capturing of solar energy underneath specialized plastic covers, thereby increasing soil temperature to levels lethal to propagules of many plant pathogens (Etxeberria et al., Citation2011). In Florida, soil solarization was shown to reduce populations of P. capsici by almost 50% compared with non-solarized control soil (French-Monar et al., Citation2007). Similarly, Cimen et al. (Citation2009) showed that mortality of pepper in solarized soil was reduced. Solarization, albeit effective and attractive, is not widely used in field production of many vegetables, probably due to lack of understanding of the benefits and application technology.
Integrated management
As previously stated, combinations of methods are needed to effectively manage P. capsici. There are few studies that have examined the effect of combining management methods on the incidence of Phytophthora blight (). The information in the table was derived from a bibliometric search of the Web of Knowledge databases (Version 5.4, Thomson Reuters), CABI abstracts, CABI Abstract Archives, Agricola, and Plant Disease Management Reports published by the Plant Management Network. Additional information was derived from reviews (Ristaino & Johnston, Citation1999; Hausbeck & Lamour, Citation2004) and book chapters (Erwin & Ribeiro, Citation1996). An arbitrary three-level numerical rating scale was developed with 0, 1 and 2, indicating that information was absent, scarce (fewer than five peer-reviewed articles), or extensive (more than five peer-reviewed articles), respectively.
Table 1. Available information on combination of management options for Phytophthora capsici on solanaceous and cucurbitaceous crops. A bibliometric search was conducted and an arbitrary three-level numerical rating scale was developed with 0 or 1 indicating that information was absent or scarce (fewer than five peer-reviewed articles), respectively
Some of the studies that examined the effect of combining approaches on P. capsici and Phytophthora blight focused on host resistance used in conjunction with fungicides (Foster & Hausbeck, Citation2010), biological control agents (Camp et al., Citation2008) or with irrigation (Café-Filho & Duniway, Citation1995). Other combinations of approaches include cultural methods, such as raised beds, plastic mulching or alternate-row irrigation with fungicides (Biles et al., Citation1992; Kousik et al., Citation2011); solarization with other tactics including soil fumigants (French-Monar et al., Citation2007), mycorrhizal fungi (Cimen et al. Citation2009), organic soil amendments (McGrath, Citation1997; Coelho et al., Citation1999), and organic soil amendments and fungicides (Chávez-Alfaro et al., Citation1995); plastic mulching with organic soil amendments (Núñez-Zofío et al., Citation2011) and induced resistance with fungicides (Ji et al., Citation2011).
The attractiveness of combining control methods resides, among many other factors, in the potential synergistic effects that may be generated from such combinations. Few studies have shown synergistic effects among combinations of strategies. Host resistance, in conjunction with fungicides applied as a drench, was shown to reduce the incidence of crown and root rot on pepper (Foster & Hausbeck, Citation2010). On tolerant bell pepper and squash, application of Muscodor albus significantly reduced Phytophthora blight (Camp et al., Citation2008). It was suggested that combining host resistance with less frequent irrigation would be ideal for control of P. capsici (Café-Filho & Duniway, Citation1995).
Soil solarization may be used in conjunction with mycorrhizal fungi to decrease the impact of Phytophthora blight. In plots infested with P. capsici, yield of pepper was increased by 42.8% when plots were solarized and inoculated with mycorrhizal fungi (Cimen et al., Citation2009). Combining solarization with an effective soil fumigant equivalent to methyl bromide may reduce the incidence of crown blight in pepper caused by P. capsici (Yucel, Citation1995). Solarization using transparent polyethylene plastic in conjunction with either chicken manure or the fungicide fosetyl-aluminium, or in conjunction with both chicken manure and fosetyl-aluminium, provided the lowest disease levels and the highest yield in pepper grown in field soil naturally infested with P. capsici (Chávez-Alfaro et al., Citation1995). The marginal rates of economic returns were highest with application of chicken manure alone, chicken manure used in conjunction with transparent polyethylene, and chicken manure in combination with both fosetyl-aluminium and transparent polyethylene (Chávez-Alfaro et al., Citation1995). This study underscores the need to evaluate management strategies in terms of not only their efficacy against P. capsici but also their economic soundness.
Synergistic effects were also reported when irrigation was used in combination with soil fumigation, and plant activators with fungicides. In pepper fields under furrow irrigation, Biles et al. (Citation1992) showed that using the fumigant metam-sodium with alternate-row irrigation was, in some cases, better than alternate-row irrigation alone in reducing disease incidence caused by P. capsici and in increasing pepper yield. Phytophthora blight was significantly reduced in squash plants treated with acibenzolar-S-methyl (ASM), an inducer of plant systemic acquired resistance, in conjunction with standard fungicides such as mefenoxam, copper hydroxide and mandipropamid, compared with plants treated with these fungicides alone, and yield of squash was highest under the combined use of ASM and fungicides (Ji et al., Citation2011).
Gaps in knowledge and research needs
Extensive research has been conducted since P. capsici was first described by Leonian (Citation1922). Previous research has focused on cultural practices, fungicides and genetic resistance in reducing crop damage caused by P. capsici (McGrath, Citation1997; Ristaino & Johnston, Citation1999; Matheron & Porchas, Citation2000; Lamour & Hausbeck, Citation2003; Gisbert et al., Citation2010; Jackson et al., Citation2010). No single method has demonstrated long-term efficacy for control of P. capsici on vegetables, and this pathogen remains a serious challenge in vegetable production. This underscores the necessity of an increased understanding of the biology of P. capsici and more effective strategies for disease management.
A synopsis of the status of knowledge concerning the biology of P. capsici in relation to a wide array of production environment variables is presented in . The information in the table was derived from a bibliometric search and a three-level numerical rating scale as described previously. In the first step of the search procedure, the term ‘Phytophthora capsici’ was used alone as a topic or subject area, and in the follow-up steps, the term ‘Phytophthora capsici’ was searched in conjunction with other terms listed in the first column of . For example, when ‘Phytophthora capsici’ was searched in conjunction with ‘weed’ or ‘weeds’, results yielded 11 items. These results were screened further based on information in the abstract of each study, with focus on whether the study examined specifically the survival, spread or reproduction of P. capsici or plant infection by P. capsici in relation to ‘weed’ or ‘weeds’. It is clear that on the one hand, there is a tremendous knowledge gap on the relationship between many production environment variables and the incidence of disease incited by P. capsici. On the other hand, a large amount of information has accumulated on the relationship of a few variables, such as edaphic variables, and phenology of P. capsici and its pathogenic activities.
Table 2. Status of knowledge on the effects of production environment variables on biological attributes of Phytophthora capsici. A bibliometric search was conducted and an arbitrary three-level numerical rating scale was developed with 0, 1 and 2, indicating that information was absent, scarce (fewer than five peer-reviewed articles) or extensive (more than five peer-reviewed articles), respectively
Three variables for which there exists a large volume of information are moisture, chemical fungicides and genetic resistance. However, there are shortcomings associated with each variable. Undoubtedly, moisture is one of the environmental variables that has been most studied in relation to the biology of P. capsici and development of Phytophthora blight. The salient information from numerous studies is that moisture must be reduced to effectively manage P. capsici. Cultural practices aimed at managing irrigation to create a less favourable environment for the pathogen to infect and disperse may not be highly effective, particularly under favourable environmental conditions such as high rainfall.
Development of chemical fungicides provides a useful means to effectively control this pathogen. However, some fungicides have become ineffective with the emergence of resistant P. capsici strains. For instance, resistance in P. capsici to mefenoxam is already widespread in different vegetable production areas (Lamour & Hausbeck, Citation2000; Parra & Ristaino, Citation2001; Ploetz et al., Citation2002; Keinath, Citation2007; Jackson et al., Citation2010). Continuous research is needed to search for new effective fungicides with different modes of actions and to develop integrated management strategies using different fungicides in combination or rotation as well as fungicides and other disease control tactics.
Genetic resistance to P. capsici has been studied for several decades. Although much information has been obtained on the inheritance and identification of lines with genetic resistance, very few commercial vegetable cultivars are available with tolerance or resistance to this disease. A further understanding of the impact of moisture, fungicides and genetic resistance is warranted to alleviate shortcomings associated with these variables.
Similarly, there is a need for an increased understanding of the relationships of many other variables identified in to the biology of P. capsici. For example, very little is known about the interaction between P. capsici and arthropods, especially insects. Hyder et al. (Citation2009) reported that larvae of fungus gnats, and larvae and adults of shore flies, can ingest and excrete sporangia of P. capsici. Although the excreted sporangia were not viable, the findings suggest that arthropods may play an important role in the dispersal of P. capsici, and this needs to be taken into account in management of the pathogen.
There is virtually no study that has been conducted on soil hydraulic conductivity and movement of P. capsici in soil. This is an important variable that has bearing on the movement of propagules through soil. Soil management methods that affect hydraulic conductivity may be used to reduce dispersal through soil and thereby, inoculum potential.
Very little has been done on the side effects of pesticides on P. capsici. This is important because it may provide additional avenues for control of P. capsici. Park et al. (Citation1992) showed that Phytophthora rot of red pepper was reduced by the combination of the fungicide metalaxyl and the insecticide dithianon. This study points to the benefit of exploring the combination of pest and disease control methods.
As one of the major pests in crop production systems, weeds have been shown to serve as reservoirs for P. capsici (French-Monar et al., Citation2006), but weed management with herbicides and its impact on P. capsici has not been examined. Herbicides have been shown to affect diseases incited by several pathogens (Lévesque & Rahe, Citation1992; Sanogo et al., Citation2000). However, there has been no study on the interaction between herbicides and P. capsici. It is important to examine this interaction from at least two perspectives. First, herbicides could reduce the inoculum potential of P. capsici. In this case, there is benefit to herbicide usage. Second, herbicides may increase the pathogen inoculum potential, which would represent a serious threat to management of P. capsici. Therefore, it is important to examine the effects of commonly used herbicides and fungicides on the ecology of P. capsici and its control. Determination of the basis of pesticide interactions with this pathogen could help identify more efficient, environmentally friendly, and economically viable pest management systems.
In terms of cover and rotational crops, there is a paucity of information on the effect of poaceous crops on P. capsici. Depending on the regions, poaceous cover and rotational crops used include corn (Zea mays), oat (Avena sativa), sorghum (Sorghum bicolor) including sudangrass, and forage and grain sorghum, ryegrass (Lolium sp.) and wheat (Triticum sp.). Except for a few studies that have examined the effect of wheat on dispersal of P. capsici (Ristaino et al., Citation1997) and sudangrass on Phytophthora blight (McGrath, Citation1997), little is known about other crops in relation to the biology of P. capsici.
Concluding remarks
Phytophthora capsici remains a serious challenge to the production of solanaceous and cucurbitaceous crops and other vegetables in the USA and worldwide. This mini-review has provided a repertory of management tools with several new perspectives while delineating the gaps in knowledge on integrated systems for managing P. capsici. Efficient management of this oomycete pathogen demands development of integrated disease management programmes incorporating treatments of seeds, soils and plants. An important aspect in the development of such integrated management systems is the economic soundness of these systems. Very few studies (Chávez-Alfaro et al., Citation1995; Daniell & Falk, Citation1994) have been conducted to provide such assessment for management of P. capsici in vegetables. Daniell & Falk (Citation1994) used programming models to provide management strategies for farmers, based on their attitudes toward risk; strategies differed for farmers who wished to maximize income regardless of risk, and those who wished to reduce exposure to risk. Further research can focus on significant knowledge gaps with regard to the effects of several production environment variables on biological attributes and activities of P. capsici. With continuous and coordinated efforts of plant pathologists, horticulturists, breeders and economists, integrated and effective management of Phytophthora blight on solanaceous and cucurbitaceous crops and other vegetables should be an attainable goal.
Acknowledgements
We gratefully acknowledge critical reviews by Pam Roberts, University of Florida; Mike Matheron, University of Arizona; and Shaker Kousik, USDA-ARS, Charleston, SC. This work was supported by New Mexico Agricultural Experiment Station, Georgia Agricultural Commodity Commission for Vegetables and USDA-NIFA Special Research Grants.
References
- Abbasi , P.A. , Lazarovits , G. and Weselowski , B. 2011 . Effectiveness of AG3 phosphonate formulation in suppressing Phytophthora blight in cucumber and bell pepper plants under growth room conditions . Can. J. Plant Pathol. , 33 : 150 – 158 .
- Ahmed , A.S. , Sánchez , C.P. and Candela , M.E. 2000 . Evaluation of induction of systemic resistance in pepper plants (Capsicum annuum) to Phytophthora capsici using Trichoderma harzianum and its relation with capsidiol accumulation . Eur. J. Plant Pathol. , 106 : 817 – 824 .
- Ando , K. and Grumet , R. 2006 . Evaluation of altered cucumber plant architecture as a means to reduce Phytophthora capsici disease incidence on cucumber fruit . J. Am. Soc. Hortic. Sci. , 131 : 491 – 498 .
- Ando , K. , Hammar , S. and Grumet , R. 2009 . Age-related resistance of diverse cucurbit fruit to infection by Phytophthora capsici . J. Am. Soc. Hortic. Sci. , 134 : 176 – 182 .
- Babadoost , M. 2000 . Outbreak of Phytophthora foliar blight and fruit rot in processing pumpkin fields in Illinois . Plant Dis. , 84 : 1345
- Babadoost , M. and Islam , S. Z. 2003 . Fungicide seed treatment effects on seedling damping-off of pumpkin caused by . Phytophthora capsici. Plant Dis. , 87 : 63 – 68 .
- Bae , H.H. , Roberts , D.P. , Lim , H.S. , Strem , M.D. , Park , S.C. , Ryu , C.M. , Melnick , R.L. and Bailey , B.A. 2011 . Endophytic Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms . Mol. Plant-Microbe Interact. , 24 : 336 – 351 .
- Bajpai , V.K. and Kang , S. 2010 . Antifungal activity of leaf essential oil and extracts of Metasequoia glyptostroboides Miki ex Hu . J. Am. Oil Chem. Soc. , 87 : 327 – 336 .
- Bajpai , V.K. , Kang , S. and Lee , T. 2009 . Chemical composition and in vitro control of agricultural plant pathogens by the essential oil and various extracts of Nandina domestica Thunb . J. Sci. Food Agr. , 89 : 109 – 116 .
- Baysal , O. , Turgut , C. and Mao , G. 2005 . Acibenzolar-S-methyl induced resistance to Phytophthora capsici in pepper leaves . Biol. Plant. , 49 : 599 – 604 .
- Bi , Y. , Jiang , H. , Hausbeck , M.K. and Hao , J.J. 2012 . Inhibitory effects of essential oils for controlling Phytophthora capsici . Plant Dis. , 96 : 797 – 803 .
- Biles , C.L. , Liddell , C.M. and Lindsey , D.L. 1992 . Control of Phytophthora root rot of chile peppers by irrigation practices and fungicides . Crop Prot. , 11 : 225 – 228 .
- Biles , C.L. , Wall , M.M. , Waugh , M. and Palmer , H. 1993 . Relationship of Phytophthora fruit rot to fruit maturation and cuticle thickness of new Mexican-type peppers . Phytopathology , 83 : 607 – 611 .
- Blum , M. , Boehler , M. , Randall , E. , Young , V. , Csukai , M. , Kraus , S. , Moulin , F. , Scalliet , G. , Avrova , A.O. , Whisson , S.C. and Fonne-Pfister , R. 2010 . Mandipropamid targets the cellulose synthase-like PiCesA3 to inhibit cell wall biosynthesis in the oomycete plant pathogen . Phytophthora infestans. Mol. Plant Pathol. , 11 : 227 – 243 .
- Bosland , P.W. and Lindsey , D.L. 1991 . A seedling screen for Phytophthora root rot of pepper . Capsicum annuum. Plant Dis. , 75 : 1048 – 1050 .
- Café-Filho , A.C. and Duniway , J.M. 1995 . Effects of furrow irrigation schedules and host genotype on Phytophthora root rot of pepper . Plant Dis. , 79 : 39 – 43 .
- Camp , A.R. , Dillard , H.R. and Smart , C.D. 2008 . Efficacy of Muscodor albus for the control of Phytophthora blight of sweet pepper and butternut squash . Plant Dis. , 92 : 1488 – 1492 .
- Candole , B.L. , Conner , P.J. and Ji , P. 2010 . Screening of Capsicum annuum accessions for resistance to six isolates of Phytophthora capsici . HortScience , 45 : 254 – 259 .
- Chavez , D.J. , Kabelka , E.A. and Chaparro , J.X. 2011 . Screening of Cucurbita moschata Duchesne germplasm for crown rot resistance to Floridian isolates of Phytophthora capsici Leonian . HortScience , 46 : 536 – 540 .
- Chávez-Alfaro , J.J. , Zavaleta-Mejía , E. and Téliz , O.D. 1995 . Integrated control of blight in pepper (Capsicum annuum L.) caused by the fungus Phytophthora capsici Leo., in the Valsequillo region, Puebla, Mexico . Fitopatología , 30 : 47 – 55 .
- Cimen , I. , Pirinc , V. , Sagır , A. , Akpinar , C. and Guzel , S. 2009 . Effects of solarization and vesicular arbuscular mycorrhizal fungus (VAM) on phytophthora blight (Phytophthora capsici leonian) and yield in pepper . Afr. J. Biotechnol. , 8 : 4884 – 4894 .
- Coelho , L. , Chellemi , D.O. and Mitchell , D.J. 1999 . Efficacy of solarization and cabbage amendment for the control of Phytophthora s pp. in North Florida . Plant Dis. , 83 : 293 – 299 .
- Daniell , I.R. and Falk , C.L. 1994 . Economic comparison of Phytophthora root rot control methods . Crop Prot. , 13 : 331 – 336 .
- Diaz , J. , Silvar , C. , Varela , M.M. , Bernal , A. and Merino , F. 2005 . Fusarium confers protection against several mycelial pathogens of pepper plants . Plant Pathol. , 54 : 773 – 780 .
- Erwin , D.C. and Ribeiro , O.K. 1996 . Phytophthora Diseases Worldwide , St. Paul , MN : American Phytopathological Society .
- Etxeberria , A. , Mendarte , S. and Larregla , S. 2011 . Thermal inactivation of Phytophthora capsici oospores . Rev. Iberoam. Micol. , 28 : 83 – 90 .
- Fang , X.L. , Li , Z.Z. , Wang , Y.H. and Zhang , X. 2011 . In vitro and in vivo antimicrobial activity of Xenorhabdus bovienii YL002 against Phytophthora capsici and Botrytis cinerea . J. Appl. Microbiol. , 111 : 145 – 154 .
- Foster , J.M. and Hausbeck , M.K. 2010 . Managing Phytophthora crown and root rot in bell pepper using fungicides and host resistance . Plant Dis. , 94 : 697 – 702 .
- French-Monar , R.D. , Jones , J.B. and Roberts , P.D. 2006 . Characterization of Phytophthora capsici associated with roots of weeds on Florida vegetable farms . Plant Dis. , 90 : 345 – 350 .
- French-Monar , R.D. , Jones , J.B. , Ozores-Hampton , M.M. and Roberts , P.D. 2007 . Survival of inoculum of Phytophthora capsici in soil through time under different soil treatments . Plant Dis. , 91 : 593 – 598 .
- French-Monar , R.D. , Rodrigues , F.A. , Korndörfer , G.H. and Datnoff , L.E. 2010 . Silicon suppresses Phytophthora blight development on bell pepper . J. Phytopathol. , 158 : 554 – 560 .
- Gevens , A.J. , Donahoo , R.S. , Lamour , K.H. and Hausbeck , M.K. 2007 . Characterization of Phytophthora capsici from Michigan surface irrigation water . Phytopathology , 97 : 421 – 428 .
- Gisbert , C. , Sánchez-Torres , P. , Raigon , M.D. and Nuez , F. 2010 . Phytophthora capsici resistance evaluation in pepper hybrids: agronomic performance and fruit quality of pepper grafted plants . J. Food Agr. Environ. , 8 : 116 – 121 .
- Glosier , B.R. , Ogundiwin , E.A. , Sidhu , G.S. , Sischo , D.R. and Prince , J.P. 2008 . A differential set of pepper (Capsicum annuum) lines delineates fourteen physiological races of Phytophthora capsici . Euphytica , 162 : 23 – 30 .
- Granke , L.L. and Hausbeck , M.K. 2010 . Effects of temperature, concentration, age, and algaecides on Phytophthora capsici zoospore infectivity . Plant Dis. , 94 : 54 – 60 .
- Hausbeck , M.K. and Lamour , K.H. 2004 . Phytophthora capsici on vegetable crops: research progress and management challenges . Plant Dis. , 88 : 1292 – 1303 .
- Hyder , N. , Coffey , M.D. and Stanghellini , M.E. 2009 . Viability of oomycete propagules following ingestion and excretion by fungus gnats, shore flies, and snails . Plant Dis. , 93 : 720 – 726 .
- Islam , S.Z. and Babadoost , M. 2002 . Effect of red-light treatment of seedlings of pepper, pumpkin, and tomato on the occurrence of Phytophthora damping-off . HortScience , 37 : 678 – 681 .
- Jackson , K.L. , Yin , J. , Csinos , A.S. and Ji , P. 2010 . Fungicidal activity of fluopicolide for suppression of Phytophthora capsici on squash . Crop Prot. , 29 : 1421 – 1427 .
- Jackson , K. L. , Yin , J. and Ji , P. 2012 . Sensitivity of Phytophthora capsici from vegetable crops in Georgia to mandipropamid, dimethomorph, and cyazofamid . Plant Dis. , 96 : 1337 – 1342 .
- Ji , P. , Csinos , A.S. , Hickman , L.L. , McMillan , J. and Hargett , U. 2008 . Integrated use of Ridomil Gold and other compounds for management of Phytophthora blight in squash, 2007 . Plant Dis. Manag. Rep. , 2 : V178
- Ji , P. , Yin , J. and Koné , D. 2011 . Application of acibenzolar-S-methyl and standard fungicides for control of Phytophthora blight on squash . Crop Prot. , 30 : 1601 – 1605 .
- Ji , P. , Koné , D. , Yin , J. , Jackson , K. L. and Csinos , A. S. 2012 . Soil amendments with Brassica cover crops for management of Phytophthora blight on squash . Pest Manag. Sci. , 68 : 639 – 644 .
- Keinath , A. P. 2007 . Sensitivity of population of Phytophthora capsici from South Carolina to mefenoxam, dimethomorph, zoxamide, and cymoxanil . Plant Dis. , 91 : 743 – 748 .
- Khan , J. , Ooka , J.J. , Miller , S.A. , Madden , L.V. and Hoitink , H.A.J. 2004 . Systemic resistance induced by Trichoderma hamatum 382 in cucumber against Phytophthora crown rot and leaf blight . Plant Dis. , 88 : 280 – 286 .
- Kim , K.D. , Nemec , S. and Musson , G. 1997 . Effects of composts and soil amendments on soil microflora and Phytophthora root and crown rot of bell pepper . Crop Prot. , 16 : 165 – 172 .
- Kim , Y.J. , Hwang , B.K. and Park , K.W. 1989 . Expression of age-related resistance in pepper plants infected with Phytophthora capsici . Plant Dis , 73 : 745 – 747 .
- King , S.R. , Levi , A. , Liu , W. and Davis , A.R. 2008 . Grafting for disease resistance . HortScience , 43 : 1673 – 1676 .
- Koné , D. , Csinos , A.S. , Jackson , K.L. and Ji , P. 2009 . Evaluation of systemic acquired resistance inducers for control of Phytophthora capsici on squash . Crop Prot. , 28 : 533 – 538 .
- Kousik , C.S. and Subramanya , S.R. 2001 . Evaluation of Actigard 50WG treatments for management of Phytophthora blight and Erwinia soft rot on bell pepper 2000 . Fung. & Nemat. Tests , 56 : V34
- Kousik , C.S. , Adams , M.L. , Jester , W.R. , Hassell , R. , Harrison , H.F. and Holmes , G.J. 2011 . Effect of cultural practices and fungicides on Phytophthora fruit rot of watermelon in the Carolinas . Crop Prot. , 30 : 888 – 894 .
- Kuck , K.-H. and Gisi , U. 2008 . “ FRAC mode of action classification and resistance risk of fungicides ” . In Modern Crop Protection Compounds , Edited by: Krämer , W. and Schirmer , U. 415 – 432 . Weinheim : Wiley-VCH Verlag GmbH & Co. KGaA .
- Lamour , K.H. and Hausbeck , M.K. 2000 . Mefenoxam insensitivity and the sexual stage of Phytophthora capsici in Michigan cucurbits field . Phytopathology , 90 : 396 – 400 .
- Lamour , K.H. and Hausbeck , M.K. 2003 . Effect of crop rotation on the survival of Phytophthora capsici in Michigan . Plant Dis. , 87 : 841 – 845 .
- Lee , B.K. , Kim , B.S. , Chang , S.W. and Hwang , B.K. 2001 . Aggressiveness to pumpkin cultivars of isolates of Phytophthora capsici from pumpkin and pepper . Plant Dis. , 85 : 497 – 500 .
- Leonian , L.H. 1922 . Stem and fruit blight of peppers caused by Phytophthora capsici sp. nov . Phytopathology , 12 : 401 – 408 .
- Lévesque , C.A. and Rahe , J.E. 1992 . Herbicide interactions with fungal root pathogens, with special reference to glyphosate . Annu. Rev. Phytopathol. , 30 : 579 – 602 .
- Liu , B. , Gumpertz , M.L. , Hu , S. and Ristaino , J.B. 2008 . Effect of prior tillage and soil fertility amendments on dispersal of Phytophthora capsici and infection of pepper . Eur. J. Plant Pathol. , 120 : 273 – 287 .
- Louws , F.J. , Kubota , C. and Rivard , C.L. 2010 . Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds . Sci. Hortic. , 127 : 127 – 146 .
- Ma , Y. , Chang , Z.Z. , Zhao , J.T. and Zhou , M.G. 2008 . Antifungal activity of Penicillium striatisporum Pst10 and its biocontrol effect on Phytophthora root rot of chilli pepper . Biol. Cont. , 44 : 24 – 31 .
- Mao , W. , Lewis , J.A. , Lumsden , R.D. and Hebbar , K.P. 1998 . Biocontrol of selected soilborne diseases of tomato and pepper plants . Crop Prot. , 17 : 535 – 542 .
- Matheron , M.E. and Porchas , M. 2000 . Comparison of five fungicides on development of root, crown, and fruit rot of chile pepper and recovery of Phytophthora capsici from soil . Plant Dis. , 84 : 1038 – 1043 .
- Matheron , M.E. and Porchas , M. 2002 . Suppression of Phytophthora root and crown rot on pepper plants treated with acibenzolar-S-methyl . Plant Dis. , 86 : 292 – 297 .
- Matheron , M.E. and Porchas , M. 2007 . Comparison of fungicides for management of the soil phase of Phytophthora blight, 2005 . Plant Dis. Manag. Rep. , 1 : V137
- Mazzola , M. , Brown , J. , Izzo , A. and Cohen , M.F. 2007 . Mechanism of action and efficacy of seed meal-induced suppression of pathogens inciting apple replant disease differ in a Brassicaceae species and time-dependent manner . Phytopathology , 97 : 454 – 460 .
- McGrath , M.T. 1997 . Evaluation of solarization and sorghum grass for managing Phytophthora crown rot and fruit rot in pumpkin, 1995–1996 . Biol. Cult. Tests , 12 : 159
- McGrath , M.T. 2004 . Evaluation of yard-waste compost for managing Phytophthora blight of pumpkin . Biol. Cult. Tests , 20 : V010
- McGrath , M.T. 2009 . “ Managing plant diseases with crop rotation ” . In Crop Rotation on Organic Farms: A Planning Manual , Edited by: Mohler , C.L. and Johnson , S.E. 32 – 41 . Ithaca , NY : Cooperative Extension, Cornell University . NRAES-177, Natural Resource, Agriculture, and Engineering Services
- McGrath , M.T. and Rangarajan , A. 2002 . Evaluation of brewery-waste compost for managing Phytophthora blight of pumpkin . Biol. Cult. Tests , 17 : V20
- Mercier , J. and Manker , D.C. 2005 . Biocontrol of soil-borne diseases and plant growth enhancement in greenhouse soilless mix by the volatile-producing fungus Muscodor albus . Crop Prot , 24 : 355 – 362 .
- Mitani , S. , Araki , S. , Yamaguchi , T. , Takii , Y. , Ohshima , T. and Matsuo , N. 2001 . Antifungal activity of the novel fungicide cyazofamid against Phytophthora infestans and other plant pathogenic fungi in vitro . Pestic. Biochem. Physiol. , 70 : 92 – 99 .
- Mojica-Marin , V. , Luna-Olvera , H.A. , Sandoval-Coronado , C.F. , Pereyra-Alferez , B. , Morales-Ramos , L.H. , Gonzalez-Aguilar , N.A. , Hernandez-Luna , C.E. and Alvarado-Gomez , O.G. 2009 . Biological control of chili pepper root rot (Capsicum annuum L.) by Bacillus thuringiensis . Phyton-Int. J. Exp Bot. , 78 : 105 – 110 .
- Monroy-Barbosa , A. and Bosland , P.W. 2011 . Identification of novel physiological races of Phytophthora capsici causing foliar blight using the New Mexico recombinant inbred pepper lines set as a host differential . J. Am. Soc. Hort. Sci. , 136 : 205 – 210 .
- Muller-Riebau , F.F. , Yegen , O.O. and Berger , B.B. 1995 . Chemical composition and fungitoxic properties to phytopathogenic fungi of essential oils of selected aromatic plants growing wild in Turkey . J. Agr. Food Chem. , 43 : 2262 – 2266 .
- Nemec , S. , Datnoff , L.E. and Strandberg , J. 1996 . Efficacy of biocontrol agents in planting mixes to colonize plant roots and controls root diseases of vegetables and citrus . Crop Prot. , 15 : 735 – 742 .
- Núñez-Zofío , M. , Larregla , S. and Garbisu , C. 2011 . Application of organic amendments followed by soil plastic mulching reduces the incidence of Phytophthora capsici in pepper crops under temperate climate . Crop Prot. , 30 : 1563 – 1572 .
- Oelke , L.M. , Bosland , P.W. and Steiner , R. 2003 . Differentiation of race specific resistance to Phytophthora root rot and foliar blight in Capsicum annuum . J. Am. Soc. Hort. Sci. , 128 : 213 – 218 .
- Ozgonen , H. and Erkilic , A. 2007 . Growth enhancement and Phytophthora blight (Phytophthora capsici Leonian) control by arbuscular mycorrhizal fungal inoculation in pepper . Crop Prot. , 26 : 1682 – 1688 .
- Padley , L.D. , Roberts , P.D. , French , R. and Kabelka , E.A. 2008 . Evaluation of Cucurbita pepo accessions for crown rot resistance to isolates of . Phytophthora capsici. HortScience , 43 : 1996 – 1999 .
- Park , Y-H. , Song , B-H. , Kim , Y-K. , Park , Y-S. and Shin , C-S. 1992 . Development of mixed pesticide for phytophthora rot and anthracnose control in red pepper . Res. Rep. Rural Dev. Admin. , 34 : 121 – 126 .
- Parra , G. and Ristaino , J.B. 2001 . Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper . Plant Dis. , 85 : 1069 – 1075 .
- Ploetz , R.C. , Heine , G. , Haynes , J.L. and Watson , M. 2002 . Investigating biological attributes that may contribute to Phytophthora capsici’s importance as a vegetable pathogen in Florida . Ann. Appl. Biol. , 140 : 61 – 67 .
- Quesada-Ocampo , L.M. and Hausbeck , M.K. 2010 . Resistance in tomato and wild relatives to crown and root rot caused by Phytophthora capsici . Phytopathology , 100 : 619 – 627 .
- Reifschneider , F.J.B. , Boiteux , L.S. , Dellavecchia , P.T. , Poulos , J.M. and Kuroda , N. 1992 . Inheritance of adult-plant resistance to Phytophthora capsici in pepper . Euphytica , 62 : 45 – 49 .
- Ristaino , J.B. and Johnston , S.A. 1999 . Ecologically based approaches to management of Phytophthora blight on bell pepper . Plant Dis. , 83 : 1080 – 1089 .
- Ristaino , J.B. , Gregory , P. and Campbell , C.L. 1997 . Suppression of Phytophthora blight in bell pepper by a no-till wheat cover crop . Phytopathology , 87 : 242 – 249 .
- Roberts , P.D. , Urs , R.R. , French-Monar , R.D. , Hoffine , M.S. , Seijo , T.E. and McGovern , R.J. 2005 . Survival and recovery of Phytophthora capsici and oomycetes in tailwater and soil from vegetable fields in Florida . Ann. Appl. Biol. , 146 : 351 – 359 .
- Romero , A.M. , Kousik , C.S. and Ritchie , D.F. 2001 . Resistance to bacterial spot in bell pepper induced by acibenzolar-S-methyl . Plant Dis. , 85 : 189 – 194 .
- Ros , C. , Guerrero , M.M. , Martínez , M.A. , Barceló , N. , Martínez , M.C. , Rodríguez , I. , Lacasa , A. , Guirao , P. and Bello , A. 2005 . Resistant sweet pepper rootstocks integrated into the management of soilborne pathogens in greenhouse . Acta Hortic. , 698 : 305 – 310 .
- Sang , M.K. and Kim , K.D. 2012 . The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper . J. Appl. Microbiol. , 113 : 383 – 398 .
- Sang , M.K. , Kim , J.G. and Kim , K.D. 2010 . Biocontrol activity and induction of systemic resistance in pepper by compost water extracts against Phytophthora capsici . Phytopathology , 100 : 774 – 783 .
- Sanogo , S. 2006 . Predispositional effect of soil water saturation on infection of chile pepper by Phytophthora capsici . HortScience , 41 : 172 – 175 .
- Sanogo , S. and Clary , M. 2006 . Occurrence of Phytophthora blight on pumpkin in New Mexico . Plant Dis. , 90 : 1110
- Sanogo , S. , Yang , X.B. and Scherm , H. 2000 . Effects of herbicides on Fusarium solani f. sp. glycines and development of sudden death syndrome in glyphosate-tolerant soybean . Phytopathology , 90 : 57 – 66 .
- Shafi , P.M. , Sarma , Y.R. , Veena , S.S. , Nambia , M.G. and Clery , R.A. 2004 . Composition and antifungal activity of the oil of Artemisia nilagirica (Clarke) Pamp . J. Essent. Oil Res. , 16 : 377 – 379 .
- Sholberg , P.L. , Walker , M.C. , O'gorman , D.T. and Jesperson , G.D. 2007 . First report of Phytophthora capsici on cucurbits and peppers in British Columbia . Can. J. Plant Pathol. , 29 : 153 – 158 .
- Sy , O. , Steiner , R. and Bosland , P.W. 2008 . Recombinant inbred line differential identifies race-specific resistance to Phytophthora root rot . Capsicum annuum. Phytopathology , 98 : 867 – 870 .
- Tahboub , M.B. , Sanogo , S. , Bosland , P.W. and Murray , L. 2008 . Heat level in chile pepper in relation to root and fruit infection by . Phytophthora capsici. HortScience , 43 : 1846 – 1851 .
- Tian , D. and Babadoost , M. 2004 . Host range of Phytophthora capsici from pumpkin and pathogenicity of isolates . Plant Dis. , 88 : 485 – 489 .
- Toquin , V. , Barja , F. , Sirven , C. and Bffa , R. 2008 . “ Fluopicolide, a new anti-oomycetes fungicide with a new mode of action inducing perturbation of a spectrin-like protein ” . In Modern Crop Protection Compounds , Edited by: Krämer , W. and Schirmer , U. 675 – 682 . Weinheim : Wiley-VCH Verlag GmbH & Co. KGaA .
- Wang , Z. , Langston , D.B. , Csinos , A.S. , Gitaitis , R.D. , Walcott , R.R. and Ji , P. 2009 . Development of an improved isolation approach and simple sequence repeat markers to characterize Phytophthora capsici in irrigation ponds in southern Georgia . Appl. Environ. Microbiol. , 75 : 5467 – 5473 .
- Xie , J. , Cardenas , E.S. , Sammis , T.W. , Wall , M.M. , Lindsey , D.L. and Murray , L.W. 1999 . Effects of irrigation method on chile pepper yield and Phytophthora root rot incidence . Agric. Water Manag. , 42 : 127 – 142 .
- Yin , J. , Jackson , K.L. , Candole , B.L. , Csinos , A.S. , Langston , D.B. and Ji , P. 2012 . Aggressiveness and diversity of Phytophthora capsici on vegetable crops in Georgia . Ann. Appl. Biol. , 160 : 191 – 200 .
- Yucel , S. 1995 . A study on soil solarization and combined with fumigant application to control Phytophthora crown blight (Phytophthora capsici Leonian) on peppers in the East Mediterranean region of Turkey . Crop Prot. , 14 : 653 – 655 .