Abstract
Bacterial angular leaf spot of strawberry caused by Xanthomonas fragariae is an increasingly important disease for which few management strategies are available. The resistance status of most cultivars is unknown and of those cultivars tested, few have significant levels of resistance. Copper formulations are available and have limited effectiveness but phytotoxicity is a problem with repeated applications. This controlled environment study examined the efficacy of foliar applications of various antioxidants, such as α-tocopherol and mannitol, along with the plant activators acetylsalicylic acid and acibenzolar-S-methyl, and the fungicide fosetyl-Al, for disease control. Mannitol and acibenzolar-S-methyl consistently provided excellent control of disease, while fosetyl-Al and α-tocopherol were significantly less effective. Other antioxidants and sugar alcohols similar to mannitol did not provide the same level of angular leaf spot control. Interestingly, interactions were observed between various sugar alcohols and strawberry cultivars in suppressing disease. The mode of action of sugar alcohols against angular leaf spot is likely not related to their antioxidant activity. Sugar alcohols, especially mannitol, may represent a new and non-toxic approach to bacterial angular leaf spot control.
Résumé
La tache anguleuse de la fraise, causée par Xanthomonas fragaria, est une maladie de plus en plus courante contre laquelle il existe peu de stratégies de lutte. Le degré de résistance de la plupart des cultivars est inconnu et, chez ceux testés, rares sont ceux qui affichent un degré de résistance significatif. Des préparations à base de cuivre sont offertes, mais leur efficacité est limitée. Par ailleurs, les applications répétées engendrent des problèmes de phytotoxicité. Cette étude, menée en milieu contrôlé, a examiné l'efficacité d'applications foliaires de divers antioxydants tels que l'α-tocophérol et le mannitol, de concert avec des phytoactivateurs comme l'acide acétylsalicylique et l'acibenzolar-S-méthyl, ainsi qu'avec le fongicide fosétyl aluminium, pour lutter contre la maladie. Le mannitol et l'acibenzolar-S-méthyl ont invariablement enrayé la maladie, tandis que le fosétyl aluminium et l'α-tocophérol ont été significativement moins efficaces. D'autres antioxydants et alcools de sucre semblables au mannitol n'ont pas permis de gérer aussi efficacement la tache anguleuse. Fait intéressant, des interactions visant à supprimer la maladie ont été observées entre divers alcools de sucre et cultivars de fraise. Il est peu probable que le mode d'action des alcools de sucre contre la tache anguleuse soit associé à leur activité antioxydante. Les alcools de sucre, particulièrement le mannitol, peuvent constituer une nouvelle approche non toxique à la lutte contre la tache anguleuse.
Introduction
Bacterial angular leaf spot of strawberry (Fragaria ×ananassa Duch. Ex Rozier), caused by Xanthomonas fragariae Kenn. & King (Kennedy & King, Citation1962) is a disease of increasing importance. Since its discovery in Minnesota in 1960, angular leaf spot has been found in North and South America, Europe, North Africa, Great Britain and Australasia (Gillings et al., Citation1998). Reported yield losses from this disease range from 8% to 80% (Epstein, Citation1966; Roberts et al., Citation1997). Current disease control measures include cultural practices and the use of copper compounds and antibiotics, but none provide consistent disease reduction.
The primary consideration in managing angular leaf spot is the use of disease-free planting material. The bacteria are systemic and much of the spread of this disease worldwide has been blamed on the importation of symptomless infected plants (Milholland et al., Citation1996; Roberts et al., Citation1996; Gillings et al., Citation1998). In Europe, X. fragariae is considered a quarantinable pathogen and as such movement of nursery stock is regulated (Smith et al., Citation1992). Eradication may be possible in small outbreaks of angular leaf spot (Matthews-Berry & Reed, Citation2009), but in regions where the pathogen is more widespread, this is not possible. Treatment of nursery plants with hot water has been studied, but the temperature and time required to inactivate the pathogen may result in phytotoxicity (Turechek & Peres, Citation2009). No commercial cultivars have yet been described as completely resistant to angular leaf spot (Maas et al., Citation2000), but quantitative differences in disease susceptibility exist among several of them (Hildebrand et al., Citation2005), as well as in wild sources (Maas et al., Citation2002; Xue et al., Citation2005). Recent attempts at crossing wild strawberry (F. virginiana) selections showing high levels of disease resistance (Maas et al. Citation2002; Lewers et al., Citation2003) with commercial cultivars has shown promise for disease control (Jamieson et al., Citation2013).
Chemical controls such as copper compounds and the antibiotics streptomycin sulphate and oxytetracycline can reduce disease severity, but they have drawbacks (Stall & Thayer, Citation1962; Roberts et al., Citation1997). Copper compounds and antibiotics are not registered for use in all countries against angular leaf spot disease and the former have limited efficacy and may cause phytotoxicity when used repeatedly (Lynch & Tremblay, Citation1996). Thus, additional control strategies, particularly non-toxic means of controlling angular leaf spot, would be desirable.
A number of non-toxic chemical control strategies have been proposed for a range of plant pathogens that may be applicable to angular leaf spot in strawberry. Norris (Citation1991) patented the use of antioxidants and in particular ascorbates, tocopherols, reduced glutathione and cysteines to control pathogens and plant pests. Elad (Citation1992) tested 18 antioxidants for the control of Botrytis cinerea Pers. Fr. and Sclerotinia sclerotiorum (Lib.) de Bary and found that most of them had some effect on reducing disease severity. Schmitz & Noga (Citation1998) demonstrated that α-tocopherol reduced environmental stress on plants and the severity of Venturia inaequalis (Cooke) Wint. on apple trees. Pierpoint et al. (Citation1981) were among the early workers who reported that tobacco leaves treated with acetylsalicylic acid (ASA) or mannitol produced pathogenesis-related (PR) proteins which are now known to be associated with disease resistance in many plant/pathogen systems (Vlot et al., Citation2009). Acibenzolar-S-methyl (ASM), a synthetic analog of salicylic acid, has since been shown to have activity against several bacterial pathogens, including X. campestris pv. vesicatoria (Doidge) Dye on tomato and pepper (Louws et al., Citation2001; Romero et al., Citation2001), Xanthomonas oryzae pv. oryzae (Ishiyama) Swings et al. on rice (Babu et al., Citation2003) and X. fragariae on strawberry (Mertley, Citation2010). The fungicide fosetyl-Al has also been shown to have some activity against X. campestris pv. dieffenbachiae (McCulloch & Pirone) Dye on greenhouse-grown ornamentals (Chase, Citation1993).
Here we report on a series of experiments that tested the efficacy of ASA, ASM, fosetyl-Al, α-tocopherol, mannitol and several other sugar alcohols and antioxidants to determine the potential of these alternative chemicals for the control of angular leaf spot on strawberry.
Materials and methods
Plant material and inoculum
Plant material, maintenance, inoculum production and inoculation methods have been previously described in detail (Hildebrand et al., Citation2005). In brief, autumn-dug strawberry plants were purchased from a local nursery and stored at 0 °C until needed. Crowns were planted in 10-cm diameter pots containing a soil mix of peat moss, pasteurized soil, and sand (2:1:1, by volume) and grown in a greenhouse at 20 to 24 °C with supplemental lighting giving a 14-h photoperiod. Plants were fertilized bi-weekly with 40 mL of a 20:20:20 N-P-K soluble fertilizer (7.5 g L−1) and used in experiments when they had 5–7 fully expanded trifoliate leaves (∼4–7 wks old). Plants were inoculated with X. fragariae (isolate W2) grown on potato dextrose agar at 20 °C for 3–5 days. Bacterial cells were washed from the agar surface and suspended in sterile distilled water (SDW) to form a suspension with an absorbance of 0.05 units at 600 nm which approximated 2.8×107 colony-forming units per mL.
Source of chemicals and treatment of plants
Actigard™ (acibenzolar-S-methyl, 50 WG, Syngenta, Guelph, ON) was a gift from Syngenta and Aliette® (fosetyl-Al, 80 WDG, Bayer CropScience, Research Triangle Park, NC) was purchased from a local pesticide supplier. All other chemicals were purchased from Sigma-Aldrich (Oakville, ON).
In all experiments, spray solutions were applied to run-off on the upper and lower leaf surfaces of all strawberry plants with a DeVilbiss®(Toledo, OH) mister (model 15-RD). The mister was slightly modified by removing the rubber bulb and replacing it with a compressed air line operating at ∼ 140 kPa. Appropriate water controls were included for each experiment. Treated plants were returned to the greenhouse for 48 h and then the abaxial surface of all three leaflets of the oldest non-senescent trifoliate leaf, youngest fully expanded trifoliate leaf and a trifoliate leaf midway in age between the two age extremes of each plant were inoculated. The inoculated leaves were tagged for later identification. The inoculum was applied to plants during mid-late morning (≥ 4 h of light exposure) to ensure that stomata were open and receptive to inoculum. Twenty-three μL of inoculum were applied to a 9 cm2 area with a pressurized paint sprayer (620 kPa) as described previously (Hildebrand et al., Citation2005). Inoculated plants were immediately placed in a humid chamber (RH > 90%) operating with a 20 °C day and 10 °C night temperature regime and a 14-h photoperiod. Lesion densities were evaluated after 21 days by estimating them to the nearest scale value of 0–7 where 0 = 0.3, 1 = 0.7, 2 = 1.3, 3 = 2.7, 4 = 5.3, 5 = 10.7, 6 = 21.3 and 7 = 42.7 lesions cm−2 (Hildebrand et al., Citation2005). The strawberry cultivar ‘Honeoye’ was used in all experiments except the final one where numerous cultivars were used ().
Efficacy of plant activators, antioxidants and sugar alcohols
The systemically acquired resistance activators, ASA and ASM, and antioxidants α-tocopherol and mannitol were applied to strawberry plants. ASA, ASM and mannitol were prepared in 100 mL of sterile distilled water (SDW) as 10 mM, 0.17 mM and 50 mM solutions, respectively. A stock solution of α-tocopherol (100 mM ) in absolute ethanol stored in the dark at 4 °C was diluted 1 : 5 with SDW immediately before use and suspended with vigorous agitation.
The enhancement of angular leaf spot control by the addition of Agral 90® (Norac Concepts Inc. Ottawa, ON), a wetting agent, to ASM, mannitol and α-tocopherol was examined. ASM (0.166 mM), mannitol (50 mM) and α-tocopherol (20 mM) were applied with or without the addition of Agral 90 (0.1% v/v).
The effect on lesion density of a range of ASM and mannitol concentrations and their possible interaction was tested. All possible combinations of ASM at 0.04 mM, 0.08 mM, 0.17 mM and 0.33 mM, and mannitol at 1.9 mM, 5.6 mM, 16.7 mM and 50 mM were applied.
The efficacy of ASM (0.17 mM), fosetyl-Al (12.4 mM), mannitol (50 mM), glycerol (100 mM), glucitol (100 mM) and combinations of the sugar alcohols with ASM or fosetyl-Al were also tested.
To determine if common antioxidants in addition to mannitol could effectively reduce lesion density, ascorbic acid (1.0 mM), benzoic acid (1.0 mM), citric acid (5.0 mM) and dimethylsulfoxide (DMSO; 1.0 mM) were applied individually or in combination with mannitol (50 mM).
To determine if a wider range of sugar alcohols had a similar effect as mannitol on disease control and whether the effect was consistent across strawberry cultivars, 16 commercial cultivars () were treated with mannitol, ribitol, inositol or glucitol at 50 mM.
Statistical analysis
For all experiments, there were four plants per treatment (36 leaflets) and all experiments were run 2–4 times. The runs were considered as blocks in a randomized complete block design. For ease of recording data, the 0–7 density scale was used. These rating values were then converted to the corresponding lesions/cm2 and transformed to the log2 scale since lesion densities doubled with each increase in rating value. The data were then analysed using the ANOVA directive in Genstat® 8.2 (VSN International Ltd, Hemel Hempstead, UK).
Results
For all experiments, there were no significant differences (P > 0.05) among the responses of the three leaf age classes to the various treatments (data not shown) and so the data were averaged over leaf age.
In the first experiment where ASA, ASM, α-tocopherol and mannitol were examined for their ability to control angular leaf spot, mannitol reduced lesion density by > 90% while ASM reduced lesion density by > 84% compared with the water control (). Alpha-tocopherol provided moderate disease control while ASA was ineffective at reducing lesion density. The combination of mannitol plus α-tocopherol was not more effective than mannitol alone. The addition of Agral 90 did not significantly (P = 0.05) improve disease control efficacy (data not shown).
Table 1. Effect of acetylsalicyclic acid (ASA), acibenzolar-S-methyl (ASM), α-tocopherol, mannitol and α-tocopherol plus mannitol on the density of angular leaf spot lesions on ‘Honeoye’ strawberry leaves
Combinations of increasing concentrations of ASM and mannitol did not result in a significant interaction (P > 0.05) with respect to disease control, but the main effect of concentration was significant. Increasing the concentration of ASM from 0.04 mM to 0.33 mM resulted in a linear decrease in lesion density from 0.4 lesions cm−2 to 0.2 lesions cm−2 and a similar linear decrease of 0.9 lesions cm−2 to 0.1 lesions cm−2 occurred with increasing concentrations of mannitol from 1.9 mM to 50 mM ().
Table 2. Effect of increasing concentrations of either acibenzolar-S-methyl (ASM) or mannitol alone and in combination on the density of angular leaf spot lesions on ‘Honeoye’ strawberry leaves
When ASM and fosetyl-Al were combined with mannitol, glycerol or glucitol, disease control was not improved compared with treatments where the chemicals were applied alone (). ASM and fosetyl-Al significantly (P < 0.005) reduced lesion density compared with the water control, but of the sugar alcohols, only mannitol reduced lesion density.
Table 3. Effect of acibenzolar-S-methyl (ASM), fosetyl-Al, and the sugar alcohols, mannitol, glycerol and glucitol alone and in combination with ASM or fosetyl-Al on the density of angular leaf spot lesions on ‘Honeoye’ strawberry leaves
In the experiment where mannitol was tested alone or in combination with the other antioxidants ascorbic acid, benzoic acid, citric acid and DMSO, mannitol was the only chemical that significantly reduced lesion density and the addition of the other antioxidants had no effect ().
Table 4. Effect of mannitol, ascorbic acid, benzoic acid, citric acid and dimethylsulfoxide alone and in combination with mannitol on the density of angular leaf spot lesions on ‘Honeoye’ strawberry leaves
Table 5. Effect of four sugar alcohols on the density of angular leaf spot lesions on leaves of 16 strawberry cultivars
A continuum of susceptibility to angular leaf spot occurred among the 16 non-treated strawberry cultivars ranging from 5 lesions cm−2 for ‘Mira’ to 43 lesion cm−2 for ‘Micmac’ (). On average, the sugar alcohols reduced disease compared with the non treated control, but a significant interaction occurred between the sugar alcohols and cultivars. On most cultivars, mannitol provided substantially better disease control than the other sugar alcohols. However, a notable exception occurred with ‘Mira’ where mannitol failed to control disease. Disease was reduced by ribitol on this cultivar. Good disease control also occurred with ribitol and glucitol on ‘Glooscap’ and ‘Brunswick’ and with inositol on ‘Cavendish’, ‘Evangeline’ and ‘Glooscap’, but mannitol was still more effective on each of these cultivars. Slight phytotoxicity appearing as necrotic flecks occurred on ‘Cabot’, ‘Sparkle’ and ‘Jewel’ and more severe necrotic spots occurred on ‘Brunswick’ especially when higher concentrations of mannitol were tested (data not shown).
Discussion
An application of ASM, mannitol or fosetyl-Al to strawberry leaves 48 h before challenge inoculation with X. fragariae resulted in a substantial reduction in angular leaf spot symptoms. This supports a previous report that ASM has the potential to control this disease (Mertley et al., Citation2010) as well as observations on the control of related Xanthomonads with ASM and fosetyl-Al on other crops (Chase, Citation1993; Louws et al., Citation2001; Romero et al., Citation2001; Babu et al., Citation2003). However, to our knowledge, this is the first demonstration that mannitol, a sugar alcohol, is highly effective against a bacterial disease. There was a linear relationship between the concentration of ASM and mannitol and disease control, with the highest rates of 0.33 mM and 50 mM, respectively, resulting in at least 98% disease control (). Even after 40 days, plants that had been treated with mannitol prior to inoculation remained essentially disease-free (). Fosetyl-Al was less effective and reduced lesion density by 77.3% (). Interestingly, ASA, an activator of plant disease defence mechanisms (Pierpoint et al., Citation1981; López- López et al., Citation1995), and the model for the development of ASM, failed to reduce disease severity ().
Fig. 1. Mannitol treated strawberry ('Honeoye') leaf (left) compared with a water-treated control (right). Both plants were challenge inoculated with Xanthomonas fragariae 48 h after treatment and incubated in a humid chamber for 40 days. Individual lesions have coalesced in the control.

Norris (Citation1991) postulated that, in general, diseases and insect pests could be controlled by antioxidants like tocopherols and ascorbates and it was later demonstrated that α-tocopherol could protect apple trees against the fungal pathogen Venturia inaequalis (Cooke) Wint. that causes apple scab (Schmitz & Noga, Citation1998). However, in our study, α-tocopherol was not highly effective compared to mannitol and reduced angular leaf spot symptoms by only 50%.
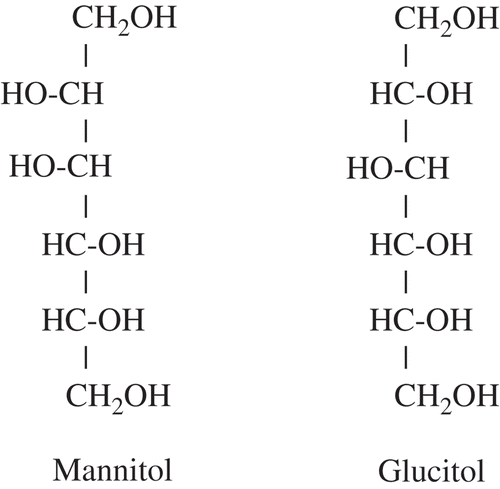
The remarkable efficacy of mannitol against angular leaf spot suggested that there may be other antioxidants or sugar alcohols effective as bacterial disease control agents. However, glucitol, a sugar alcohol with the same chemical formula (C6H14O6) and structure as mannitol, except for the position of one hydroxyl group (), had no effect on suppressing disease on ‘Honeoye’. Likewise glycerol (C3H8O3), ascorbic acid, benzoic acid, citric acid and dimethylsulfoxide, the other antioxidants tested, were ineffective. In addition, combining these antioxidants with mannitol did not increase disease control over mannitol alone ( and ).
Of the sugar alcohols tested, mannitol provided excellent disease control on most of the 16 strawberry cultivars, but failed to provide control on ‘Mira’. Interestingly, ‘Mira’ was also the least susceptible cultivar in this study. Ribitol (C5H12O5), glucitol (C6H14O6) and inositol (C6H12O6) provided some reduction in lesion density, but the effect was cultivar dependent. Herman et al. (Citation2007) observed a cultivar dependent control of Xanthomonas campestris pv. vesicatoria on tomato with ASM. They demonstrated that the level of induced resistance coincided with increased levels of induced PR1a protein in some cultivars.
While the mode of action of ASM in strawberry plants no doubt is related to its plant activating ability (Oostendorp et al., Citation2001), the mode of action of mannitol is less obvious. Its mode of action does not appear related to its antioxidant activity since other tested antioxidants had little or no effect on disease severity. A possible mode of action may be that mannitol, like ASM, induces a resistance response associated with PR proteins. Early work by Pierpoint et al. (Citation1981) showed that mannitol could induce PR proteins in tobacco, but since that time, there have been no other reports on this form of activity by mannitol. Additionally, in a recent review, Bolouri-Moghaddam & Van den Ende (Citation2012) concluded that sugar and sugar-like compounds were involved in induced resistance responses in some host/pathogen systems and could be an alternative to toxic agrochemicals.
In conclusion, this study demonstrated that ASM and mannitol have good potential for controlling angular leaf spot in strawberry. The excellent level of control afforded by mannitol, a common and innocuous substance, has not been previously reported. The interaction between the sugar alcohols and strawberry cultivars in suppressing disease symptoms is curious and is possibly related to a cultivar-dependent induction of PR proteins. Further research is in progress to elucidate the mode of action of mannitol on this bacterial disease and its effect on other bacterial plant/pathogen systems. Mannitol or a combination of mannitol with reduced copper concentrations may afford field level control of angular leaf spot while avoiding copper phytotoxicity or resistance.
Acknowledgements
The authors thank A. Braun and W.E. Renederos for technical support.
References
- Babu , R.M. , Sajeena , A. , Samundeeswari , A.V. , Sreedhar , A. , Vidhyasekeran , P. and Reddy , M.S. 2003 . Induction of bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in rice by treatment with acibenzolar-S-methyl . Ann. Appl. Biol. , 143 : 333 – 340 .
- Bolouri-Moghaddam , M.R. and Van den Ende , W. 2012 . Sugars and plant innate immunity . J. Exp. Bot. , 63 : 3989 – 3998 .
- Chase , A.R. 1993 . Efficacy of fosetyl-Al for control of some bacterial diseases on ornamentals . Plant Dis. , 77 : 771 – 776 .
- Elad , Y. 1992 . The use of antioxidants (free radical scavengers) to control grey mould (Botrytis cinerea) and white mould (Sclerotinia sclerotiorum) in various crops . Plant Pathol. , 41 : 417 – 426 .
- Epstein , A.H. 1966 . Angular leaf spot of strawberry . Plant Dis. Rep. , 50 : 167
- Gillings , M.R. , Fahy , P.C. and Bradley , J. 1998 . Identification of Xanthomonas fragariae, the cause of an outbreak of angular leaf spot on strawberry in South Australia, and comparison with the cause of previous outbreaks in New South Wales and New Zealand . Australas. Plant Pathol. , 27 : 97 – 103 .
- Herman , M.A.B. , Restrepo , S. and Smart , C.D. 2007 . Defense gene expression patterns of three SAR-induced tomato cultivars in the field . Physiol. Mol. Pathol. , 71 : 192 – 200 .
- Hildebrand , P.D. , Braun , P.G. , Renderos , W.E. , Jamieson , A.R. , McRae , K.B. and Binns , M.R. 2005 . A quantitative method for inoculating strawberry leaves with Xanthomonas fragariae, factors affecting infection, and cultivar reactions . Can. J. Plant Pathol. , 27 : 16 – 24 .
- Jamieson , A.R. , Hildebrand , P.D. and Renderos , W.E. 2013 . “ Breeding strawberry plants resistant to angular leaf spot disease ” . In Intern. J. Fruit Sci doi: 10.1080/15538362.2012.696959
- Kennedy , B.W. and King , T.H. 1962 . Angular leaf spot of strawberry caused by Xanthomonas fragariae sp. nov . Phytopathology , 52 : 873 – 875 .
- Lewers , K.S. , Maas , J.L. , Hokanson , S.C. , Gouin , C. and Hartung , J.S. 2003 . Inheritance of resistance in strawberry to bacterial angular leafspot disease caused by Xanthomonas fragariae . J. Am. Soc. Hort. Sci. , 128 : 209 – 212 .
- López-López , M.J. , Liébana , E. , Marcilla , P. and BeltrÁ , R. 1995 . Resistance induced in potato tubers by treatment with acetylsalicylic acid to soft rot produced by Erwinia carotovora subsp. carotovora . J. Phytopathol. , 143 : 719 – 724 .
- Louws , F.J. , Wilson , M. , Campbell , H.L. , Cuppels , D.A. , Jones , J.B. , Shoemaker , P.B. , Sahin , F. and Miller , S.A. 2001 . Field control of bacterial spot and bacterial speck of tomato using a plant activator . Plant Dis. , 85 : 481 – 488 .
- Lynch , K.V. and Tremblay , R.J.A. 1996 . Control of angular leaf spot (Xanthomonas fragariae) in strawberry using tribasic copper sulphate . Adv. Strawberry Res. , 15 : 18 – 20 .
- Maas , J.L. , Gouin-Behe , C. , Hartung , J.S. and Hokanson , S.C. 2000 . Sources of resistance to two differentially pathogenic strains of Xanthomonas fragariae in Fragaria genotypes . HortScience , 35 : 128 – 131 .
- Maas , J.L. , Gouin , C.C. , Hokanson , S.C. and Hartung , J.S. 2002 . Strawberry parent clones US 4808 and US 4809 resistant to bacterial angular leafspot disease caused by Xanthomonas fragariae . HortScience , 37 : 716 – 717 .
- Matthews-Berry , S.S. and Reed , P.J. 2009 . Eradication of the first outbreak of Xanthomonas fragariae in the United Kingdom . EPPO Bulletin , 39 : 171 – 174 .
- Mertley , J.C. 2010 . Efficacy of acibenzolar-S-methyl and copper fungicides for the control of angular leaf spot of strawberry . Phytopathology , 100 : S83 (Abstr.)
- Milholland , R.D. , Ritchie , D.F. , Daykin , M.E. and Gutierrez , W.A. 1996 . Multiplication and translocation of Xanthomonas fragariae in strawberry . Adv. Strawberry Res. , 15 : 13 – 17 .
- Norris , D.M. 1991 . Method for inducing resistance in plants using environmentally safe antioxidants , U.S. Patent No. 5,004,493 .
- Oostendorp , M. , Kunz , W. , Dietrich , B. and Staub , T. 2001 . Induced disease resistance in plants by chemicals . Eur. J. Plant Pathol. , 107 : 19 – 28 .
- Pierpoint , W.S. , Robinson , N.P. and Leason , M.B. 1981 . The pathogenesis-related proteins of tobacco: their induction by viruses in intact plants and their induction by chemicals in detached leaves . Physiol. Plant Pathol. , 19 : 85 – 97 .
- Roberts , P.D. , Jones , J.B. , Chandler , C.K. , Stall , R.E. and Berger , R.D. 1996 . Survival of Xanthomonas fragariae on strawberry in summer nurseries in Florida detected by specific primers and nested polymerase chain reaction . Plant Dis. , 80 : 1283 – 1288 .
- Roberts , P.D. , Berger , R.D. , Jones , J.B. , Chandler , C.K. and Stall , R.E. 1997 . Disease progress, yield loss, and control of Xanthomonas fragariae on strawberry plants . Plant Dis. , 81 : 917 – 921 .
- Romero , A.M. , Kousik , C.S. and Ritchie , D.F. 2001 . Resistance to bacterial spot in bell pepper induced by acibenzolar-S-methyl . Plant Dis. , 85 : 189 – 194 .
- Schmitz , M. and Noga , G. 1998 . Tocopherol reduced environmental stress and improved fruit quality . Acta Hortic. , 466 : 89 – 94 .
- Schmitz , I.M. , McNamar , D.G. , Scott , P.R. and Harris , K.M. 1992 . “ Xanthomonas fragariae ” . In Quarantine Pests for Europe. Data Sheets on European Communities and for the European and Mediterranean Plant Protection Organization , 829 – 833 . Wallingford : CAB International .
- Stall , R.E. and Thayer , P.L. 1962 . Streptomycin resistance of the bacterial spot pathogen and control with streptomycin . Plant Dis. Rep. , 16 : 389 – 392 .
- Turechek , W.W. and Peres , N.A. 2009 . Heat treatment effects on strawberry plant survival and angular leaf spot, caused by Xanthomonas fragariae, in nursery production . Plant Dis. , 93 : 299 – 308 .
- Vlot , A.C. , Dempsey , D.A. and Klessig , D.F. 2009 . Salicylic acid, a multifaceted hormone to combat disease . Annu. Rev. Phytopathol. , 47 : 177 – 206 .
- Xue , S. , Bors , R.H. and Strelkov , S.E. 2005 . Resistance sources to Xanthomonas fragariae in non-octoploid strawberry species . HortScience , 40 : 1653 – 1656 .